Back to Journals » OncoTargets and Therapy » Volume 12
Predictive Significance Of Preoperative Systemic Immune-Inflammation Index Determination In Postoperative Liver Metastasis Of Colorectal Cancer
Received 16 July 2019
Accepted for publication 6 September 2019
Published 20 September 2019 Volume 2019:12 Pages 7791—7799
DOI https://doi.org/10.2147/OTT.S223419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Yao Lu, Dao Xin, Feng Wang
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China
Correspondence: Feng Wang
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East Road, Erqi District, Zhengzhou 450052, People’s Republic of China
Tel +86 139 3824 4776
Email [email protected]
Purpose: Systemic inflammation and immune dysfunction have been proved to be significantly associated with cancer progression and metastasis in colorectal cancer (CRC). The aim of this retrospective study was to investigate the association between preoperative systemic immune-inflammation index (SII) and postoperative liver metastasis in CRC.
Patients and methods: This retrospective study evaluated 182 patients with CRC who underwent surgical resection. The inflammation-based prognostic factors, including SII, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR) and prognostic nutritional index (PNI), were calculated based on preoperative laboratory data. The univariate and multivariate logistic regression analysis was performed to identify the risk factors correlated with postoperative liver metastasis in CRC. Receiver operating characteristic (ROC) curves and decision curve analysis (DCA) were respectively used to assess the predictive ability and clinical usefulness of SII for postoperative liver metastasis in CRC.
Results: The univariate and multivariable analysis confirmed SII was independently correlated with postoperative liver metastasis in CRC (p<0.001), and the ROC and DCA analysis demonstrated SII was superior to other inflammation-based factors in terms of predictive ability.
Conclusion: SII is an independent predictive indicator of postoperative liver metastasis for patients with colorectal cancer.
Keywords: colorectal cancer, systemic immune inflammation index, liver metastasis, predictive factor
Introduction
Colorectal cancer (CRC) was one of the most prevalent malignant tumors in the world, accounting for 1.4 million new cases and almost 700,000 deaths in 2012.1 Moreover, the incidence of new cases with colorectal cancer in China has increased in recent years.2 In 2015, there were 376,300 new cases estimated for CRC in China.3 Despite immense efforts in developing advanced treatments for this disease,4,5 the overall survival of colorectal cancer remained poor, with approximately 40% of patients who underwent curative surgery dying from their diseases, especially those with distant metastasis.6,7
Distant metastasis is one of the strongest independent prognostic factors of 5-year survival rate in CRC. The liver is the most common site for metastasis of CRC.3 About 15% of patients have liver metastasis at the time of diagnosis and an additional 20–23% developed into liver metastasis over time.8 Colorectal cancer patients with liver metastasis had no obvious symptoms at the early stage. Surgical resection is potentially curable in 25% and 5-year survival is approximately 50%.9 Therefore, the development and optimization of a simple and convenient prediction index for liver metastasis in CRC can not only provide clinical data for the basic research, but also acquire new ideas for the prevention of liver metastasis and form a complete prediction and intervention system.10
The invasion and migration of tumor are inextricably linked with inflammation. Systemic inflammation has been recognized as a part of tumor immune microenvironment and played an important role in the development and progression of many solid tumors.11,12 The existence of systemic inflammation, as measured by parameters such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR) and prognostic nutritional index (PNI), was reported to be correlated with poor prognosis of colorectal cancer.13–15 Recently, the systemic immune-inflammation index (SII), which is based on platelets, neutrophils, and lymphocytes in the complete blood count, has been shown to have independent prognostic value in many cancer types.16,17 It is of also interest that the relationship between the systemic immune environment and postoperative liver metastasis in CRC patients. Therefore, this study aimed to investigate the significance of SII as a possible marker to predict postoperative liver metastasis in patients with colorectal cancer and compare the clinical values with other inflammation-based prognostic factors (NLR, PLR, LMR and PNI).
