Back to Journals » Cancer Management and Research » Volume 12
Predictive Factors and Long-Term Outcomes of Early Gastric Carcinomas in Patients with Non-Curative Resection by Endoscopic Submucosal Dissection
Authors Xu P , Wang Y, Dang Y, Huang Q, Wang J, Zhang W, Zhang Y, Zhang G
Received 20 May 2020
Accepted for publication 21 August 2020
Published 4 September 2020 Volume 2020:12 Pages 8037—8046
DOI https://doi.org/10.2147/CMAR.S263525
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Ping Xu,1,2,* Yun Wang,1,* Yini Dang,1,* Qin Huang,3 Jianhua Wang,2 Weifeng Zhang,1 Yifeng Zhang,4 Guoxin Zhang1
1Department of Gastroenterology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Gastroenterology, Yancheng City No.1 People’s Hospital, Yancheng, Jiangsu, People’s Republic of China; 3Department of Pathology and Laboratory Medicine, VA Boston Healthcare System and Harvard Medical School, Boston, MA, USA; 4Department of Gastroenterology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guoxin Zhang Department of Gastroenterology
The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, People’s Republic of China
Email [email protected]
Qin Huang
Department of Pathology and Laboratory Medicine, VA Boston Healthcare System, MA 02132, USA
, Tel +1 857-203-5020
Fax +1 857-203-5623
Email [email protected]
Purpose: Non-curative resection (NCR) remains problematic in some cases of early gastric carcinomas (EGCs) treated by endoscopic submucosal dissection (ESD). The aim of this study was to identify predictors of NCR, especially of eCura C1 and eCura C2 resections, before ESD and study long-term outcomes of EGC patients with NCR.
Patients and Methods: A retrospective review of medical records was conducted over an 8-year period for EGCs undergoing ESD. Clinicopathologic and endoscopic characteristics and patients’ survival were analyzed. Risk factors for NCR and eCura C1 and C2 resections were assessed by logistic analyses. Survival of patients was estimated with the Kaplan–Meier method with a Log rank test.
Results: A total of 463 patients with 472 lesions were qualified. By univariate and multivariate analyses, the predictors for NCR and eCura C2 resections were tumor size > 20 mm, tumors located in cardia-fundus, uneven surface, margin elevation, and mixed and undifferentiated types, and those for eCura C1 resection were tumors located in cardia-fundus, negative lifting sign, and mixed and undifferentiated types. The 5-year cancer-specific and cancer-free survival rates were 100.0% and 94.2%, and 95.3% and 83.4% in the curative resection (CR) and NCR groups, respectively. The 5-year cancer-specific and cancer-free survival rates were significantly greater in the CR group than that in the NCR group (P < 0.0001).
Conclusion: In this cohort, we identified various endoscopic and pathologic features of EGCs to predict NCR, especially eCura C1 and eCura C2 resections before ESD. These clinically valuable factors would be very informative to endoscopists and surgeons who perform ESD to resect EGCs.
