Back to Journals » Patient Preference and Adherence » Volume 13
Predictive factors of adherence to an association of glucosamine sulfate, copper, and ginger extracts in patients with symptomatic osteoarthritis: a prospective open-label French noninterventional study (the PREDOA study)
Authors Conrozier T, Renevier JL , Parisaux JM, Balblanc JC
Received 9 January 2019
Accepted for publication 2 May 2019
Published 7 June 2019 Volume 2019:13 Pages 915—921
DOI https://doi.org/10.2147/PPA.S200892
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Thierry Conrozier,1 Jean-Luc Renevier,2 Jean-Marc Parisaux,3 Jean-Charles Balblanc1
1Service de Rhumatologie, Hôpital Nord Franche-Comté, Belfort, France; 2Centre Médico Chirurgical du Mantois, Mantes-la-Joli, France; 3Institut Monégasque de Médecine et Chirurgie Sportive, Monaco, Principauté de Monaco
Background: Osteoarthritis (OA) management needs a combination of nonpharmacological and pharmacological modalities. However, as in many chronic conditions, the main concern with OA therapy is the difficulty in obtaining good medication compliance over a long period. The PREDOA study aimed to investigate the predictive factors of adherence to treatment in patients with OA treated with glucosamine sulfate (GS)–copper sulfate–ginger root (GCu), a symptomatic slow-acting drug for OA.
Methods: Ambulatory patients with a clinical diagnosis of OA were included in a prospective (6 months) multicenter open-label observational study. All patients received two capsules of GS-GCu once daily for 6 months. Demographics, disease features (OA location, symptom duration, concomitant therapies, comorbidities), and patient self-assessment of pain (0–10) were obtained at baseline. At month 6, the investigator collected patient self-assessments of treatment observance, reasons for nonadherence, pain scores, patient perceptions of treatment efficacy, changes in analgesic intake, and occurrence of adverse events. Predictors of observance were studied in univariate and multivariate analyses.
Results: A total of 2,030 patients were included in the study. At baseline, the average pain score was 6.4±1.7. Observance was good in 80% of patients. It was weaker in active than retired patients (P=0.005) in patients not taking concomitant treatment (P=0.008) or who had never been treated for OA (P=0.001). Observance was correlated with pain decrease (P<0.0001) and with lack of adverse effects (P<0.001). Age, sex, pain level at baseline, OA location, and number of painful joints were not related to treatment compliance.
Conclusion: Medication compliance with GS-GCu depends both on the safety–efficacy balance and several patient related-factors. To improve adherence, detailed information about therapeutic objectives is necessary in active patients who do not get any other medications and for whom it is their first treatment for OA.
Clinical trial identifier: CCTIRS 14-371 B.
Keywords: compliance, symptomatic slow acting drug for osteoarthritis (SYSADOAs), pain, knee OA, hip OA, hand OA
Introduction
Osteoarthritis (OA) is the most frequent musculoskeletal cause of pain and disability in adults aged >50 years.1 Guidelines recommend that OA treatment must be based on a combination of nonpharmacological and pharmacological measures, including analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), symptomatic slow-acting drugs for OA (SYSADOA), and intra-articular injections of corticosteroids and/or hyaluronic acid.2–6 Many food supplements and phytotherapy drugs (eg, curcumin, ginger extracts, and harpagophytum) are also frequently used as adjunctive treatments, and have been demonstrated to provide moderate but clinically meaningful effects on pain and function in knees and hips.7–9 SYSADOA, including glucosamine sulfate (GS), chondroitin sulfate (CS) and avocado and soybean unsaponifiables, are primarily recommended to reduce pain and thusavoid the use of NSAIDs,10 that must be used with caution, because of a high risk of serious adverse events (AEs), especially in the elderly.11–13 The clinical effectiveness of GS and CS has been demonstrated in many randomized controlled trials, meta-analyses, and systematic reviews that concluded GS and CS demonstrated a moderate but statistically significant analgesic effect in patients with knee and hip OA.14–19 A structure-modifying effect has also been shown in knee OA.20–22 However, this chondroprotective effect is at best mild, and would requireclinical relevance to be used as a long-term