Back to Journals » Journal of Inflammation Research » Volume 15
Predictive Value of Ionized Calcium for Prognosis of Sepsis in Very Low Birth Weight Infants
Authors Zheng X , Li Y, Cheng Q, Wang L
Received 7 April 2022
Accepted for publication 23 June 2022
Published 1 July 2022 Volume 2022:15 Pages 3749—3760
DOI https://doi.org/10.2147/JIR.S369431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Xuejie Zheng, Yuanzhi Li, Qiyuan Cheng, Lili Wang
Department of Pediatrics, the First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
Correspondence: Lili Wang, Department of Pediatrics, the First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China, Tel +86 13075578390, Email [email protected]
Purpose: Previous studies have shown that ionized calcium (iCa) is strongly correlated with critical illnesses, including sepsis. However, there are few studies on the association of iCa levels and sepsis in very low birth weight infants (VLBWI). Therefore, the aim of this study was to investigate the role of iCa in assessing the severity of sepsis and in predicting the prognosis of sepsis in VLBWI.
Patients and Methods: 249 eligible VLBWI with sepsis were included in the present study and were divided into good and poor prognosis groups according to prognosis. We collected complete laboratory and clinical data. The lowest iCa measured during the first 24h from sepsis onset was recorded, and the Pediatric Risk of Mortality (PRISM III) score was calculated for each newborn.
Results: Neonatal mortality was higher in the hypocalcemia group (32.80% vs 12.80%, P < 0.001), and iCa levels were negatively correlated with PRISM III scores (r= − 0.819, P < 0.001). The result of multiple logistic regression analysis showed that iCa was an independent predictor of poor prognosis (odds ratio [OR]= 0.558, 95% confidence interval [CI], 0.406– 0.768, P < 0.001). Furthermore, our data demonstrated that iCa was also an independent predictor for the occurrence of death in VLBWI with sepsis who have a poor prognosis (OR= 0.659, 95% CI, 0.445– 0.977, P =0.038). ROC curve analysis showed that iCa had good discriminatory power in predicting the poor prognosis (AUC=0.739, 95% CI, 0.664– 0.813, P < 0.001) in VLBWI with sepsis.
Conclusion: iCa levels correlate with the severity of sepsis and can be an independent predictor of poor prognosis in VLBWI with sepsis.
Keywords: ionized calcium, sepsis, very low birth weight infants, poor prognosis
Introduction
Neonatal sepsis is a common deadly problem with a global impact including significant morbidity and mortality, especially in developing countries. Fleischmann-Struzek et al1 showed that neonatal sepsis has a mortality rate of up to 19% and is one of the leading causes of neonatal death. The morbidity of sepsis is closely related to birth weight, with the lower birth weight, the higher incidence.2 In addition to that, for very low birth weight infants (VLBWI), even if survival rates are gradually improving, the high rate of disability among survivors remains a concern.3–5 Therefore, for VLBWI with sepsis, death is not the only serious adverse outcome, and the risk of other severe complications such as cerebral injury should also be quantified.
Since better outcomes are closely related to timely treatment, it is important to find an indicator that can assess the severity of the disease and predict its prognosis of the disease. For this purpose, clinical risk index for babies (CRIB), the Score for Acute Neonatal Physiology—Perinatal Extension (SNAPPE), the Pediatric Risk of Mortality score (PRISM III) have been established to evaluate the severity of neonatal diseases in the NICU population.6 However, the CRIB score is calculated based on data within the first 12 hours of life and is more appropriate for assessing neonatal admission.7 The SNAPPE score requires the use of factors that are less sensitive for VLBWI, such as urine output.8 In addition this, since the PRISM III score was formulated, it has been confirmed by many studies to be related to the severity of the disease.9–13 The intuitive performance is that the higher the score, the more serious the condition is.9–13 Therefore, the PRISM III score is more appropriate for assessing disease severity at the onset of sepsis than the CRIB, SNAPPEII score. However, it requires the collection of a large amount of data to finally obtain a score, which is both clinically cumbersome and difficult.
Calcium is one of the most important elements of the human body and is an indispensable ion for various physiological activities in the body, participating in all vital activities and maintaining the normal physiological functions of cells. Calcium exists in the body in two forms: the bound state and the ionic state. However, only ionized calcium (iCa) is physiologically active.14,15 iCa is involved in blood coagulation, muscle contraction, synthesis, and release of neurotransmitters, and also plays an important role in cell adhesion and maintenance of cell membrane function. Carlstedt et al16 pointed out that the iCa correlated with the severity of the disease in critically ill patients, as many studies presented the same results.17–19 Some studies have confirmed that hypocalcemia was correlated with increased mortality, prolonged hospitalization in sepsis.14,17,18 Liu et al20 also pointed out that iCa was correlated with neonatal sepsis mortality. However, to date, no study has pointed out that iCa can be used as a predictor of poor prognosis in VLBWI with sepsis.