Keywords: early gastric carcinoma, endoscopic submucosal dissection, non-curative resection, long-term outcome, stomach
Introduction
Gastric carcinoma is the fifth most common cancer and the third leading cause of cancer-related deaths in the world.1 At present, the only hope for better survival of gastric cancer patients is early detection and prompt resection of early gastric carcinoma (EGC), which is defined as invasive carcinoma restricted to the mucosa and submucosa of the stomach, regardless of the presence or absence of lymph node metastasis (LNM).2 With the advancement of endoscopic techniques and instruments, early detection of EGCs has been improved dramatically over the past decade.3 For EGC cases with a negligible risk of LNM, endoscopic submucosal dissection (ESD) has been recognized as a beneficial procedure for resection because of high therapeutic efficacy, low prevalence of complications, and excellent quality of postoperative life, compared to surgical resection.4–9
In reality, despite a strict application of those “absolute” and “expanded” rules for ESD resection of EGC tumors in the pre-operative assessment, many cases with residual tumors or the risk of LNM are discovered by histopathologic examination after ESD resection. For those outliers, the ESD resection is considered as non-curative resection (NCR). Previous studies have shown NCR rates ranging from 11.9% to 21.4% of ESD-resected EGC cases.10–15. After non-curative ESD resection, the incidence of residual carcinomas and the rate of LNM range 24.6–34.9% and 5.2–9.3%, respectively.16–19 In the latest Japanese gastric cancer treatment guidelines (ver. 5), according to complete resection of the primary EGC lesions and possibility of LNM, the NCR is subdivided into eCura C1 and eCura C2 resections.20 Although there have been some studies focused on the relationship between tumor size, location and, ulcer and NCR, few studies explored other pre-procedural endoscopic findings for ESD treatment-related NCR in EGCs, not to mention eCura C1 and eCura C2 resections.11,12,21 To minimize the risk of NCR and related unnecessary additional resection procedures, it is critically important for endoscopists to thoroughly evaluate the risk for NCR before any ESD procedure. The aim of this study was to identify clinically useful risk factors that may predict the risk of NCR, especially of eCura C1 and eCura C2 resections, before the start of an ESD procedure in EGC patients.
Patients and Methods
Patients
We retrospectively reviewed electronic medical records of EGC patients, whose tumors were resected with the ESD procedure between January 2010 and December 2017 at the First Affiliated Hospital of Nanjing Medical University in China. We identified 496 patients for the study. Exclusion criteria were: 1. ESD in stump EGCs (n=7); 2. Synchronous cancers in other organs (n=25); 3. Cases with incomplete clinical or endoscopic data (n=2). As a result, a total of consecutive 463 patients with 472 EGC lesions were included in this observational study (Figure 1).
Due to the retrospective nature of the study, informed consent was waived. The privacy of all the participants was maintained with confidentiality. The study protocol adhered to the ethical principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (approval no. 2018-SR-234).
Endoscopic Procedure and Data Extraction
We acquired the clinical data, endoscopic features, and pathological information of the enrolled cases through a review of medical records. The clinical data included age and gender. In each EGC tumor, tumor epicenter location and conventional white light endoscopic features were recorded and tabulated. EGC locations were divided into the cardia-fundus, corpus, angularis, and antrum-pylorus. The tumor circular locations were grouped into the greater curve, lesser curve, anterior wall, and posterior wall. The gross macroscopic features were classified into three major types: elevated (0-I, IIa and IIa + IIc), flat (0-IIb), and depressed (0-IIc, III and IIc + IIa). Also recorded and analyzed were other endoscopic findings, such as ulcer, remarkable redness, uneven surface, margin elevation, enlarged folds, spontaneous bleeding, and negative lifting signs.22
Pathological Assessment
The tumor maximum microscopic dimension was measured. Resected EGC tumors were histologically categorized into differentiated, undifferentiated, or mixed types. The differentiated type comprised well-moderately differentiated tubular and papillary adenocarcinomas and the undifferentiated type included poorly differentiated adenocarcinoma, poorly cohesive carcinoma/signet-ring cell carcinoma, and mucinous adenocarcinoma. The mixed type contained both differentiated and undifferentiated components. The submucosal infiltration (SM) of EGCs was sub-categorized as SM1 (<500 μm from the muscularis mucosae) or SM2 (≥500 μm from the muscularis mucosae) subgroups.
