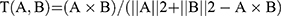Back to Journals » Drug Design, Development and Therapy » Volume 14
Prediction of Targets of Curculigoside A in Osteoporosis and Rheumatoid Arthritis Using Network Pharmacology and Experimental Verification
Authors Han J , Wan M, Ma Z , Hu C , Yi H
Received 15 September 2020
Accepted for publication 29 October 2020
Published 26 November 2020 Volume 2020:14 Pages 5235—5250
DOI https://doi.org/10.2147/DDDT.S282112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Jiawen Han,1,2,* Minjie Wan,1,3,* Zhanchuan Ma,1,2 Cong Hu,1,2,4 Huanfa Yi1,2
1Central Laboratory, The First Hospital of Jilin University, Changchun, Jilin 130031, People’s Republic of China; 2Key Laboratory of Organ Regeneration and Transplantation Ministry of Education, Changchun, Jilin 130021, People’s Republic of China; 3Department of Hepatology, The First Hospital of Jilin University, Changchun, Jilin 130021, People’s Republic of China; 4Center for Reproductive Medicine, Center for Prenatal Diagnosis, The First Hospital of Jilin University, Changchun, Jilin 130021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huanfa Yi
Central Laboratory, The First Hospital of Jilin University, Changchun, Jilin 130031, People’s Republic of China
Tel +86-431-84808391
Email [email protected]
Purpose: Network pharmacology is considered to be the next-generation drug development model that uses bioinformatics to predict and identify multiple drug targets and interactions in diseases. Here, network pharmacology was used to investigate the mechanism by which Curculigoside A (CA) acts in rheumatoid arthritis (RA) and osteoporosis.
Methods: First, TCMSP and SwissADME were applied to predict the druggability of CA. Then, potential targets were identified from overlapping data in SwissTarget and TargetNet, and targets were analyzed using Genemania and DAVID6.8 to obtain information about the GO and KEGG pathways. Ultimately, the drug-target-pathway network was identified after using Cytoscape 3.0 for visualization. Besides, qPCR was used to validate the predicted five major genes targets (EGFR, MAP2K1, MMP2, FGFR1, and MCL1).
Results: The results of TCMSP and SwissADME demonstrated that CA exhibits good druggability; 26 potential protein targets were classified by SwissTarget and TargetNet. The results of Genemania and DAVID6.8 indicated that CA probably caused anti-osteoporosis and anti-RA effects by regulating some biological pathways, especially nitrogen metabolism, estrogen signaling pathway, Rap1 signaling pathway, and PI3K/Akt signaling pathway. Besides, the result of Cytoscape 3.0 showed that the 26 targets participate in osteoporosis and RA-related pathways, metabolism, and other physiological processes. In vitro induced inflammation cell model experiments, the qPCR results showed that CA pretreatment significantly decreased the expression of EGFR, MAP2K1, MMP2, FGFR1, and MCL1 genes.
Conclusion: These results suggested that network pharmacology may provide possible mechanism of how CA exerts therapeutic effects in osteoporosis and RA.
Keywords: Curculigoside A, network pharmacology, target identity, osteoporosis, rheumatoid arthritis
Introduction
Osteoporosis is a main public health problem that is becoming more and more common in the aging of the population around the world. Osteoporosis is a skeletal disease characterized by impaired bone strength, making individuals susceptible to fractures of the hips, spine, and other bone parts. Osteoporotic fractures are associated with many risk factors, mainly including hormonal factors, the use of certain drugs (such as glucocorticoids), smoking, low physical activity, and low intake of calcium and vitamin D.1 In addition, the clinical consequences and financial burden of the disease require effective measures to provide appropriate and continuous intervention in high-risk populations.
Arthritis is quite prevalent now and has more than 100 types, including rheumatoid arthritis (RA), osteorheumatoid arthritis, psoriatic arthritis, inflammatory arthritis, and so on.2 Among them, RA is the most common type of immune-mediated arthritis,3 but the exact cause of RA is still unknown and there is no effective treatment. Anti-inflammatory drugs are often used to treat RA, but they usually accompany significant side effects. Therefore, these patients urgently need new treatments and alternative drugs with known and controllable side effects.
As a traditional Chinese medicinal plant that has been used for the treatment of osteoporosis and RA for a long time, the toxic and side effects of Curculigo orchioides Gaertn are known and controllable. Thus, it is undoubtedly that Curculigo orchioides Gaertn is an available and effective drug source for osteoporosis and RA.