Therefore, we hypothesized that iCa could reflect the disease severity and prognosis of neonatal sepsis in VLBWI. To test this hypothesis, we investigated the correlation between iCa levels and Prism III in septic neonates, whether there was a difference in prognosis between the hypocalcemia and normal iCa groups, and whether iCa could be used as an independent predictor of poor prognosis.
Materials and Methods
Study Design and Definition
The study was a case-based cross-sectional study. We used the prognosis of VLBWI with sepsis at a corrected gestational age of 40 weeks as an outcome variable and collected laboratory indicators at the onset of the sepsis and basic characteristics of the VLBWI. In this study, iCa levels were obtained from arterial blood gases measured by the blood gas analyzer. We selected the lowest iCa value and the highest PRISM III score within the first 24 hours after the onset of the disease and before the complications had occurred yet. The severity of neonatal sepsis was assessed by calculating the Prism III score. To determine whether iCa levels correlated with the severity of sepsis, we compared neonatal outcomes and PRISM III scores in the normal iCa and hypocalcemia groups. In addition to this, the correlation between iCa levels and PRISM III scores were investigated. We also compared iCa levels in different prognostic groups to determine whether iCa could be used as a predictor of poor prognosis in VLBWI with sepsis.
iCa concentration< 4mg/dl(1mmol/L) was defined as hypocalcemia.21 Based on the published International Pediatric Sepsis Consensus, newborn sepsis is defined as suspected or confirmed infection with ≥2 systemic inflammatory response syndromes (SIRS).22
Study Population
In this study, we collected clinical data of 602 septic neonates treated in the Neonatal Intensive Care Unit (NICU), the First Affiliated Hospital of Anhui Medical University from January 2015 to December 2021. It is a comprehensive hospital. The neonatology department is set up with 95 beds (including 35 NICU beds) and has a professional team with excellent professional skills. There are 103 doctors and nurses. We are equipped with various types of monitoring and emergency equipment, including laminar flow wards, multiple types of ventilators, far-infrared radiation resuscitation tables, blue light therapy cabinets, multi-functional warming cabinets, multi-functional monitors, bedside blood gas analyzers, bedside X-ray, bedside ultrasound, bedside brain function monitoring, sub-cryogenic equipment, etc. We can complete peripherally inserted central catheter, umbilical venous catheter, neonatal respiratory support, peripheral arterial-venous exchange, and perioperative care for neonates in pediatric surgery, cardiac surgery, and brain surgery. Every year, we receive about 3500 cases of critically ill newborns from all over the province and neighboring provinces, with a success rate of over 95% in critical care. The lowest GA of premature infants survived was 24+4 weeks, and the lowest BW was 610 g. The level of neonatal care is leading in the province.
Study Grouping
These newborns were divided into normal iCa group (iCa≥4mg/dl) and hypocalcemia group (iCa< 4mg/dl) according to calcium levels.21 According to the prognosis, neonates were divided into a death group and a survival group, and the surviving neonates were further divided into a group with severe complications, and a group with mild complications or no complications. We defined newborns with severe complications and death as poor prognosis, and newborns with mild complications and no complications as good prognosis. We defined the occurrence of one or more of the following diseases after sepsis as a combined severe complication: grade 3 or 4 intraventricular hemorrhage (IVH),23 periventricular leukomalacia (PVL),24 grade 3 or higher retinopathy of prematurity (ROP),25 grade 3 necrotizing enterocolitis (NEC)26 or severe bronchopulmonary dysplasia (BPD).27 These diseases are related to long-term acute and chronic outcomes and neurodevelopmental disorders, which usually require rehospitalization and continuous medical treatment.28,29 We defined the occurrence of grade 1 or 2 IVH, stage 1 or 2 ROP, stage 2 NEC, and mild BPD as mild complications. Generally, newborns with these diseases only need follow-up after discharge and will not leave long-term sequelae.28 These complications were evaluated at 40 weeks of corrected gestational age. The procedure was shown in Figure 1.
 |
Figure 1 Flow chart of this study. |
Inclusion Criteria and Exclusion Criteria
The inclusion criteria were: (1) birth weight<1500g; (2) newborns within 0–28 days; (3) diagnosis of neonatal sepsis; and (4) completion of iCa measurement within the first 24 hours at the onset; (5) absence of calcium metabolism disorders before the onset of the disease; (6) without BPD, ROP, NEC, or cerebral injury before sepsis occurred.