treatment (several years). In order to optimize the efficacy of GS, a new therapeutic association consisting of a combination of GS, copper sulfate, and ginger root (GS-GCu), has been developed. Ginger-root extracts have been shown to inhibit proinflammatory mediators in human and arthritic cartilage23 and to be superior to placebo in relieving pain in subjects with knee OA.24,25 Copper sulfate has demonstrated its ability to promote the synthesis of collagen by human articular chondrocytes in vitro.26 The antioxidant effect of GS-GCu has been investigated on HEK293 TRex cells to study Nox4 activity.27 Nox4 activity decreased significantly by 30% after incubation with GS-GCu. Conversely a decrease in Nox4 activity was not observed with GS alone, indicating that Nox4 inhibition was triggered by ginger and copper sulfate. In human C20/A4 chondrocytes, increased expression of ADAMTS5, observed upon IL1β activation was reversed in the presence of both GS and GS-GCu. On the other hand, GS-GCu, copper sulfate and ginger extracts, but not CS alone, had a significant inhibitory effect on MMP1 expression. The same authors also observed a positive impact of GS-GCu on HO1) expression, in IL1β-treated C20/A4 chondrocytes. HO1 overexpression was twice as high with GS-GCu than with GS alone. Oxidative stress has been proven to be a main contributor to OA severity and development. As HO1 has been shown to play a major role in the oxidative stress response in chondrocytes, stimulating HO1 may be considered a relevant target for OA treatment.28
Because most OA patients are elderly and suffering from comorbidities, contraindicating the use of NSAIDs,13 it is logical to prescribe CS or GS as first-line therapies, giventheir excellent tolerance profile, in OA patients whose pain is not sufficiently relieved by nonpharmacological modalities and analgesics.29 However, as in many other chronic conditions, the main concern with OA therapy is obtaining good medication compliance over a long period.30–32
The PREDOA study (PREDictive factors of Observance in OA), was designed to investigate whether some patients or disease characteristics (eg, sex, age, professional status, OA severity and duration, affected joint[s], comorbidities, and co-prescriptions) might impact compliance with a background OA treatment of GS-GCu.
Methods
Study design and regulations
Patients with symptomatic OA were included in a prospective, observational, multicenter, 6-month study. To ensure good representation of the French population, 141 independent physicians or hospital rheumatologists homogeneously distributed all over France (seven regions: northwest, southwest, north and Ile-de-France, northeast, south, and southeast) participated in this study. PREDOA received the approval of the French Comité Consultatif sur le Traitement de l’Information en Matière de Recherche dans le Domaine de la Santé and the Commission Nationale de l’Informatique et des Libertés. The study wasconducted in accordance with good clinical practice (decision of November 24, 2006) and the Declaration of Helsinki (ethical principles for medical research involving human subjects).
Patient selection
Inclusion criteria
All ambulatory patients, females and males, whatever their age, with a clinical diagnosis of OA who required treatment with SYSADOA according to a rheumatologist’s opinion were included.
Exclusion criteria
Patients with a history of hypersensitivity to glucosamine or shellfish, known adverse reactions to ginger or copper, those in whom screening could not be performed reliably (cognitive disorders or language problem), or who were unable to give informed consent were not included in the study.
Study design
During the screening visit, the investigator handed the patient a document providing key information about the study and obtained their written informed consent for participating in the trial. Then he/she collected demographic characteristics (age, sex, weight, height, body-mass index), disease features (OA location, symptom duration, previous and current treatments for OA, concomitant therapies for comorbidities). Patient self-assessment of pain at rest and during exercise was obtained using an 11-point numeric rating scale (0–10). At the end of the screening visit, the investigator prescribed the patient two capsules of GS-Cu (Cuiramine; Laboratoire Labrha, Lyon, France) once daily for 6 months. Each capsule of GS-CU contained 750 mg GS, 1 mg copper sulfate, 50 mg ginger-root extracts, and 12 mg vitamin C.