Traditional Chinese medicine (TCM) is relatively safe and effective, with few side effects, and can prevent and treat various diseases. Over the past several thousand years, TCM has been used widely for clinical treatment in Asian countries. To date, TCM is still the most reliable resource for drug discovery and design.4–7 At present, TCM uses an accumulated wealth of clinical and practical experience that has been established and gradually improved, to obtain a unique and complex medical system.8 However, due to the complexity of Chinese herbal medicine and the human body, the mechanisms of most clinically effective Chinese medicines remain unclear. Curculigo orchioides Gaertn is a small herb widely distributed in China, India, Malaysia, Japan, and Australia. Its rhizome is known as Curculigo orchioides or “Xian Mao” and is a traditional Chinese medicine used in strengthening tendons and bones.9 Furthermore, Curculigoside A (CA) is the major bioactive compound isolated from the rhizome of Curculigo orchioides Gaertn. CA is reported to have potent anti-RA and anti-osteoporotic properties.10–12 However, relevant molecular mechanisms remain unclear.
The concept of network pharmacology was first defined by Hopkins in 2007 to raise awareness about the effects of drugs.13 It is a new approach toward drug design that takes system biology, network analysis, connectivity, redundancy, and pleiotropy into account.14 Now, in the new model, TCM, in combination with network pharmacology, provides a new research approach, which can be used as a powerful method to modify Chinese medicines from traditional experience-based medicines to modern evidence-based medicines. This system accelerates the discovery of TCM and improves current drug discovery strategies.8 In recent years, network pharmacology has greatly promoted the investigation of pharmacological mechanisms to explore Chinese medicine, such as in the use of the Guizhi Fuling pill for treating uterine fibroids,15 Baihe Dihuang decoction for improving the psychological sub-health state,16 and Danggui Buxue decoction for its blood-related functions.17 In summary, network pharmacology may be used as a powerful method to determine the overall pharmacological mechanism of how CA is used to treat osteoporosis and RA.
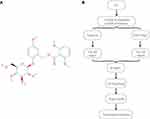
The network pharmacology approach has been used to determine the active components and potential targets of Curculigo orchioides in the treatment of osteoporosis.18 Using this approach, the mechanisms for the effects of Curculigo orchioides in the prevention and treatment of osteoporosis have been investigated, which inspired us to further explore the mechanism of CA, the main component of Curculigo orchioides, in treating osteoporosis and related RA. In our study, we first used TCMSP and SwissADME to predict the druggability of CA. Then, we applied 2D and 3D similarity measurements, using the SwissTarget server and TargetNet, to predict potential targets. These targets were then used for gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Ultimately, we established a drug-target-pathway network and visualized the molecular mechanisms and pathways by which the CA plays its anti-osteoporosis and anti-RA role; this might provide guidance for the clinical application of CA. In addition, we verified five key target genes associated with anti-osteoporosis and anti-RA effects predicted in the network through qPCR analysis. The chemical structure of CA and the flow chart for this study are shown in Figure 1.
Materials and Methods
Evaluation of the Druggability the TCMSP Server and SwissADME
In drug discovery and development, early ADME screening is critical. The proper use of ADME results can give preference to drug candidates that are more likely to have good pharmacokinetic properties, minimize potential drug–drug interactions, and avoid costly late-stage failures in drug development.19
The TCMSP server (https://tcmspw.com/tcmsp.php) is a drug screening and evaluation platform established by the Wang research team, based on the pharmacology of herbal systems. It provides information about important features related to ADME, such as human oral bioavailability, half-life, Caco-2 permeability, blood-brain barrier, and Lipinski’s rule of five.20
By analyzing the physicochemical properties and structural characteristics of drug candidates, the researchers summarized the concept of drug-like properties, which has been widely used to identify drug candidates for the screening of poor absorption, distribution, metabolism, excretion, and toxicity properties. Thus, as an established concept for drug design, drug-likeness (DL) can estimate which compounds have “drug-like” features. A model that can filter out drug candidates is formulated using the molecular descriptors and Tanimoto coefficient (displayed below), as follows:20
In this formula, A represents the molecular descriptor of the drug candidate and B represents the average molecular properties of all molecules in the Drug-Bank database. When investigating oral drugs, oral bioavailability (OB) and Caco-2 permeability are the critical pharmacokinetic properties. They are critical for evaluating the efficacy of the distributed delivery of oral drugs that enter the systemic circulation. Their value was, respectively, estimated using the OB prediction model OBioavail1.1 and the Caco-2 permeability prediction model preCaco2 in the TCMSP database.20
The SwissADME server (http://www.swissadme.ch/) is another network tool that provides a set of mature and efficient predictive models to users for calculating physicochemical properties, pharmacokinetics, ADME, DL, and medicinal chemistry friendliness with a focus on parameters such as BOILED-Egg, iLOGP, and Bioavailability Radar.21
In this study, “Curculigoside A” and the Canonical Simplified Molecular-Input Line-Entry System (SMILES) of “Curculigoside A” (PubChem CID: 158845) were, respectively, entered as keywords in the search box and its druggability was evaluated at the molecular level.