The exclusion criteria were: (1) with other diseases, such as congenital heart disease, hematological system diseases, and major congenital malformation; (2) hypoparathyroidism; and (5) incomplete information.
Data Collection
The data collected for VLBWI with sepsis were as follows: (1) basic clinical data, including gender, gestational age(GA), birth weight(BW), mode of delivery, length of hospital stay, and so on; (2) Blood glucose, blood pressure(BP), heart rate(HR), body temperature and other data at the time of onset; (3) Laboratory indexes within the first 24 hours of onset, including white blood cell(WBC) count, neutrophil percentage, platelet(PLT) count, hemoglobin(Hb), hematocrit(Hct), high sensitivity C-reactive protein (hsCRP), procalcitonin(PCT), total protein(TP), albumin(ALB), total bilirubin(TBIL), alanine aminotransferase(ALT), aspartate aminotransferase(AST), iCa, total calcium(TCa), PH, blood sodium, blood potassium, blood phosphorus, and blood magnesium, etc; (4) Combined complications, and calculate PRISM III value.
PRISM III scores were calculated according to the following four parts:6,30 including four parts: cardiovascular/neurological vital signs, acid-base balance/blood gas, biochemical indexes, and hematological tests. Specific variables to be collected include systolic blood pressure (SBP), HR, body temperature, pupillary reflex, mental state, pH or bicarbonate ion concentration, oxygen partial pressure, carbon dioxide partial pressure, blood glucose, blood potassium, blood creatinine, blood urea nitrogen, leukocyte count, platelet count, prothrombin or partial thromboplastin time.
Statistical Analysis
The SPSS 25.0 software package (SPSS Inc, Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software Inc, San Diego, CA, USA) were used for data processing, and measures that conformed to a normal distribution were expressed as mean ± standard deviation (x ± s), and comparisons between two independent sample groups were made using the paired t-test. Non-normally distributed measures were expressed as median and interquartile spacing [P50 (P25, P75)], and group comparisons between two independent samples were performed using the Mann–Whitney U-test. Count data were expressed as the number of cases and percentages (%), and group comparisons were performed using the χ2 test or Fisher’s exact probability test. Univariate and multivariate binary logistic regression analyses were conducted to determine potential predictors of neonatal sepsis prognosis. The ROC curve was plotted and the area under the curve was calculated to determine the critical value. The correlation between quantitative data was analyzed by Spearman correlation test. P<0.05 was considered a statistically significant difference.
Results
Characteristics of Participants in Different Prognostic Groups
From January 2015 to December 2021, we collected clinical data on 602 septic neonates. Of the 602 septic neonates, 289 neonates were excluded due to birth weight ≥1500g, 41 were excluded with congenital anomalies or combined thyroid function abnormalities, and 23 were excluded for incomplete information. The remaining 249 eligible VLBWI with sepsis were included in this study. We divided these VLBWI with sepsis into three groups according to their outcome: death (n = 55), severe complications (n = 88), and mild or no complications (n = 106). The death and severe complication groups were collectively referred to as the poor prognosis group (n = 143), and the mild complication or no complication group was referred to as the good prognosis group (n = 106). Table 1 summarized the participant characteristics in different prognostic groups. As shown in Table 1, there was no statistical difference between the good prognosis group and the poor prognosis group in terms of gender, mode of delivery, temperature and heart rate, WBC, N, Hct, Hb, hsCRP, PCT, TP, TBIL, PH, Na, Mg at presentation, and maternal gestational factors. The BW, GA, PLT, ALB, TCa, iCa were lower in the poor prognosis group than in the good prognosis group (1.05 vs 1.27, P<0.05; 28.5 vs 30.2, P<0.05; 189.0 vs 192.5, P<0.05; 26.1 vs 28.6, P<0.05;2.28 vs 2.31, P<0.05; 3.76 vs 5.20, P<0.05, respectively), blood potassium and blood phosphorus were higher than in the good prognosis group (4.55 vs 4.20, P<0.05; 2.02±0.71 vs 1.82±0.46, P < 0.05, respectively). Further analysis showed that iCa, PLT, and ALB levels were lower in the death group compared to the severe complications group (3.40 vs 3.84, P<0.05; 69.0 vs 212.0, P<0.05; 25.8 vs 26.8, P<0.05, respectively). We found that iCa showed a significant progressive decrease among the three groups (5.20 vs 3.84 vs 3.40, P < 0.05). The indicators of the three groups of newborns could be shown more clearly in Figure 2.