Definition of ESD Curative or Non-Curative Resection
Curative ESD resection of tumors was defined as en bloc resection with negative horizontal and vertical margins, without lymphovascular invasion, and fulfilling one of the following conditions: (a) an intramucosal differentiated carcinoma without ulcer, regardless of tumor size; (b) an intramucosal differentiated carcinoma with ulcer, with the size smaller than 30 mm; (c) an intramucosal undifferentiated carcinoma without ulcer, with the size smaller than 20 mm; and (d) a SM1 invasive differentiated carcinoma with the size smaller than 30 mm.20 Otherwise, the ESD resection was classified as NCR, which were sub-categorized as eCura C1 and C2 resections.20 An eCura C1 resection referred to cases only with piecemeal resection or positive horizontal margins, while an eCura C2 resection was used for cases with other non-curative conditions, such as a positive vertical margin, SM2 or lymphovascular invasion. As for mixed-type carcinomas, the curability was based on that of the predominantly histological type. However, if the undifferentiated carcinoma was not the predominant histological type, an ESD resection for a mixed-type carcinoma with undifferentiated component larger than 20 mm in size or with submucosal invasion was regarded as NCR.20
Long-Term Outcomes After ESD
We estimated the survival based on the number of enrolled EGC patients. All patients were followed up annually or biannually with upper endoscopy after ESD resection to assess tumor recurrence and other pathology. If the medical records were incomplete without required information, a telephonic interview to the patient or the family members was conducted. If the telephone interview failed three times at different time periods, the patient was considered to be lost to follow-up and excluded from statistical analysis. Patient survival was calculated from the date of the first ESD resection procedure for EGCs until the date of death of all causes or the last date of alive at an interview before December 31, 2018.
Statistical Analysis
Data analyses were conducted with R version 3.4.3 (The R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were presented as mean ± standard deviation. The Mann–Whitney U-test was used to compare the two groups. Numbers with percentages were used for categorical variables. Univariate and multivariate logistic regression analyses were executed to determine independent risk factors for non-curative, eCura C1 and eCura C2 resections. Survival rates were estimated with the Kaplan–Meier method with a Log rank test. A P-value < 0.05 was defined as statistical significance.
Results
Clinical Characteristics
As shown in Figure 1, a total of 472 EGCs from 463 patients were enrolled in the study, which were divided into curative resection (CR) (372 EGCs from 364 patients) and NCR (100 EGCs from 99 patients) groups. In the NCR group, 44 EGCs from 43 patients and 56 EGCs from 56 patients were classified into eCura C1 and C2 groups, respectively. Overall, the percentages of CR, NCR, eCura C1 and C2 were 78.8% (372/472), 21.2% (100/472), 9.3% (44/472), and 11.9% (56/472), respectively. As shown in Table 1, age and gender in the CR group were not significantly different from those in the NCR, eCura C1 and eCura C2 groups (P >0.05).
 |
Table 1 Clinicopathologic Features of the Enrolled Early Gastric Carcinomas (Curative Resection Group vs Non-Curative, eCura C1 and eCura C2 Resection Groups, Respectively) |
Comparison of Endoscopic Features Between Curative and Non-Curative Groups
From Table 1, the mean tumor size was significantly lager in the NCR, eCura C1 and eCura C2 groups than that in the CR group (19.1 ± 7.1mm, 17.7 ± 5.4mm, 19.1 ± 8.1mm vs 15.1 ± 6.6mm, respectively; P < 0.001). The most common location of EGC tumors was, in a descending order, the cardia-fundus (36.0%, 170/472), the antrum-pylorus (33.3%, 157/472), angularis (21.8%, 103/472), and corpus (8.9%, 42/472). Tumors located in the cardia-fundus were more common in the NCR (55.0%) group than in the CR group (30.9%) (P < 0.001). There were also more tumors located in the cardia-fundus in the eCura C1 and eCura C2 groups (P < 0.05). Among various endoscopic findings, in comparison with the CR group, gross morphology, ulcers, remarkable redness, uneven surface, margin elevation, and spontaneous bleeding were significantly more common in the NCR and eCura C2 groups (P < 0.05); remarkable redness and negative lifting sign were significantly more common in the eCura C1 group (P < 0.05).
Pathologic Features for Non-Curative Resection
As for the final histologic types shown in Table 1, there were more mixed and undifferentiated carcinomas in the NCR, eCura C1 and eCura C2 groups than in the CR group (22.0%, 10.0%, 32.1% vs 3.0%, respectively), and the differences were significant. (P < 0.05). The top three reasons for NCR included horizontal margin involvement (n = 56), SM2 invasion (n = 43), and vertical margin involvement (n = 14) (Supplementary Table 1).