Computational Target Fishing Using TargetNet and SwissTarget
TargetNet is a user-friendly server that is widely used at present to connect or integrate connections between multiple targets of molecules that need to be detected. TargetNet has built a large number of QSAR models based on proven chemogenomic data for prediction.22 Furthermore, the SwissTarget server can also be used to evaluate the most probable macromolecular targets of a small molecule, which are considered to be bioactive compounds. This prediction is based on the 2D and 3D similarity alignment in the established active substance library.23 Both are effective tools that are commonly used to predict targets.
We downloaded the SDF file of CA (PubChem CID: 158845) from the PubChem database and input it into the TargetNet and SwissTarget servers. Default values were set for all parameter settings and the protein targets identified by the two servers were allowed to overlap. Thus, they were considered as pre-selected targets for further studies.
Analysis by GeneMANIA
GeneMANIA (http://www.genemania.org) is an open-source, highly usable web server, used for predicting and identifying gene functions, investigating gene lists, and determining gene priorities for further functional analysis. After entering a list of genes, GeneMANIA expands the gene clusters that display similar functions based on known and trusted genomics and proteomics information. GeneMANIA will also show the predictive value of each selected data set for the query.24 In our study, after selecting Homo sapiens from the nine options for the study species, we entered the name of the genes to be tested into the search box and obtained the results of the prediction process.
GO and Pathway Analyses, and Network Construction
The DAVID database includes an exhaustive biological knowledgebase; the accompanying analytics tools can be used to explore biological information from complex gene/protein lists.25 The first step in the use of this database is to upload or enter a list of genes for any type of identifier that needs to be studied. Next, one or more mining methods is selected from the gene functional classification, functional annotation chart or clustering chart, and functional annotation table. This allows the researcher to gain an in-depth biological understanding of the list of the genes to be tested. In our study, in the first step, we entered the gene list into the search box, subsequently selected identifier “OFFICIAL GENE SYMBOL” and chose list type “Gene List”, then submitted list. In the second step, we selected “Homo sapiens” to limit annotations and selected “List 1”. In the third step, we selected the background “Homo sapiens”. In the last step, we chose four parameters “GOTERM-BP-DIRECT”, “GOTERM-CC-DIRECT”, “GOTERM-MF-DIRECT”, and “KEGG-PATHWAY” below the Annotation Summary Results, then selected “Functional Annotation Chart” to obtain GO and KEGG Pathway Analysis results.
Finally, we used Cytoscape 3.0 to build and explore a network to get a more accurate and in-depth insight into the internal complexities of the drugs to be tested, corresponding targets, and related diseases.
Pharmacological Verification of Network Analysis
Materials
Curculigoside A (purity >99%, as determined using high-performance liquid chromatography), which was obtained from Tokyo Chemical Industry (Tokyo Japan), was prepared as a 10 mM stock solution in dimethyl sulfoxide and stored in small aliquot at −80°C.
Cells and Cell Culture
The RAW 264.7 murine macrophage and human monocyte THP1 cells were obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). These cells were cultured in RPMI1640 medium (Hyclone, Logan, Utah, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, U.S.A.), glutamine (2 mmol/L), penicillin (100 U/mL), and streptomycin (100 µg/mL) and were maintained at 37°C in humidified 5% CO2 incubators. Cells in the mid-log phase were used for further experiments.