 |
Table 1 Comparison of Clinical Data of Neonates in Different Prognostic Groups |
Relationship Between iCa Levels and Prognosis of Neonatal Sepsis
According to iCa levels, subjects were divided into a hypocalcemia group (n=116, iCa<4mg/dl) and a normal iCa group (n= 133, iCa≥4mg/dl). As shown in Table 2, CRP and PCT were not statistically significant between the two groups. Further analysis showed that the incidence of severe BPD (55.20% vs 12.80%; P < 0.001), grade 3 or higher ROP (31.90% vs 19.70%, P = 0.028), and cerebral injury (20.9% vs 8.3%, P < 0. 001) were significantly higher in the hypocalcemia group than in the normal iCa group. The incidence of grade 3 NEC was not statistically different between the two groups of neonates. In addition, mortality and PRISM III scores were significantly higher in the neonates of the hypocalcemia group than in the normal iCa group (12.8% vs 32.8%, P < 0. 001;6.00 vs 14.00, P < 0.001, respectively).
 |
Table 2 Clinical and Demographic Characteristics in the Hypocalcemia and Normal iCa Groups |
Ionized Calcium as an Independent Predictor in Determining the Prognosis of Sepsis in the VLBWI
Variables with P values < 0.05 in the univariate analysis, including GA, BW, PLT, ALB, blood potassium, blood phosphorus, iCa, and total calcium at presentation (Table 1), were selected for multivariate analysis. The results of the multivariable binary logistic regression analysis were revealed in Tables 3 and 4, GA, BW, ALB, and iCa were independent predictors of poor prognosis in VLBWI with sepsis. After excluding the PLT, blood potassium, blood phosphorus, and TCa (P≥0.05) indicators in Table 3, stepwise logistic analysis was again analyzed for GA, BW, ALB, iCa, and according to the results of stepwise logistic analysis (Table 5), it was assumed that iCa=X1, GA=X2, BW=X3, ALB=X4, the regression equation model was established: 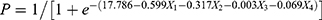 .
.
 |
Table 3 Logistic Analysis Results of Predictors for Poor Outcomes in VLBWI with Sepsis |
 |
Table 4 Results of Stepwise Logistic Regression Analysis of Predictors for Poor Outcomes in VLBWI with Sepsis |
 |
Table 5 Logistic Analysis Results of Predictors for Death in the Poor Prognosis Group |
Furthermore, as shown in Table 5, our data showed that iCa was an independent predictor for the occurrence of death in VLBWI with a poor prognosis for sepsis.
ROC curve analysis was used to assess the predictive value of iCa on the prognosis of sepsis. As shown in Figure 3A, the area under the ROC curve showed that iCa (AUC=0.727, 95% CI, 0.664–0.791, P <0.001) had a rather good predictive value for the poor prognosis of neonatal sepsis and was superior to GA(AUC=0.726, 95% CI, 0.663–0.788, P<0.001), BW(AUC=0.723, 95% CI, 0.656–0.790, P<0.001), ALB(AUC=0.591, 95% CI, 0.521–0.662, P=0.001), but inferior to PRISM III(AUC=0.743, 95% CI, 0.681–0.806, P<0.001). The optimal diagnostic threshold for predicting poor prognosis of iCa in VLBWI with sepsis was 5.02mg/dl, with a sensitivity of 79.7% and specificity of 57.5%. Moreover, we tested the value of iCa in predicting death in the poor prognosis group. As shown in Figure 3B, according to the AUCs and 95% CIs assessed, the rank order of variables predicting death in the poor prognosis group was PRISM III(AUC = 0.733, 95% CI, 0.647–0.820, P < 0.001), iCa(AUC = 0.666, 95% CI, 0.573–0.759, P = 0.001), PLT(AUC = 0.662, 95% CI, 0.569–0.759, P = 0.001), K(AUC = 0.650, 95% CI, 0.552–0.748, P = 0.002). Of these, the best cut-off value for iCa was 3.18 mg/dl, with a sensitivity of 40.0% and specificity of 88.6%.
Correlation Study of iCa Levels with PRISM III Scores
We divided sepsis patients into two groups according to PRISM III scores (the cut-off for grouping is the median). The level of iCa tended to decrease as the PRISM III score increased. iCa levels were lower in the PRISM III ≥ 9 group than in the PRISM III < 9 group (P < 0.05, Figure 4A). Meanwhile, Pearson correlation analysis revealed that iCa levels were negatively correlated with PRISM III scores (r= −0.819, P <0.001), as shown in Figure 4B.