At the last study visit 6 months later, the investigator recorded on the clinical report formthe patient's self-assessment of treatment observance and possible reasons for noncompliance, pain scores at rest and exercise, patient perception of treatment efficacy (0 for not effective, 1 slightly effective, 2 effective, and 3 very effective), change in analgesic intake (<25%, 26%–50%, 51%–75%, >75%) and occurrence of any AE.
All concomitant OA treatments (ie, analgesics, NSAIDs, intra-articular injections, physiotherapy, spa therapy) excepted other SYSADOA and all therapies prescribed for associated pathology were allowed during the 6-month study. These were recorded on the clinical report form.
Statistics
The primary end point was patient self-reported treatment compliance. This was estimated in a binary manner: “yes” if they had taken 90% or more of the treatment and “no” if they had taken <90% during the 6 month follow-up. Noncompliant patients were divided into two groups: those who discontinued the treatment and those who took it intermittently (<90% of the time). Predictors of treatment observance (demographic, clinical, pathological, therapeutic) were studied in univariate and multivariate analyses from the per protocol population. Multivariate analysis included sex, age, pain at baseline, body-mass index, and all variables with P<0.2 in the univariate analysis. Regression coefficients of the multivariate models (ANCOVA and mixed model) were considered significant if the degree of significance was <5%. Statistics were analyzed using Xlstats software (Addinsoft, Paris, France).
Results
A total of 2,030 patients were included in the study, and 1,730 (85%) completed the 6-month visit. The population was in accordance with what was expected: 72% female, average age 66.5 years, mean body-mass index 26.3 kg/m2, 70% not working, and 51% with multisite OA. Knee OA was the main reason for GS-GCu prescription (66%). Cervical and lumbar spine (42%), hand (33%), and hip (18%) were the next–most frequent OA locations. Sixty percent of patients suffered from comorbidities (the most frequent were arterial hypertension, dyslipidemia, and diabetes), 62% were taking one or more medications (average pills/day 3.6±2.8). At baseline, the average pain score was 3.6±2.2 at rest and 6.4±1.7 on exercise without significant differences according to OA location. Patient characteristics at baseline are summarized in Table 1.
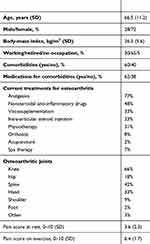 | Table 1 Patient characteristics at baseline (n=2,030) |
Medication compliance, the primary end point of the trial, was self-reported as good by 80% of patients. Among the 20% of nonobservant patients, 5.2% had temporarily suspended the treatment before resuming it and 14.8% had discontinued treatment prematurely.
At the end of the study, pain scores were significantly lower than at baseline (1.8±2.0 and 3.5±3.2 at rest and during exercise, respectively; P<0.001). Overall, 83% reported improvement in pain. Regarding efficacy, 13% of patients rated the treatment as highly effective, 50% effective, 25% moderately effective, and 12% ineffective. Tolerability was rated as excellent in 97% of patients. Among patients with AEs (3%), about half (1.7%) reported gastrointestinal disturbances (diarrhea, bloating, constipation, epigastric pain) and 0.5% allergic skin reactions. No severe AE was reported.
Observance to medication was significantly weaker in active subjects than in retired patients (P=0.005), in patients not taking concomitant treatment (P=0.008) and in patients who had not been treated before with SYSADOA (P=0.001). Unsurprisingly, observance was strongly correlated with pain decrease over time (P<0.0001), but not with relief-occurrence delay. Observance was also related to the lack of AEs. In nonobservant patients, 18% had experienced an AE, while this datum was only 2% in observant patients (P<0.001). Neither age, sex, pain level at baseline, OA location, nor number of painful joints were related to treatment compliance. In multivariate analysis, observance was still related to pain decrease, absence of AEs, and lack of concomitant medications (P=0.003).