Predictive Risk Factors for NCR, eCura C1 and eCura C2 Resections
By multivariate logistic regression analyses, we identified significant independent risk factors for NCR as follows: tumor size >20 mm (Odds Ratio (OR): 2.1, 95% Confidence Interval (CI): 1.2–3.7, P = 0.015), tumors located in cardia-fundus (OR: 3.9, 95% CI: 2.2–6.8, P < 0.001), uneven surface (OR: 2.0, 95% CI: 1.1–3.7, P = 0.028), margin elevation (OR: 2.5, 95% CI: 1.1–5.9, P = 0.037), and mixed and undifferentiated types (OR: 9.6, 95% CI: 3.9–23.9, P < 0.001) (Table 2). Significant risk factors for eCura C1 resection included tumors located in cardia-fundus (OR: 2.8, 95% CI: 1.4–5.6, P = 0.004), negative lifting sign (OR: 2.8, 95% CI: 1.3–6.1, P = 0.010), and mixed and undifferentiated types (OR: 4.0, 95% CI: 1.1–14.5, P = 0.038) (Table 3). Significant risk factors for eCura C2 resection were: tumor size >20 mm (OR: 2.4, 95% CI:1.1–5.2, P = 0.033), tumors located in cardia-fundus (OR: 6.8, 95% CI: 2.9–15.7, P < 0.001), uneven surface (OR: 3.4, 95% CI: 1.6–7.6, P = 0.002), margin elevation (OR: 3.6, 95% CI: 1.3–10.1, P = 0.015), and mixed and undifferentiated types (OR: 21.5, 95% CI: 5.3–87.2, P < 0.001) (Table 4).
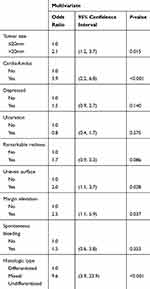 |
Table 2 Associated Risk Factors with Non-Curative ESD Resection |
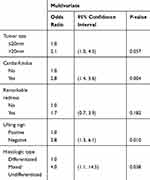 |
Table 3 Associated Factors with eCura C1 Resection of ESD |
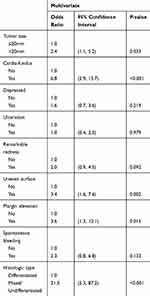 |
Table 4 Associated Factors with eCura C2 Resection of ESD |
Long-Term Outcomes
In this cohort, the average number of follow-up months after ESD resection was 35.7 ± 23.4 (range: 0.0–102.0). The 5-year cancer-specific survival rates were 100.0%, 87.5%, 91.1% and 82.1% in the CR, NCR, eCura C1, and eCura C2 groups, respectively. The 5-year cancer-specific survival rate was significantly worse for patients in the NCR group than for those in the CR group (P < 0.0001) (Figure 2A). In the subgroup analysis, there were no significant differences in the cancer-specific survival rate between the eCura C1 and C2 groups (P = 0.82) (Figure 2B), and compared to patients without additional surgery after non-curative ESD resection, the cancer-specific survival rate of patients with additional surgery was significantly better (P = 0.033) (Figure 2C). The 3- and 5-year cancer-free survival rates were 97.1% and 95.3%, and 85.8% and 83.4% in the CR and NCR groups, respectively. The 5-year cancer-free survival rate was significantly lower for patients in the NCR group than for those in the CR group (P < 0.0001) (Figure 3).
 |
Figure 3 Cancer-free survival for the patients in the curative and non-curative resection groups. |
Discussion
ESD has been recognized as a preferred treatment for EGCs, but is not a perfect technique because of a high prevalence of NCR.15 In our cohort, the rates of NCR, eCura C1 and eCura C2 were 21.2%, 9.3%, and 11.9%, respectively, which are similar to those reported by others in recent studies.11,12,23 Our results showed that tumor size >20 mm, tumors location in cardia-fundus, uneven surface, margin elevation, and mixed and undifferentiated types were closely associated with a high risk for NCR, in particular for eCura C2 resection, while the risks for the eCura C1 resection included tumors located in cardia-fundus, negative lifting sign, and mixed and undifferentiated carcinomas. As expected, the 5-year cancer-specific survival and cancer-free survival rates were better in the CR group than those in the NCR group, and the patients with additional surgery after non-curative ESD resection had better cancer-specific survival than those without additional surgery.