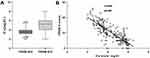 |
Figure 4 Correlation of iCa levels with PRISM III. (A) iCa levels in different PRISM III score groups; (B) scatter plot of correlation between iCa and PRISM III score. |
Discussion
Neonatal sepsis is an important cause of neonatal death,31 especially in VLBWI. Many studies as well have confirmed that BPD,32 cerebral injury,33–36 ROP,37 and NEC are serious complications closely related to sepsis. Mercier et al23 also showed that these complications were associated with later disability rates, this conclusion was also confirmed by many studies.23–27 In addition, in our hospital, the mortality rate of VLBWI with sepsis in our hospital was still as high as 17.5% in the past seven years, with a poor prognosis of 48.5%. Therefore, it is crucial to find an indicator that can determine the severity and prognosis of sepsis.
CRP, PCT, and other inflammatory markers are commonly used to diagnose and assess the severity of disease in patients with sepsis,38 but each has its advantages and disadvantages. CRP is synthesized in the liver, is an acute phase reactant protein, and takes longer to peak in neonatal sepsis.39,40 PCT is a glycoprotein produced by extra-thyroidal organs and is released into the blood in large quantities when a serious infection occurs.41 It can be detected after 3 hours and reaches a peak at 6–12 hours, which can be used as an early assessment indicator,39,41,42 but the basal secretion level of PCT varies with age, suggesting variable PCT criteria for sepsis.43,44 Some studies have shown that nonspecific inflammatory conditions, such as respiratory distress syndrome and inhalation injury, can also cause neonatal with elevated serum PCT concentrations.45,46 In the present study, CRP and PCT values did not differ statistically significantly among different groups of iCa levels and different prognostic groups, so both are more difficult to use as uniform indicators for early prognostic assessment.
Numerous adult studies have identified that iCa is correlated with a variety of diseases such as acute pancreatitis, hypoparathyroidism, and sepsis.14,47,48 The incidence of hypocalcemia in patients with disease varies considerably across studies.48 Of concern is that pediatric patients with sepsis may have a particularly high frequency of decreased iCa concentrations,49 which is consistent with the present study. In addition, a study conducted on neonates indicated that iCa was correlated with sepsis mortality.20 However, that study showed a 13.61% mortality rate, different from our study. The difference in the study population may explain this situation, as the study by Liu et al included a population with BW ≥ 1500 g compared to the VLBWI of our study. In addition, different from the outcome variables by Liu et al, we included VLBWI with severe complications in the poor prognosis group.
However, in patients with sepsis, the etiology or pathophysiology of hypocalcemia is not clear. Zaloga et al50 reported for the first time the mechanism of the inability to mobilize calcium in patients with sepsis. This study pointed out that hypocalcemia in patients with sepsis resulted from the efflux of calcium from the vascular space not met by the concomitant influx. The cause for defective calcium influx was multifactorial and resulted from acquired parathyroid gland insufficiency, renal la-hydroxylase insufficiency, dietary vitamin D deficiency, and acquired tissue resistance to calcitriol.50 In addition to this, many studies have shown that sepsis induces increased levels of various cytokines that can induce hypocalcemia.51–53 An animal study by Canaff et al51 showed that interleukin 1b induced hypocalcemia through upregulation of calcium-sensitive receptor (CASR) mRNA levels in the physical parathyroid, kidney, and parathyroid glands. It is consistent with the mechanism of interleukin 6-induced hypocalcemia in the study of Canaff et al.52 Patients with toxic shock syndrome or gram-negative bacterial sepsis have elevated levels of tumor necrosis factor-a (TNF-a), which is also associated with hypocalcemia.53 Therefore, the hypocalcemic effect caused by the combined upregulation of CASR by TNF-α, IL-1, and IL-6 in patients with sepsis is one of the possible mechanisms of hypocalcemia. In conclusion, multiple pathophysiological changes can lead to the development of hypocalcemia in sepsis.
In the present study, we first explored the correlation between iCa and the severity of neonatal sepsis. Because PRISM III scores were used clinically to assess the severity of disease,11–13,54 we investigated the correlation between iCa and PRISM III scores in the present study. The results of the present study showed that PRISM III scores were higher in the hypocalcemia group than in the normal iCa group. In the hypocalcemia group, there were a significantly higher number of neonates with poor prognosis than in the normal iCa group. Consistent with the results of previously reported studies in adults,16–19,55 the results of the adult study showed that iCa was correlated with disease severity. Meanwhile, the results of our study showed a negative correlation between iCa values and PRISM III scores, which further suggested that iCa could be used to assess disease severity in septic neonates. Given the negative correlation between iCa levels and disease severity, it was not surprising that hypocalcemia was correlated with a poor prognosis.