Discussion
Adherence to antirheumatic medications is known to be low, as in many chronic conditions. Factors that have been shown to influence adherence to OA treatments include dosing frequency, pain and efficacy levels, medication price, confidence in the doctor, fear of addiction, previous treatment effectiveness and safety, and increased pill numbers.32 By enhancing medication compliance, it is likely that treatment efficacy is improved. This has been shown in a cohort of 4,822 patients with OA in which compliant patients significantly improved their quality of life compared to the noncompliant patients.33
In the PREDOA trial, medication adherence was high (80%), much higher than usually observed in this therapeutic area.34 However, our results are possibly biased. One can state the hypothesis that patients consulting a rheumatologist for participation in a clinical study are more likely to be compliant with their treatment than patients followed in daily medicine.
The main strength of our study is the sample size and lack of stringent inclusion/exclusion criteria, which allowed the study population to be well representative of the “real-life” one. Another important point to underline is that in the PREDOA study, patients had to pay the cost of treatment (€26/month). This is very important, since it has been shown that in controlled trials, during which patients do not have to pay to be treated, adherence to treatment was better than in “real life”.
We identified several criteria that strongly influenced treatment observance. The main predictive factors of strong observance were (unsurprisingly) treatment efficacyperceived by the patient and tolerability. The study highlights the very high correlation between decrease in pain and medication compliance, as well as between lack of AEs and observance. However, relief-occurrence delay was not correlated with medication compliance, probably because patients had been well informed of the mode of action of the symptomatic long-acting antiarthritics and the relief-occurrence delay to be expected. The other interesting points of this study, to our knowledge never reported in OA, were that medication compliance was significantly worse in active than retired patients, in patients who were not taking concomitant treatments for comorbidities, and in patients who had never been previously treated with SYSADOA. These data suggest that to get good adherence to OA treatment, detailed information about objectives and how to take the treatment is necessary, especially in active patients without comorbidities or concomitant treatment and in subjects for whom it is the first prescription of a SYSADOA.
Our study suffers from several limitations. The main one was the patients' self-reporting of observance, which may have overstated actual adherence to treatment. Another limitation is the absence of function assessment. We only evaluated pain variation over time, but not disability, so we cannot prove that CS-GCu also improved joint function. We did not take into account possible interactions between CS-GCu and other drugs in patients taking polymedication either. Indeed, it has been shown that many botanical dietary supplements may interact with therapeutic agents with respect to absorption, transport, and metabolism.35 The design of the study, which aimed to study compliance but not effectiveness, and the absence of a control group do not allow a conclusion on the real effectiveness of CS-GCu. However, it can be argued that better adherence was statistically related to better efficacy, as perceived by patients.
Lastly the good observance reported in our study may have been due at least in part to a strong therapeutic alliance (TA) between rheumatologists and patients. TA is defined as the relationship between the health-care provider and the patient, developed during the care process, that begins to develop from the first visit and continues developing throughout the treatment course. Initially described in psychiatry, this analytic concept has been extended to several chronic conditions requiring a strong relationship between caregiver and patient. Its measurement is important, since several studies have demonstrated that TA is predictive of treatment success.36–39 Indeed, it has been established that good medication compliance improves the long-term prognosis of the disease, while weak medication adherence increases the risk of treatment failure, symptom worsening, and disease progression.36 Unfortunately, in our work we did not specifically evaluate TA, assessment of which requires the use of complex questionnaires.39
In conclusion, this large-scale prospective trial shows that adherence to CS-GCu treatment depends both on the safety–efficacy balance of the treatment and on several patient related-factors. To improve adherence to SYSADOA, detailed information about therapeutic objectives is particularly necessary in active patients, especially in those who do not usually get medications and/or in patients for whom it is the first prescription of a SYSADOA. However, the results of the present study with GS-GCu must be carefully extrapolated to the other SYSADOA, the investigated treatment having specific antiarthritic mechanisms of action.