Materials And Methods
Patients
This is a retrospective, cross-sectional study of diagnosed CRC patients in the First Affiliated Hospital of Zhengzhou University between January 2010 and December 2017. The eligibility criteria: 1) patients were diagnosed with primary colorectal cancer; 2) patients underwent radical resection in the First Affiliated Hospital of Zhengzhou University; 3) clinical records, including pathologic diagnosis, treatment strategy, follow-up information and laboratory data, were available and complete; and 4) first-line chemotherapy was provided after surgery. The exclusion criteria: 1) inflammatory bowel disease-related CRC, known hereditary CRC syndrome; 2) synchronous distant metastasis at the time of the initial diagnosis; 3) the patient received neoadjuvant chemo-radiotherapy or radiation therapy; 4) other malignant tumors in other organs; 5) hematologic malignancies, acute or chronic inflammatory disease, and other diseases that can affect inflammatory markers. The specific screening process of patients is shown in Figure 1. The study’s retrospective protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. All data were obtained in accordance with the Declaration of Helsinki. And we maintained the confidentiality of all patient privacy. For the retrospective study, the data were analyzed anonymously. The need for consent to participate was waived by Ethics Commission of the First Affiliated Hospital of Zhengzhou University.
 |
Figure 1 Flow chart for screening of patients. |
Clinical Data Collecting And Processing
Baseline data, including demographic information, routine blood test results, tumor markers, and pathologic data, were reviewed. Tumor characteristics, including tumor size, location and differentiation (well or poor), were noted based on both imaging and pathology reports from the surgical procedure. Tumor (T) and nodal stage (N) were based on the American Joint Committee on Cancer (AJCC) staging. SII, NLR, PLR, LMR and PNI were, respectively, calculated as follows: SII=P×N/L; NLR=N/L; PLR=P/L; LMR=N/M; PNI=Alb(g/L)+ 5×L(×109/L), where P, N, L and M stood for platelet, neutrophil, lymphocyte and monocyte counts, respectively, and Alb stood for serum albumin. Optimal cutoff values of SII, NLR, PLR, LMR and PNI were calculated according to receiver operating characteristic (ROC) curves. Cutoff values of age, tumor size and number of lymph nodes removed during surgery were adopted from median in this data. Cutoff values of CA125, CA199 and CEA were acquired according to inspection standard in our hospital. All diagnoses of liver metastasis were independently confirmed by CT scan by two radiologists. Patients with colorectal cancer were divided into positive group and negative group according to whether liver metastasis after surgery.
Statistical Analysis
SPSS 21 software and R software were used for data analysis. Relationships between liver metastasis and clinicopathologic factors were analyzed using the Chi square test or Fisher’s exact test. The Mann–Whitney U-test was used to analyze differences in the SII distributions between the patient groups. Significant risk factors for liver metastasis were analyzed first by univariate logistic regression analysis and then by multivariate logistic regression analysis. All tests were two-sided and p values less than 0.05 were considered significant. ROC analysis was performed to calculate the area under ROC curve (AUC) into evaluating the predictive significance of SII. Decision curve analysis (DCA) was conducted to determine the clinical usefulness by quantifying the net benefits at different threshold probabilities.
Results
Patient Characteristic
The clinical and pathologic characteristics are shown in Table 1. In the present study, we included 182 patients, 144 patients did not develop into liver metastasis and 38 patients developed into liver metastasis at the last follow-up. NLR, PLR, SII, LMR, PNI, CA125 and CEA were significantly associated with postoperative liver metastasis in CRC (all p<0.05).
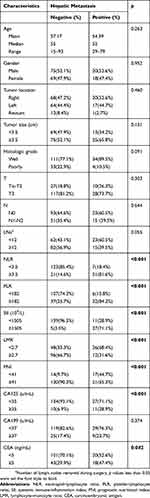 |
Table 1 Patients Demographics And Clinicopathological Characteristics |
Univariate And Multivariate Logistic Regression Analysis For Postoperative Liver Metastasis In CRC
As shown in Table 2, in univariate analysis, number of lymph nodes removed during surgery, NLR, PLR, SII, LMR, PNI, CA125 and CEA were significant prognostic factors (all p<0.1). Significant variables in univariate analysis were included in multivariate logistic regression analysis. We found that postoperative liver metastasis was independently associated with PLR (OR=8.21, 95% CI 2.22–30.33, p<0.05) and SII (OR=21.79, 95% CI 4.18–113.7, p<0.05).