Determination of Pro-Inflammatory Cytokines
The cells were plated at an appropriate density and allowed to grow to anastomosis for drug treatment. CA was dissolved in complete culture medium to different concentrations (0, 1, 5, 10, 20, 40, 80 µg/mL) and then filtered through a 0.22 µm membrane filter. Firstly, RAW264.7 cells were pretreated with CA (0, 1, 5, 10, 20, 40, 80 µg/mL) for 4 hours, and the solution was changed and stimulated with LPS 1 µg/mL (Sigma, Burlington, Massachusetts, USA) for 24 hours. All the experiments were repeated three times. Real-time PCR (qPCR) assay was performed for the expression of pro-inflammatory cytokines (IL-1β and IL-6) in RAW264.7 cells. Total RNA was harvested using Trizol reagent (Yeason, ShangHai, China). The RNA quality was checked using the ratio A260/A280, and pure RNA samples were converted to cDNA using PrimeScript RT Reagent Kit (Takara Bio, Kusatsu, Japan) with gDNA Eraser in accordance with the manufacturer’s instructions. The polymerase chain reaction was performed using SYBR Green based on the instruction manual (Takara Bio, Kusatsu, Japan). The primer sequences (Comate Bioscience, Changchun, China) of pro-inflammatory cytokines (IL-1β and IL-6) are presented in Table 1. The relative mRNA expression of different genes was quantified using the ∆∆Ct method. β-actin was used as housekeeping gene.
 |
Table 1 Oligonucleotide and Primer Sequence |
Determination of Predicted Five Major Gene Targets
The cells were plated at an appropriate density and allowed to grow to anastomosis for drug treatment. CA was dissolved in complete culture medium to a concentration of 40 µg/mL and then filtered through a 0.22 µm membrane filter. In RAW264.7 cell culture, first, cells were pretreated with CA (40 µg/mL) for 4 hours (control group did not add CA), and the solution was changed and stimulated with LPS 1 µg/mL (Sigma, Burlington, Massachusetts, USA) for 24 hours. In THP1 cell culture, first, pre-treated the cells with CA (40 µg/mL) for 4 hours (control group treat without CA), changed the solution with PMA 60 ng/mL (Sigma, Burlington, Massachusetts, USA) and incubated for 48 hours, then in the presence of LPS (0.5 µg/mL), the differentiated cells were incubated for an additional 24 hours. All the experiments were repeated three times. Real-time PCR (qPCR) assay was performed for the expression of predicted five major gene targets in RAW264.7 cells and THP1 cells pretreated or not pretreated at concentrations of CA (40 µg/mL). Total RNA was harvested using Trizol reagent (Yeason, ShangHai, China). The RNA quality was checked using the ratio A260/A280, and pure RNA samples were converted to cDNA using PrimeScript RT Reagent Kit with gDNA Eraser in accordance with the manufacturer’s instructions (Takara Bio, Kusatsu, Japan). The polymerase chain reaction was performed using SYBR Green based on the instruction manual (Takara Bio, Kusatsu, Japan). The primer sequences of these predicted five major gene targets enriched on these putative osteoporosis and RA-related KEGG pathways are presented in Table 1 (Comate Bioscience, Changchun, China). The relative mRNA expression of different genes was quantified using the ∆∆Ct method. β-actin was used as housekeeping gene.
Statistical Analysis
All data represent at least three independent experiments, and statistical analyses were performed with Prism (version 7.0, GraphPad Software, San Diego California, U.S.A.). All results are shown as mean ± SD. Statistical comparisons were performed using one-way analysis of variance. P<0.05 were considered to denote statistical significance.
Results
The Druggability of Curculigoside A
To predict the druggability of CA, we analyzed the parameters related to Lipinski’s rule of five, provided by TCMSP and SwissADME servers. The data obtained are, respectively, shown in Table 2 and Figure 2. TCMSP showed that CA was basically in line with the Lipinski’s rule of five (MW≤500, AlogP≤5, Hdon≤5, Hacc≤10, RBN≤10).26 Consistently, SwissADME server also showed that CA generally fulfilled Lipinski’s rule of five. The results of TCMSP and SwissADME analysis enabled us to conclude that CA exhibited good druggability.