Our study showed that iCa levels were lower in the poor prognosis group compared to the good prognosis group. The iCa levels progressively decreased in the mild complication or no complication group, the severe complication group, and the death group. Multivariate analysis also showed that not only iCa, but also ALB levels, GA, and BW were independent predictors of poor prognosis in VLBWI with sepsis.BW and GA were correlated with poor prognosis, which was consistent with the results of many previous studies.56,57 Still, it is clear that BW and GA were constant indicators and did not vary with disease severity. Yang et al58 also noted that lower ALB levels might be associated with a poorer prognosis, but as our findings show, the AUC of ALB was lower than iCa. In addition, AUC curves showed the rather good discriminatory ability of iCa in predicting the death and combined severe complication groups. We also compared the iCa with the PRISM III score. Although the AUC of the PRISM III score was slightly higher than the iCa in predicting poor prognosis, the difference between both was not significant. The PRISM III score requires multiple indicators to calculate, which is cumbersome. Sometimes, the PRISM III could not be calculated due to the lack of individual indicators. Therefore, we considered the iCa superior to the PRISM III score in terms of clinical applicability.
Thus, iCa can be used as a predective indicator measured early in the onset of sepsis. It was fast, inexpensive, and required little skill. By predicting neonatal outcomes in sepsis, we may be able to take more aggressive measures early on to reduce the occurrence of this poor prognostic outcome. However, it is worth noting that iCa was only part of the multivariate regression analysis, and clinicians should still combine the other three variables (BW, GA, ALB) to provide a more comprehensive, meaningful guidance for clinical treatment.
There are several limitations of our study. First, this was a cross-sectional study conducted in a single center. Second, the iCa value we selected was the lowest value within the first 24 hours at onset, and the PRISM III score obtained was the highest score within the first 24 hours at onset, and we did not dynamically follow changes in either. Third, we only followed the infant at 40 weeks of corrected gestational age and did not follow the development of the infant at three months of age, one year, three years, or even beyond. Therefore, iCa can only be used as a short-term poor prognostic predictor for VLBWI with sepsis. Afterward, we will attempt to validate our findings in a multicenter and conduct a long-term follow-up of these children.
Conclusion
The iCa level was negatively correlated with the severity of neonatal sepsis and had a rather good predictive value for the prognosis of sepsis in VLBWI. Pediatricians should be aware that hypocalcemia alerts them to the risk of poor prognosis in VLBWI with sepsis.
Ethics Approval and Consent to Participate
Our study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. All manipulations involving humans in this study were under the principles in the Declaration of Helsinki. We confirm that all data are anonymous and confidential. Therefore, due to the retrospective nature of the current study, the requirement for informed consent has been waived.
Funding
There was no funding directly for this manuscript.
Disclosure
No potential conflicts of interest in relation to this work was reported by the authors.
References
1. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–230. doi:10.1016/S2213-2600(18)30063-8
2. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. doi:10.1016/S0140-6736(17)31002-4
3. Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi:10.1056/NEJMoa041367
4. van Haastert IC, Groenendaal F, Uiterwaal CS, et al. Decreasing incidence and severity of cerebral palsy in prematurely born children. J Pediatr. 2011;159(1):86–91. doi:10.1016/j.jpeds.2010.12.053
5. Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, Van den Broeck C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol. 2018;60(4):342–355. doi:10.1111/dmcn.13675
6. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24(5):743–752. doi:10.1097/00003246-199605000-00004
7. The International Neonatal Network. The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet. 1993;342(8865):193–198. doi:10.1016/0140-6736(93)92296-6
8. Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi:10.1067/mpd.2001.109608
9. Gemke RJ, van Vught J. Scoring systems in pediatric intensive care: PRISM III versus PIM. Intensive Care Med. 2002;28(2):204–207. doi:10.1007/s00134-001-1185-2
10. Shen Y, Jiang J. Meta-analysis for the prediction of mortality rates in a pediatric intensive care unit using different scores: PRISM-III/IV, PIM-3, and PELOD-2. Front Pediatr. 2021;9:712276. doi:10.3389/fped.2021.712276
11. Gonçalves JP, Severo M, Rocha C, Jardim J, Mota T, Ribeiro A. Performance of PRISM III and PELOD-2 scores in a pediatric intensive care unit. Eur J Pediatr. 2015;174(10):1305–1310. doi:10.1007/s00431-015-2533-5
12. Basu B, Sinha SK, Basu T, Mahapatra TKS. Factors predicting mortality in newborn ventilation. Pediatr Pulmonol. 2015;50(3):271–275. doi:10.1002/ppul.23019
13. Pasqualotto AC, de Moraes AB, Zanini RR, Severo LC. Analysis of independent risk factors for death among pediatric patients with candidemia and a central venous catheter in place. Infect Control Hosp Epidemiol. 2007;28(7):799–804. doi:10.1086/516658
14. Kelly A, Levine MA. Hypocalcemia in the critically ill patient. J Intensive Care Med. 2013;28(3):166–177. doi:10.1177/0885066611411543
15. Zaloga GP, Chernow B, Cook D, Snyder R, Clapper M, O’Brian JT. Assessment of calcium homeostasis in the critically ill surgical patient. The diagnostic pitfalls of the McLean-Hastings nomogram. Ann Surg. 1985;202(5):587–594. doi:10.1097/00000658-198511000-00009
16. Carlstedt F, Lind L, Rastad J, Stjernström H, Wide L, Ljunghall S. Parathyroid hormone and ionized calcium levels are related to the severity of illness and survival in critically ill patients. Eur J Clin Invest. 1998;28(11):898–903. doi:10.1046/j.1365-2362.1998.00391.x
17. Vincent JL, Bredas P, Jankowski S, Kahn RJ. Correction of hypocalcaemia in the critically ill: what is the haemodynamic benefit? Intensive Care Med. 1995;21(10):838–841. doi:10.1007/BF01700968
18. Chernow B, Zaloga G, McFadden E, et al. Hypocalcemia in critically ill patients. Crit Care Med. 1982;10(12):848–851. doi:10.1097/00003246-198212000-00008
19. Zhang Z, Xu X, Ni H, Deng H. Predictive value of ionized calcium in critically ill patients: an analysis of a large clinical database MIMIC II. PLoS One. 2014;9(4):e95204.