In this study, although the lesion size was measured based on pathologic tissue of ESD, we could use instruments to make a general assessment in preoperative endoscopic examination. Therefore, it can also provide some reference significance for endoscopists. Large tumors and proximal gastric location of EGCs have been reported as risk factors for non-curative ESD resection,11,12,21,23,24 which are confirmed in our cohort. In this cohort, the results demonstrated large tumors were more likely to contain undifferentiated carcinoma components. Therefore, we found large tumor size was prone to deep submucosal invasion and positive margins (Supplementary Table 2). The wall and submucosa of the cardia and fundus are thinner than those of other parts. Moreover, small lymphatic capillaries directly exist above the mucosal muscularis; and the cardia and fundus are difficult anatomical positions for endoscopic manipulation.25 As a result, in our study, tumors located in the cardia-fundus were more vulnerable to occurring deep submucosal invasion and positive margins (Supplementary Table 2).
For ESD resection of EGCs, our study also disclosed other endoscopic findings for NCR. Regarding to eCura C2 resection, uneven surface and margin elevation, that is to say, tumor nodularity, and a protruding edge surrounding a tumor, were independent risk factors. Abe et al considered tumors with these signs alluded potentially deep invasion.26 In the present study, the results revealed uneven surface and margin elevation were correlated with deep submucosal invasion, positive vertical margins and lymphovascular invasion. What’s more, a greater proportion of mixed and undifferentiated EGCs had these features than differentiated EGCs (Supplementary Table 2). Interestingly, negative lifting sign was a risk factor for eCura C1 resection, but not for eCura C2 resection. Negative lifting sign is usually due to scar formation at the bottom of an ulcer, or fibrosis due to tumor infiltration to the submucosa with desmoplastic reaction. In our cohort, negative lifting sign was tendentiously associated with piecemeal resection, and had a relationship to positive horizontal margins (Supplementary Table 2). Although reported as one of significant risk factors for the NCR by others,11,23,24 ulceration was not a significant risk factor for NCR in our cohort. However, we found tumors with ulcers had a correlation with positive vertical margins (Supplementary Table 2).
Mixed and undifferentiated EGC tumors have been shown to be risk factors of NCR,12,27,28 which parallel to our results. In this study, mixed and undifferentiated EGC tumors were relevant to deep submucosal invasion, positive margins and lymphovascular invasion (Supplementary Table 2). Undifferentiated EGCs are more prone to deep infiltration, and intramucosal spreading over a large area; therefore, the actual tumor size in resection specimens is often larger than the assumed size under endoscopy.29,30 The pre-ESD diagnosis of mixed EGCs was difficult and there is no unified standard. As previously reported, mixed and undifferentiated EGCs diagnosed at the final ESD resection specimens were most frequently misdiagnosed as differentiated EGCs at initial biopsies.12 If the pre-ESD biopsy showed differentiated EGCs, but the possibility of suspected undifferentiated components was observed, the mixed type should be highly suspected, and the endoscopists should be on the alert for the possibility of NCR.
The prognosis of EGC patients after ESD resection is excellent, as also shown in our cohort with the 3- and 5-year cancer-specific survival rates in the CR group over 99%.31 As expected, in comparison with the CR group, the 5-year survival rate was lower in NCR, eCura C1, and eCura C2 groups. Local recurrence along with possible nodal metastasis remain the causes of poor prognosis.10,19,32 Previous studies have shown that additional surgery after NCR can lead to a better prognosis for the patients with NCR.10,18,33,34 From our study, we also found higher cancer-specific survival rate for the patients with additional surgery than for those without additional surgery. Despite the risks associated with surgical procedures, additional surgery may result in better long-term outcomes. Local recurrence was found in patients with curative ESD resection, but as long as treated timely, they would have good prognosis. Therefore, regular surveillance is still necessary for patients after CR.