 |
Table 2 Univariate And Multivariate Analysis Of Clinicopathologic Factors And SII For Hepatic Metastasis Of Patients With Colorectal Cancer |
Association Of SII And PLR With Postoperative Liver Metastasis
Patients were divided into two groups according to PLR or SII. Liver metastasis rates were 5.3% in the group with PLR lower than 182, while 46.4% patients evolved into liver metastasis in the group with PLR equal to or higher than 182 (p<0.001; Figure 2A). At the same time, liver metastasis rates were 7.3% in the group with SII lower than 1505×109/L, while 84.4% patients evolved into liver metastasis in the group with SII equal to or higher than 1505×109/L (p<0.001; Figure 2B). This suggested that patients with high SII or high PLR have a higher risk of postoperative liver metastasis in CRC.
 |
Figure 2 Association of PLR (A) and SII (B) with postoperative liver metastasis (LM). |
Comparison Between Inflammation Factors
We evaluated the predictive significance of these inflammation factors. AUC of SII, PLR, NLR, LMR and PNI were 0.882, 0.835, 0.843, 0.72 and 0.688, respectively (p<0.05; Figure 3A). The clinical usefulness of these inflammation indexes was analyzed by DCA, which showed that the net benefit of SII was better than other inflammation factors if the threshold probability exceeded 20%. These results indicated that SII was superior to NLR, PLR, LMR and PNI for predicting liver metastasis in patients with CRC (Figure 3B). Meantime, the association of SII with clinicopathologic characteristics was demonstrated (Table 3). And the high SII was associated with large tumor size, small number of lymph nodes removed during surgery, high NLR, high PLR, low LMR, low PNI and high CA125 level (all p < 0.05).
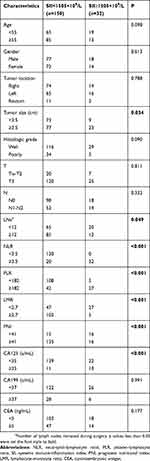 |
Table 3 Association Of SII With Clinicopathologic Characteristics In Patients With Colorectal Cancer |
Discussion
In recent years, radical surgery is still the main treatment for colorectal cancer.18,19 However, due to the limitations of current medical technology, postoperative liver metastasis of colorectal cancer plays an important role in prognosis, which is mainly attributed to the lack of reliable predictors for liver metastasis.6 This study found that SII was an independent risk factor for postoperative liver metastasis in patients with colorectal cancer, and the risk of postoperative liver metastasis in patients with high SII was significantly higher than patients with low SII. This provides a new idea for the prevention of postoperative liver metastasis in CRC.
Immune mechanisms have been associated with tumor progression in previous research.20 Elevated levels of inflammatory markers are often associated with more advanced disease, such as a greater tumor burden.21 Numerous studies have found that inflammation plays a key role in the development and progress of CRC,22,23 which may be related to the induction of DNA damage by leucocyte-derived reactive oxygen species. At the same time, the cancer cells produce large amounts of inflammatory cytokines, which induce leucocyte to accumulate around the tumor.24–26 Inflammatory cytokines have developed an important direction to study the proliferation, invasion and metastasis of colorectal cancer cells.27,28 Itzkowitz and Yio found that patients with chronic inflammation, such as ulcerative colitis and Crohn’s disease, are at increased risk for developing colorectal cancer.29 Burr et al emphasize that aspirin or non-aspirin non-steroidal anti-inflammatory drugs (NA-NSAIDs) prevent colorectal cancer (CRC) in patients with inflammatory bowel disease (IBD).30 Mariani et al examined the inflammatory pathways involved in the early steps of carcinogenesis, with particular emphasis on colorectal and suggested systemic inflammation of the large bowel (as in inflammatory bowel diseases) has been associated with the subsequent development of colorectal cancer.31 In conclusion, systemic inflammation plays an important role in the progress of colorectal cancer.