 |
Table 2 Pharmacological and Molecular Properties of CA by TCMSP |
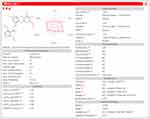 |
Figure 2 Pharmacological and molecular properties of CA by SwissADME. |
Identification of Potential Targets
Potential targets were obtained by overlapping TargetNet and SwissTarget servers, using the aforementioned method. Figure 1B shows the top 300 potential protein targets of CA from all 623 QSAR models that were obtained using TargetNet, as well as the potential 100 targets identified using the SwissTarget server. In TargetNet, the fingerprints model was set to the default value “ECFP4 fingerprints” and an area under the curve threshold of 0.7 indicated the presence of favorable targets.22 In SwissTarget, the threshold value of the similarity value was set as 0.85 and the value of 2D was 0.65.23 Finally, to increase the accuracy of the target prediction process, the overlapping 26 predicted targets obtained using these two methods were selected for further studies (Table 3).
 |
Table 3 Putative Targets of CA Identified by TargetNet and SwissTarget |
Analysis by GeneMANIA
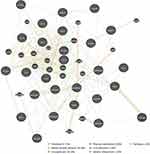
GeneMANIA generated a list of genes with similar functions to the query gene and constructs an interactive functional-association network to illustrate relationships between genes and datasets. GeneMANIA also assigned weights to data sets based on how useful they are for each query. Weights indicated the predictive value of each selected data set for the query. Individual data sets were represented as networks. The network weights were non-negative, sum to 100%. The interaction analysis of the 26 genes and their 20 interacting genes at the gene level was performed by GeneMANIA to clarify the correlations among colocalization, shared protein domains, co-expression, prediction and pathways. Among the 26 targets and their 20 interacting genes, the forecast results showed the portion that were predicted to interact with each other occupy 37.72% of the weight. Among all genes, the portion that shared the same protein domains occupy 28.28% of the weight, whereas the portion that displayed similar co-expression characteristics occupy 20.10% of the weight. The weights of these three portions add up to account for 86.1%. These results indicated that the 26 genes may comprehensively interact with each other and may function through alliance mechanisms. Other prediction outcomes, such as physical interactions, pathways, and co-localization are shown in Figure 3.
GO and Pathway Analyses, and Network Construction
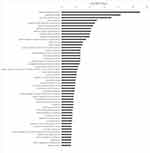
We used DAVID 6.8 to analyze the internal interaction networks of the 26 potential targets. We used -log10 (P value) for clarity to compare the P value and the top five functions were GO:0004089~carbonate dehydratase activity, GO:0015701~bicarbonate transport, GO:0006730~one-carbon metabolic process, GO:0005886~plasma membrane, and GO:0001609~G-protein-coupled adenosine receptor activity (Figure 4). The count, which represents the number of genes enriched in this pathway among the 26 target genes, showed that the 26 targets mainly participated in 17 KEGG pathways, including hsa00910: nitrogen metabolism, hsa04915: estrogen signaling pathway, and hsa04015: Rap1 signaling pathway (Table 4). More detailed pathway analysis data are provided in Supplementary Table S1. On the basis of target identification and pathway analysis, we then constructed the entire interaction network profile of how CA treats osteoporosis and RA using Cytoscape 3.0. The interaction network has 44 nodes and 95 edges (Figure 5).