Informed consent
Before enrolling in the study, all patients had to give written informed consent.
Data-sharing statement
All data related to the PREDOA trial are accessible from Labrha SAS, 19 Place Tolozan, Lyon 69001, France for a 15-year period.
All information concerning the study remains the property of the promoter (Labrha). Only people involved in the conduct of the study, who are bound by professional secrecy in accordance with article R5120 of the CSP, can have access to deidentified patient data. The investigators were allowed to use patient information only for the conduct of this study. The collection and processing of data by the investigators or members of staff were carried out respecting the anonymity of the patients. Medical information obtained for each patient during this study is considered confidential, and any disclosure to a third party is prohibited.
The promoter and the service-provider organization ensure the confidentiality of the data of study participants in accordance with European directives and the French Informatique et Libertés law. Patients were informed of their right to access and correct data concerning them.
Acknowledgments
The authors thank all the investigators who participated in the PREDOA study, Vesna Yengo for statistical assistance and Elise Murat for writing assistance. The PREDOA study was funded by Labrha SAS, 19 Place Tolozan, Lyon 69001, France
Disclosure
TC received fees from Labrha SAS for scientific consultancy and speaking services during the study. The other authors report no conflicts of interest in this work.
References
1. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi:10.1016/j.cger.2010.03.001
2. Jordan KM, Arden NK, Doherty M. Standing Committee for International Clinical Studies Including Therapeutic Trials ESCISIT. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:114555.
3. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981. doi:10.1016/j.joca.2007.06.014
4. Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:377–388.
5. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi:10.1016/j.joca.2008.02.013
6. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. doi:10.1016/j.joca.2009.11.014
7. Henrotin Y, Lambert C, Couchourel D, Ripoll C, Chiotelli E. Nutraceuticals: do they represent a new era in the management of osteoarthritis? - A narrative review from the lessons taken with five products. Osteoarthritis Cartilage. 2011;19:1–21. doi:10.1016/j.joca.2010.10.017
8. Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. 2018;52(3):167–175. doi:10.1136/bjsports-2016-097333
9. Wang A, Leong DJ, Cardoso L, Sun HB. Nutraceuticals and osteoarthritis pain. Pharmacol Ther. 2018;187:167–179. doi:10.1016/j.pharmthera.2018.02.015
10. Dougados M. Symptomatic slow acting drugs for osteoarthritis: what are the facts. Joint Bone Spine. 2006;73:606–609. doi:10.1016/j.jbspin.2006.09.008
11. Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167(4):394–399. doi:10.1001/archinte.167.4.394
12. Sudano I, Flammer AJ, Roas S, Enseleit F, Noll G, Ruschitzka F. Nonsteroidal antiinflammatory drugs, acetaminophen, and hypertension. Curr Hypertens Rep. 2012;14:304–309. doi:10.1007/s11906-012-0274-7
13. Lanas A, Tornero J, Zamorano JL. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann Rheum Dis. 2010;69:1453–1458. doi:10.1136/ard.2009.123166
14. McAlindon DM, La Valley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis. A systematic quality assessment and metaanalysis. JAMA. 2000;283:1469–1475.
15. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int. 2010;30:357–363. doi:10.1007/s00296-009-0969-5
16. Black C, Clar C, Henderson R, et al. The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1–148. doi:10.3310/hta13520
17. Chevalier X, Conrozier T. Access to highly purified chondroitin sulfate for appropriate treatment of osteoarthritis: a review. Med Access Point Care. 2017;1(1):e134–e144. doi:10.5301/maapoc.0000022
18. Bruyère O, Altman RD, Reginster JY. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S12–S17. doi:10.1016/j.semarthrit.2015.11.011
19. Simental-Mendía M, Sánchez-García A, Vilchez-Cavazos F, Acosta-Olivo CA, Peña-Martínez VM, Simental-Mendía LE. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol Int. 2018;38(8):1413–1428. doi:10.1007/s00296-018-4077-2
20. Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster J-Y. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:524–3340. doi:10.1002/art.24255
21. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256. doi:10.1016/S0140-6736(00)03610-2
22. Pavelka K, Gatterova J, Olejarova M, et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162:2113–2123. doi:10.1001/archinte.162.18.2113
23. Shen CL, Hong KJ, Kim SW. Comparative effects of ginger root (Zingiber officinale Rosc.) on the production of inflammatory mediators in normal and osteoarthrotic sow chondrocytes. J Med Food. 2005;8:149–153. doi:10.1089/jmf.2005.8.149
24. Altman RD1, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44(11):2531–2538.
25. Bartels EM, Folmer VN, Bliddal H, et al. Efficacy and safety of ginger in osteoarthritis patients: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage. 2015;23(1):13–21. doi:10.1016/j.joca.2014.09.024
26. Héraud F, Savineau C, Harmand MF. Copper modulation of extracellular matrix synthesis by human articular chondrocytes. Scand J Rheumatol. 2002;31:279–284.
27. Rousset F, Grange L, Nguyen MVU, et al. Impact of the addition of ginger extract and copper sulphate to glucosamine sulphate on Il-1β-stimulated chondrocytes. J Rheum Dis Treat. 2016;2:038. doi:10.23937/2469-5726/1510038
28. Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. doi:10.1016/j.coph.2016.02.009
29. Bruyère O, Cooper C, Pelletier JP, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2014; pii:S0049-0172(14)00108-5. doi:10.1016/j.semarthrit.2014.05.014
30. Saliba L, Boisson L, Sallerin B, Laroche M, Despas F. The lack of adherence to antiosteoporotic drugs: crossing methods, the key to adherence measurement? Therapie. 2014;69(5):383–389. doi:10.2515/therapie/2014028
31. Murage MJ, Tongbram V, Feldman SR, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence. 2018;12:1483–1503. doi:10.2147/PPA.S167508
32. Laba TL, Brien JA, Fransen M, Jan S. Patient preferences for adherence to treatment for osteoarthritis: the Medication Decisions in Osteoarthritis Study (MEDOS). BMC Musculoskelet Disord. 2013;61(14):160. doi:10.1186/1471-2474-14-160
33. Głuszko P, Stasiek M. Symptom-modifying effects of oral avocado/soybean unsaponifiables in routine treatment of knee osteoarthritis in Poland. An open, prospective observational study of patients adherent to a 6-month treatment. Reumatologia. 2016;54(5):217–226.
34. Sharma S, Roshi, Tandon VR, Mahajan A. A Study Evaluating Adherence and Compliance of Anti-rheumatic Drugs in Women Suffering from Rheumatoid Arthritis. J Clin Diagn Res. 2015;9(11):OC01–4. doi: 10.7860/JCDR/2015/15806.6729 1
35. Sprouse AA, van Breemen RB. Pharmacokinetic interactions between drugs and botanical dietary supplements. Drug Metab Dispos. 2016;44(2):162–171. doi:10.1124/dmd.115.066902
36. Hausner RS. The therapeutic and working alliances. J Am Psychoanal Assoc. 2000;48(1):155–187. doi:10.1177/00030651000480011001
37. Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J Consult Clin Psychol. 2000;68(3):438–450.
38. Benoit M, Pon J, Zimmermann MA. Comment évaluer la qualité de l’observance? L’Encéphale. 2009;Supplément 3:S87–S90. doi:10.1016/S0013-7006(09)75542-3
39. Kermarrec S, Kabuth B, Bursztejn C, Guillemin F. French adaptation and validation of the helping alliance questionnaires for child, parents, and therapist. Can J Psychiatry. 2006;51(14):913–922. doi:10.1177/070674370605101407
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