The mechanism of association between SII and liver metastasis in CRC is unclear. Neutrophils are a major subgroup of leukocytes that promote the angiogenesis, proliferation and metastasis of cancer cells through the production of angiogenic cytokines such as chemokines and vascular endothelial growth factors.32 Lymphocytes produce cytokines that inhibit the metastasis and proliferation of cancer cells and induce cytotoxic cells death.33 Lymphopenia is especially common in advanced cancer and reflects an inefficient immune system that may produce a favorable microenvironment for the spread of tumor cells.34 In the process of carcinogenesis, there is an increase in circulating neutrophils with the reduction of lymphocytes. Although the mechanism has not been clarified, several investigations have deduced its close correlations with interleukin 6, interleukin 8, vascular epidermal growth factor, and other cytokines.21,35 Inflammation induces changes in tumor microenvironment, which promotes tumor progression.36 In addition, platelets and platelet aggregation have been implicated in tumor progression, although the exact mechanism has not been elucidated.37 The mechanism of platelets in tumorigenesis might be derived from the role of platelets in promoting angiogenesis, adhesion, and invasion by increasing the production of vascular epidermal growth factor and transforming growth factor-beta in a tumor environment.35 Platelets are metastatic promoters, because of their ability to coat tumor cells making them unrecognizable for the natural killer cells produced by the immune system.38 Another research showed that platelets promote tumor metastasis by activating TGFβ and NF-κB signaling pathways.39 SII, which is calculated according to peripheral blood neutrophils, lymphocytes and platelets, is closely related to tumor microenvironment. Although the exact mechanism is not fully understood, there is more and more evidence that SII can help predict clinical outcomes for a variety of cancers.40–42 In this study, we evaluated the predictive ability of SII for postoperative liver metastasis in CRC, and the optimal cut-off value of SII was 1505×109/L, with a sensitivity of 71% and a specificity of 96.5%. These data indicate that SII has a strong ability to predict postoperative liver metastasis of CRC. Meanwhile, we compared the ability of SII with other inflammatory indicators such as NLR, PLR, LMR and PNI to predict postoperative liver metastasis in CRC. SII had significantly higher predictive ability and clinical application value for postoperative liver metastasis than other inflammatory indicators in CRC. The study indicated that compared with NLR, PLR, LMR and PNI, SII is the best indicator to predict postoperative liver metastasis in CRC.
There were several limitations to this study despite the demonstration of the predictive ability of SII in patients with CRC. First, there was a lack of follow-up and outcome data. Second, this is a retrospective analysis. Data on all patients were collected from a single institute and number of patients is relatively small. Therefore, conclusions from the present study may have a bias and larger prospective studies will need to be performed to confirm these preliminary results. Despite the above limitations, this study found the predictive significance of SII for postoperative liver metastasis in colorectal cancer patients. At the same time, because this indicator is simple, practical and economical, our results are still of great clinical application value.
Conclusion
The results of this study suggest that the SII has a moderately strong ability to accurately predict postoperative liver metastasis in patients with colorectal cancer. These findings should be carefully evaluated in future prospective studies.
Acknowledgments
This research was supported by the National Natural Science Funds of China (no. 81672442) and the Natural Science Founds of Henan Province (no. 182300410364)
Disclosure
The authors report no conflicts of interest in this work.
References
1. Buccafusca G, Proserpio I, Tralongo AC, Rametta Giuliano S, Tralongo P. Early colorectal cancer: diagnosis, treatment and survivorship care. Crit Rev Oncol Hematol. 2019;136:20–30. doi:10.1016/j.critrevonc.2019.01.023
2. Dai Z, Zheng RS, Zou XN, et al. Analysis and prediction of colorectal cancer incidence trend in China. Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46(7):598–603.
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi:10.1016/S1470-2045(14)70330-4
5. Grapsa D, Syrigos K, Saif MW. Bevacizumab in combination with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy for first-line and maintenance treatment of metastatic colorectal cancer. Expert Rev Anticancer Ther. 2015;15(11):1267–1281. doi:10.1586/14737140.2015.1102063
6. McArdle CS, Hole DJ. Outcome following surgery for colorectal cancer: analysis by hospital after adjustment for case-mix and deprivation. Br J Cancer. 2002;86(3):331–335. doi:10.1038/sj.bjc.6600120
7. Oliphant R, Nicholson GA, Horgan PG, Molloy RG, McMillan DC, Morrison DS. Contribution of surgical specialization to improved colorectal cancer survival. Br J Surg. 2013;100(10):1388–1395. doi:10.1002/bjs.9227
8. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–259. doi:10.1097/01.sla.0000217629.94941.cf
9. Coppa GF, Eng K, Ranson JH, Gouge TH, Localio SA. Hepatic resection for metastatic colon and rectal cancer. An evaluation of preoperative and postoperative factors. Ann Surg. 1985;202(2):203–208. doi:10.1097/00000658-198508000-00010
10. Wu Y, Li C, Zhao J, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol. 2016;14(1):289. doi:10.1186/s12957-016-1044-9
11. Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow inflammation outcome study. Br J Cancer. 2011;104(4):726–734. doi:10.1038/sj.bjc.6606087
12. Nguyen AV, Wu YY, Lin EY. STAT3 and sphingosine-1-phosphate in inflammation-associated colorectal cancer. World J Gastroenterol. 2014;20(30):10279–10287. doi:10.3748/wjg.v20.i30.10279
13. Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, et al. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers. 2019;2019:6036979. doi:10.1155/2019/6036979
14. Jian-Hui C, Iskandar EA, Sh I C, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277–3283. doi:10.1007/s13277-015-4008-8
15. Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative lymphocyte-to-monocyte ratio in patients with colorectal cancer. Oncol Lett. 2017;13(2):1000–1006. doi:10.3892/ol.2016.5487
16. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi:10.3748/wjg.v23.i34.6261
17. Xie QK, Chen P, Hu WM, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med. 2018;16(1):273. doi:10.1186/s12967-018-1638-9
18. Smith JA, King PM, Lane RH, Thompson MR. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90(5):583–592. doi:10.1002/bjs.4085
19. West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28(2):272–278. doi:10.1200/JCO.2009.24.1448
20. Cho YB, Chun HK, Yun HR, Lee WS, Yun SH, Lee WY. Clinical and pathologic evaluation of patients with recurrence of colorectal cancer five or more years after curative resection. Dis Colon Rectum. 2007;50(8):1204–1210. doi:10.1007/s10350-007-0247-0
21. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju061
22. Zhang H, Xia H, Zhang L, Zhang B, Yue D, Wang C. Clinical significance of preoperative neutrophil-lymphocyte vs platelet-lymphocyte ratio in primary operable patients with non-small cell lung cancer. Am J Surg. 2015;210(3):526–535. doi:10.1016/j.amjsurg.2015.03.022
23. Feng JF, Huang Y, Zhao Q, Chen QX. Clinical significance of preoperative neutrophil lymphocyte ratio versus platelet lymphocyte ratio in patients with small cell carcinoma of the esophagus. Sci World J. 2013;2013:504365. doi:10.1155/2013/504365
24. D’Inca R, Cardin R, Benazzato L, Angriman I, Martines D, Sturniolo GC. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 2004;10(1):23–27. doi:10.1097/00054725-200401000-00003
25. Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014;345(2):235–241. doi:10.1016/j.canlet.2013.07.032
26. Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–370. doi:10.1093/carcin/21.3.361
27. Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–146. doi:10.1016/j.critrevonc.2017.06.002
28. McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125(6):1298–1305. doi:10.1002/ijc.24409
29. Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G7–G17. doi:10.1152/ajpgi.00079.2004
30. Burr NE, Hull MA, Subramanian V. Does aspirin or non-aspirin non-steroidal anti-inflammatory drug use prevent colorectal cancer in inflammatory bowel disease? World J Gastroenterol. 2016;22(13):3679–3686. doi:10.3748/wjg.v22.i13.3679
31. Mariani F, Sena P, Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J Gastroenterol. 2014;20(29):9716–9731. doi:10.3748/wjg.v20.i29.9716
32. Tecchio C, Cassatella MA. Neutrophil-derived cytokines involved in physiological and pathological angiogenesis. Chem Immunol Allergy. 2014;99:123–137. doi:10.1159/000353358
33. Spicer JD, McDonald B, Cools-Lartigue JJ, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72(16):3919–3927. doi:10.1158/0008-5472.CAN-11-2393
34. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
35. Suzuki K, Aiura K, Ueda M, Kitajima M. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas. 2004;29(2):132–140. doi:10.1097/00006676-200408000-00008
36. Kurt M, Onal IK, Sayilir AY, et al. The role of mean platelet volume in the diagnosis of hepatocellular carcinoma in patients with chronic liver disease. Hepato-gastroenterology. 2012;59(117):1580–1582. doi:10.5754/hge10444
37. Takagi S, Sato S, Oh-hara T, et al. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS One. 2013;8(8):e73609. doi:10.1371/journal.pone.0073609
38. Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300.
39. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi:10.1016/j.ccr.2011.09.009
40. Liang R, Chen N, Li M, Wang X, Mao Q, Liu Y. Significance of systemic immune-inflammation index in the differential diagnosis of high- and low-grade gliomas. Clin Neurol Neurosurg. 2018;164:50–52. doi:10.1016/j.clineuro.2017.11.011
41. Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer. 2017;36(1):75. doi:10.1186/s40880-017-0243-2
42. Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15(1):221. doi:10.1186/s12967-017-1326-1
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