 |
Table 4 KEGG PATHWAY Analysis of Potential Targets by DAVID6.8 (P<0.05) |
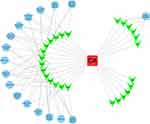 |
Figure 5 CA-target-pathway network. The red oblong, green inverted triangles, and blue circles correspond to CA, target proteins, and related pathways, respectively. |
Experimental Validation
IL-1β and IL-6 are pro-inflammatory cytokines that play a key role in osteoporosis and RA. We chose them to represent the severity of inflammation in vitro inflammation cell models. RAW264.7 cells were pretreated with CA (0, 1, 5, 10, 20, 40, 80 µg/mL) for 4 hours. RAW264.7 cells displayed a basal IL-1β and IL-6 expression in absence of CA, while RAW264.7 cells pretreated with CA (1, 5, 10, 20, 40, 80 µg/mL) for 4 hours displayed a reduced pro-inflammatory cytokines (IL-1β and IL-6) expression in a concentration-dependent manner, as shown in Figure 6. In addition, we compared the effects of CA at 40 µg/mL and 80 µg/mL. The results showed that CA at 80 µg/mL was slightly better than 40 µg/mL in value, but had no significant difference. So we selected CA at 40 µg/mL to investigate its effects on the expression of predicted five major gene targets. Based on the above predictions, to verify the results derived using the target–pathway interaction network, we chose the predicted five major gene targets (EGFR, MAP2K1, MMP2, FGFR1, MCL1) for experimental validation in vitro inflammation cell model induced by RAW264.7 cells and THP1 cells. These predicted five gene targets (EGFR, MAP2K1, MMP2, FGFR1, MCL1) have been shown to participate in pathogenesis, pathological process and related treatment of RA and osteoporosis in patients or induced RA and osteoporosis cell models and animal models.27–34 Therefore, we speculated that CA may exert anti-RA and anti-osteoporosis effects by regulating these five genes. Thus, we chose these five genes for further experimental verification, to explore if CA exerts anti-RA and anti-osteoporosis effects by regulating these five genes. Real-time polymerase chain reaction analysis results showed that compared with the control group (no CA pretreatment), EGFR, MAP2K1, MMP2, FGFR1, and MCL1 were down-regulated by treatment of 40 µg/mL CA in both RAW 264.7 cells and THP1 cells (P<0.05). (Figure 7)
Discussion
The concept of network pharmacology is relatively new and first defined by Hopkins in 2007.13 It is a new paradigm in drug discovery that takes system biology, network analysis, connectivity, redundancy, and pleiotropy into account. Besides, it can use bioinformatics to predict and identify multiple drug targets and interactions in disease.14 In recent years, with the importance of network pharmacology in drug development research and analysis, network pharmacology tools have been introduced to meet more needs. Especially in the scope of TCM network pharmacology, new tools such as BATMAN-TCM, ETCM, TCMSP, TCM-Mesh have provided great benefit for drug development and research.20,35–37 Moreover, the application of network pharmacology is more and more extensive and in-depth. For example, network pharmacology can be used to identify the combinations of clinically effective drugs for specific diseases.38 It can be used to estimate the untapped target space and therapeutic potential of natural products.39 Additionally, it is also used to guide and assist in drug repositioning to overcome the low drug productivity that has been an important issue in the pharmaceutical industry.40 In the field of TCM research, network pharmacology promotes the research on the synergistic effect of TCM in their formulas and clarifies the molecular mechanism of the formulas.41 Active drug ingredients for specific difficult diseases such as stroke and cancer from drug Banks can be screened by network pharmacology approach.42,43 It also provides new effective formulas design of Chinese medicine and effectively promotes the modernization and development of Chinese medicine.44 At present, network pharmacology is mainly used to study the effective components and action mechanism of a single Chinese medicine or Chinese medicine formulas in the treatment of complex diseases.45–49 Taken together, network pharmacology is practical in the study of single herbs, drug pairs, and traditional Chinese medicine formulas. Therefore, the development of network pharmacology techniques that can predict multiple drug–target interactions may hold the key to future drug discoveries in complex diseases such as RA and Osteoporosis.
Network pharmacology approach has been used to determine the active components and potential targets of Curculigo orchioides in the treatment of Osteoporosis.18 On the basis of this article, we further applied network pharmacology to predict the potential targets of CA to exert anti-RA and anti-osteoporosis effects, the interaction of targets with CA, the signaling pathways and networks involved. GO analysis showed that the predicted targets of CA are mainly enriched in GO: 0004089 ~ carbonate dehydratase activity, GO: 0015701 ~ bicarbonate transport, GO: 0006730 ~ one-carbon metabolic process, GO: 0005886 ~ plasma membrane, and GO: 0001609 ~ G-protein-coupled adenosine receptor activity. Several studies in vitro and in vivo suggest that inhibition of bicarbonate transport leads to changes in pH, which in turn affects the activity of pH-dependent