There are several major limitations for this observational study. First, this was a retrospective study at a single academic referral center. A selection bias might exist. However, we collected consecutive cases after a strict selection process, which minimized potential selection bias, if any. Second, endoscopic appearances of all EGC tumors were recorded as still images for this study, which might not be enough to demonstrate all aspects of an EGC tumor. In reality, still images of EGCs remain the most common materials for this type of clinical study. We look forward to adopting more advanced endoscopy technology to improve the EGC endoscopic study quality. Despite of these limitations, we believe our results are solid and helpful to digestive endoscopists dealing with EGCs in their clinical practice. Further studies are warranted to verify our preliminary findings.
Conclusion
We identified various endoscopic and pathologic features of EGCs to predict non-curative resection, especially eCura C1 and eCura C2 resections before ESD. These clinically valuable factors would be very informative to endoscopists and surgeons who perform ESD to resect EGCs.
Abbreviations
EGC, early gastric carcinoma; LNM, lymph node metastasis; ESD, endoscopic submucosal dissection; NCR, non-curative resection; SM, submucosal infiltration; CR, curative resection; OR, odds ratio; CI, confidence interval.
Disclosure
The authors declare no conflict of interests for this article.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi:10.1007/s10120-011-0041-5
3. Moon JS. Screening upper endoscopy for early detection of gastric cancer. J Korean Med Sci. 2018;33(23):e190. doi:10.3346/jkms.2018.33.e190
4. Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41(10):929–942. doi:10.1007/s00535-006-1954-3
5. Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77(Suppl 1):23–28. doi:10.1159/000111484
6. Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2010;17(1):54–58. doi:10.1111/j.1443-1661.2005.00459.x
7. Ohnita K, Isomoto H, Shikuwa S, et al. Early and long-term outcomes of endoscopic submucosal dissection for early gastric cancer in a large patient series. Exp Ther Med. 2014;7(3):594–598. doi:10.3892/etm.2014.1488
8. Choi JH, Kim ES, Lee YJ, et al. Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer. Gastrointest Endosc. 2015;82(2):299–307. doi:10.1016/j.gie.2015.01.019
9. Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19(1):198–205. doi:10.1007/s10120-015-0469-0
10. Oda I, Gotoda T, Sasako M, et al. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2008;95(12):1495–1500. doi:10.1002/bjs.6305
11. Hirasawa K, Kokawa A, Oka H, et al. Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointest Endosc. 2011;74(6):1268–1275. doi:10.1016/j.gie.2011.07.067
12. Toyokawa T, Inaba T, Omote S, et al. Risk factors for non-curative resection of early gastric neoplasms with endoscopic submucosal dissection: analysis of 1,123 lesions. Exp Ther Med. 2015;9(4):1209–1214. doi:10.3892/etm.2015.2265
13. Hatta W, Gotoda T, Oyama T, et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52(2):175–184. doi:10.1007/s00535-016-1210-4
14. Suzuki H, Oda I, Abe S, et al. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017;20(4):679–689. doi:10.1007/s10120-016-0651-z
15. Lee SH, Kim MC, Jeon SW, et al. Risk factors and clinical outcomes of non-curative resection in patients with early gastric cancer treated with endoscopic submucosal dissection: a retrospective multicenter study in Korea. Clin Endosc. 2019;53(2):196–205. doi:10.5946/ce.2019.123
16. Ryu KW, Choi IJ, Doh YW, et al. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol. 2007;14(12):3428–3434. doi:10.1245/s10434-007-9536-z