20. Liu Y, Chai Y, Rong Z, Chen Y. Prognostic value of ionized calcium levels in neonatal sepsis. Ann Nutr Metab. 2020;76(3):193–200. doi:10.1159/000508685
21. Thomas TC, Smith JM, White PC, Adhikari S. Transient neonatal hypocalcemia: presentation and outcomes. Pediatrics. 2012;129(6):2011–2659. doi:10.1542/peds.2011-2659
22. Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi:10.1097/01.PCC.0000149131.72248.E6
23. Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF; Vermont Oxford Network EIF-USG. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998–2003. Neonatology. 2010;97(4):329–338. doi:10.1159/000260136
24. Hamrick SE, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004;145(5):593–599. doi:10.1016/j.jpeds.2004.05.042
25. Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289(9):1124–1129. doi:10.1001/jama.289.9.1124
26. Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):19. doi:10.1136/adc.2006.099929
27. Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009;123(6):1562–1573. doi:10.1542/peds.2008-1962
28. Ge WJ, Mirea L, Yang J, Bassil KL, Lee SK, Shah PS. Prediction of neonatal outcomes in extremely preterm neonates. Pediatrics. 2013;132(4):2013–2702. doi:10.1542/peds.2013-0702
29. Behrman RE, Butler AS. Institute of Medicine (US) Committee on understanding premature birth and assuring healthy outcomes. In: Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US); 2007.
30. Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16(11):1110–1116. doi:10.1097/00003246-198811000-00006
31. Cantey JB, Baird SD. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics. 2017;140(4):2017–2044. doi:10.1542/peds.2017-0044
32. Ebrahimi ME, Romijn M, Vliegenthart RJS, Visser DH, van Kaam AH, Onland W. The association between clinical and biochemical characteristics of late-onset sepsis and bronchopulmonary dysplasia in preterm infants. Eur J Pediatr. 2021;180(7):2147–2154. doi:10.1007/s00431-021-03981-9
33. Gu M, Mei XL, Zhao YN. Sepsis and cerebral dysfunction: BBB damage, neuroinflammation, oxidative stress, apoptosis and autophagy as key mediators and the potential therapeutic approaches. Neurotox Res. 2021;39(2):489–503. doi:10.1007/s12640-020-00270-5
34. Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis. 2014;14(8):751–762. doi:10.1016/S1473-3099(14)70710-8
35. Villamor-Martinez E, Fumagalli M, Alomar YI, et al. Cerebellar hemorrhage in preterm infants: a meta-analysis on risk factors and neurodevelopmental outcome. Front Physiol. 2019;10:800. doi:10.3389/fphys.2019.00800
36. Wang LW, Lin YC, Wang ST, Yeh TF, Huang CC. Hypoxic/ischemic and infectious events have cumulative effects on the risk of cerebral palsy in very-low-birth-weight preterm infants. Neonatology. 2014;106(3):209–215. doi:10.1159/000362782
37. Wang X, Tang K, Chen L, Cheng S, Xu H. Association between sepsis and retinopathy of prematurity: a systematic review and meta-analysis. BMJ Open. 2019;9(5):2018–025440.