17. Jung H, Bae JM, Choi MG, Noh JH, Sohn TS, Kim S. 18 Surgical outcome after incomplete endoscopic submucosal dissection of gastric cancer. Br J Surg. 2011;98(1):73–78. doi:10.1002/bjs.7274
18. Kawata N, Kakushima N, Takizawa K, et al. 21 Risk factors for lymph node metastasis and long-term outcomes of patients with early gastric cancer after non-curative endoscopic submucosal dissection. Surg Endosc. 2017;31(4):1607–1616. doi:10.1007/s00464-016-5148-7
19. Zhao B, Zhang J, Zhang J, et al. Risk factors associated with lymph node metastasis for early gastric cancer patients who underwent non-curative endoscopic resection: a systematic review and meta-analysis. J Gastrointest Surg. 2018;23(7):1318–1328. doi:10.1007/s11605-018-3924-5
20. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020:1–21. doi:10.1007/s10120-020-01042-y
21. Fu QY, Cui Y, Li XB, Chen P, Chen XY. Relevant risk factors for positive lateral margin after en bloc endoscopic submucosal dissection for early gastric adenocarcinoma. J Dig Dis. 2016;17(4):244–251. doi:10.1111/1751-2980.12342
22. Cheng J, Wu X, Yang A, et al. Model to identify early-stage gastric cancers with deep invasion of submucosa based on endoscopy and endoscopic ultrasonography findings. Surg Endosc. 2018;32(2):855–863. doi:10.1007/s00464-017-5754-z
23. Kim EH, Park JC, Song IJ, et al. Prediction model for non-curative resection of endoscopic submucosal dissection in patients with early gastric cancer. Gastrointest Endosc. 2017;85(5):976–983. doi:10.1016/j.gie.2016.10.018
24. Ohara Y, Toshikuni N, Matsueda K, Mouri H, Yamamoto H. The superficial elevated and depressed lesion type is an independent factor associated with non-curative endoscopic submucosal dissection for early gastric cancer. Surg Endosc. 2016;30(11):4880–4888. doi:10.1007/s00464-016-4825-x
25. Akashi Y, Noguchi T, Nagai K, Kawahara K, Shimada T. Cytoarchitecture of the lamina muscularis mucosae and distribution of the lymphatic vessels in the human stomach. Med Mol Morphol. 2011;44(1):39–45. doi:10.1007/s00795-010-0503-6
26. Abe S, Oda I, Shimazu T, et al. Depth-predicting score for differentiated early gastric cancer. Gastric Cancer. 2011;14(1):35–40. doi:10.1007/s10120-011-0002-z
27. Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer. 2008;11(3):134–148. doi:10.1007/s10120-008-0476-5
28. Bang CS, Yang YJ, Lee JJ, Baik GH. Endoscopic submucosal dissection of early gastric cancer with mixed-type histology: a systematic review. Dig Dis Sci. 2020;65(1):276–291. doi:10.1007/s10620-019-05761-w
29. Katsube T, Konnno S, Hamaguchi K, et al. The efficacy of endoscopic mucosal resection in the diagnosis and treatment of group III gastric lesions. Anticancer Res. 2005;25(5):3513–3516.
30. Kim Y, Yoon HJ, Kim JH, et al. Effect of histologic differences between biopsy and final resection on treatment outcomes in early gastric cancer. Surg Endosc. 2019. doi:10.1007/s00464-019-07301-z
31. Liu Q, Ding L, Qiu X, Meng F. Updated evaluation of endoscopic submucosal dissection versus surgery for early gastric cancer: a systematic review and meta-analysis. Int J Surg. 2020;73:28–41. doi:10.1016/j.ijsu.2019.11.027
32. Nagano H, Ohyama S, Fukunaga T, et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8(3):149–154. doi:10.1007/s10120-005-0328-5
33. Jeon MY, Park JC, Hahn KY, Shin SK, Lee SK, Lee YC. Long-term outcomes after noncurative endoscopic resection of early gastric cancer: the optimal time for additional endoscopic treatment. Gastrointest Endosc. 2018;87(4):1003–1013.e2. doi:10.1016/j.gie.2017.10.004
34. Li D, Luan H, Wang S, Zhou Y. Survival benefits of additional surgery after non-curative endoscopic resection in patients with early gastric cancer: a meta-analysis. Surg Endosc. 2019;33(3):711–716. doi:10.1007/s00464-018-6570-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