38. Schuetz P, Birkhahn R, Sherwin R, et al. Serial procalcitonin predicts mortality in severe sepsis patients: results from the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) study. Crit Care Med. 2017;45(5):781–789. doi:10.1097/CCM.0000000000002321
39. Yang AP, Liu J, Yue LH, Wang HQ, Yang WJ, Yang GH. Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med. 2016;54(2):345–351. doi:10.1515/cclm-2015-0277
40. Perrone S, Lotti F, Longini M, et al. C reactive protein in healthy term newborns during the first 48 hours of life. Arch Dis Child Fetal Neonatal Ed. 2018;103(2):F163–F166. doi:10.1136/archdischild-2016-312506
41. Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta. 2002;323(1–2):17–29. doi:10.1016/S0009-8981(02)00101-8
42. Arora S, Singh P, Singh PM, Trikha A. Procalcitonin levels in survivors and nonsurvivors of sepsis: systematic review and meta-analysis. Shock. 2015;43(3):212–221. doi:10.1097/SHK.0000000000000305
43. Turner D, Hammerman C, Rudensky B, Schlesinger Y, Goia C, Schimmel MS. Procalcitonin in preterm infants during the first few days of life: introducing an age related nomogram. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):17. doi:10.1136/adc.2005.085449
44. Fukuzumi N, Osawa K, Sato I, et al. Age-specific percentile-based reference curve of serum procalcitonin concentrations in Japanese preterm infants. Sci Rep. 2016;6(1). doi:10.1038/srep23871
45. Lapillonne A, Basson E, Monneret G, Bienvenu J, Salle BL. Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet. 1998;351(9110):1211–1212. doi:10.1016/S0140-6736(05)79165-0
46. Monneret G, Labaune JM, Isaac C, Bienvenu F, Putet G, Bienvenu J. Increased serum procalcitonin levels are not specific to sepsis in neonates. Clin Infect Dis. 1998;27(6):1559–1561. doi:10.1086/517758
47. Imrie CW, Allam BF, Ferguson JC. Hypocalcaemia of acute pancreatitis: the effect of hypoalbuminaemia. Curr Med Res Opin. 1976;4(2):101–116. doi:10.1185/03007997609109289
48. Desai TK, Carlson RW, Geheb MA. Hypocalcemia and hypophosphatemia in acutely ill patients. Crit Care Clin. 1987;3(4):927–941. doi:10.1016/S0749-0704(18)30527-X
49. Zaritsky A, Nadkarni V, Getson P, Kuehl K. CPR in children. Ann Emerg Med. 1987;16(10):1107–1111. doi:10.1016/S0196-0644(87)80465-1
50. Zaloga GP, Chernow B. The multifactorial basis for hypocalcemia during sepsis. Studies of the parathyroid hormone-vitamin D axis. Ann Intern Med. 1987;107(1):36–41. doi:10.7326/0003-4819-107-1-36
51. Canaff L, Hendy GN. Calcium-sensing receptor gene transcription is up-regulated by the proinflammatory cytokine, interleukin-1beta. Role of the NF-kappaB PATHWAY and kappaB elements. J Biol Chem. 2005;280(14):14177–14188. doi:10.1074/jbc.M408587200
52. Canaff L, Zhou X, Hendy GN. The proinflammatory cytokine, interleukin-6, up-regulates calcium-sensing receptor gene transcription via Stat1/3 and Sp1/3. J Biol Chem. 2008;283(20):13586–13600. doi:10.1074/jbc.M708087200
53. Carlstedt F, Lind L, Wide L, et al. Serum levels of parathyroid hormone are related to the mortality and severity of illness in patients in the emergency department. Eur J Clin Invest. 1997;27(12):977–981. doi:10.1046/j.1365-2362.1997.2310778.x
54. Zhang L, Wu Y, Huang H, et al. Performance of PRISM III, PELOD-2, and P-MODS scores in two pediatric intensive care units in China. Front Pediatr. 2021;9:626165.
55. Egi M, Kim I, Nichol A, et al. Ionized calcium concentration and outcome in critical illness. Crit Care Med. 2011;39(2):314–321. doi:10.1097/CCM.0b013e3181ffe23e
56. Yau GSK, Lee JWY, Tam VTY, Liu CCL, Wong IYH. Risk factors for retinopathy of prematurity in extremely preterm Chinese infants. Medicine. 2014;93(28):e314. doi:10.1097/MD.0000000000000314
57. Cunningham KE, Okolo FC, Baker R, Mollen KP, Good M. Red blood cell transfusion in premature infants leads to worse necrotizing enterocolitis outcomes. J Surg Res. 2017;213:158–165. doi:10.1016/j.jss.2017.02.029
58. Yang C, Liu Z, Tian M, et al. Relationship between serum albumin levels and infections in newborn late preterm infants. Med Sci Monit. 2016;22:92–98. doi:10.12659/MSM.895435
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