cells and exerts anti-inflammatory and anti-RA effects.50 Inhibition of key enzymes in intracellular folate metabolism and further limitation of the availability of methyl groups derived from one-carbon metabolism can treat RA.51,52 The combination of adenosine and G protein-coupled adenosine receptors can exert the effects of inhibiting osteoporosis and RA, by promoting osteoblast differentiation and bone repair, regulating osteoclast function, and pathological bone remodeling.53,54 Consistent with our expectations, these GO corresponding to the predicted targets of CA is mainly related to RA and osteoporosis. Pathway analysis suggested that CA mainly regulates nitrogen metabolism, estrogen signaling pathway, Rap1 signaling pathway, and PI3K/Akt signaling pathway in RA and osteoporosis. These results were consistent with several studies in vitro and in vivo. Reactive nitrogen (RNS) indirectly induces the formation of autoantibodies, further aggravating RA55 and it is used as a potential biomarker of disease activity and antioxidant effects.56 In RA, estrogen withdrawal during menopause indirectly affects osteoclast bone resorption, accelerated bone loss, osteoporosis by increasing production of proinflammatory cytokines.1,57 Rap1 plays a vital role in promoting angiogenesis and maintaining vascular stability, and it is closely related to further RA.58 Besides, deficiency in Rap1 compromises in vivo bone resorption, resulting in an osteoporotic phenotype.59 Activation of the PI3K/Akt signaling pathway affects the anti-apoptotic properties of synovial fibroblasts to affect synovial proliferation in RA.60–62 Activation of the PI3K/Akt signaling pathway can regulate the production of RA-related inflammatory mediators.63 PI3K/Akt signaling pathway is also involved in regulating osteoporosis.64 Thus, these KEGG pathways corresponding to the predicted targets of CA were also mainly related to RA and osteoporosis. In addition, Gene interaction analysis by GeneMANIA indicated that the 26 genes may comprehensively interact with each other. Besides, it also further implied that the 26 genes may function through alliance mechanisms in anti-RA and anti-osteoporosis process. It was consistent with the results of GO and pathway analysis. GO and pathway analyses were completed by DAVID 6.8, and the network was constructed using Cytoscape 3.0. As shown in Figure 5, the network indicated that CA might act on multiple targets, further causing complex and diverse pharmacological effects to exert its anti-osteoporosis and anti-RA effects. Although network pharmacology is an efficient method for predicting multiple drug targets in complex diseases, it is still necessary to verify the predicted targets using in vitro experiments.
RA is a chronic autoimmune inflammatory arthritis associated with persistent inflammation, and osteoporosis commonly occurs in the setting of inflammatory arthritis. Thus, suppression of inflammation has been the goal of their treatment.65,66 Macrophages are essential for the pathophysiology of RA and they are a major source of pro-inflammatory cytokines and chemokines (such as TNF, IL-1β, and IL-6).67 The large number of macrophages is a major feature of inflammatory lesions, and removal of these macrophages from inflammatory tissue has profound therapeutic benefits.68 Therefore, when studying the pathogenesis of RA, we often use inflammation models induced with RAW264.7 cells and THP1 cells in vitro.69–72 According to previous experience, some drugs that work in mouse are not effective in humans. In order to avoid this mistake, we carry out qPCR verification in vitro inflammation models induced with them both, to get a more accurate and widely applicable result in this study. By detecting the expression levels of predicted gene targets, we further verified how CA exerted its anti-osteoporosis and anti-RA pharmacological effects. Experimental verification suggested that CA stimulated monocytes/macrophages in a concentration-dependent manner and effectively down-regulate the expression of the predicted five major gene targets (EGFR, MAP2K1, MMP2, FGFR1, and MCL1) enriched on these KEGG pathways associated with osteoporosis and RA, in vitro inflammation models induced with RAW264.7 cells or THP1 cells. As mentioned above, the relationship between these five gene targets and the pathogenesis, pathological process and related treatment of RA and osteoporosis had also been reported by researchers. EGFR is newly identified as a direct inhibition target of miR-573. It regulates IL-6 production and human umbilical vein endothelial cells (HUVECs) angiogenesis by miR-573, which alleviates RA.27 Consistent with the conclusion of this article, our experimental data suggested that CA may play anti-RA and anti-osteoporosis role by inhibiting EGFR expression. It confirms that our prediction may be precise that EGFR is indeed a therapeutic target of CA in RA and osteoporosis. It has been demonstrated that Cocoa Polyphenols directly inhibited the activity of MAP2K1 to reduce the TNF-α-induced up-regulation of VEGF which exasperates RA.28 Besides, MAP2K1 may be also involved in the mechanism of tomatidine in improving osteoporosis.29 Consistently, our result suggested that CA may exert anti-RA and anti-osteoporosis effects by inhibiting MAP2K1 expression. The expression of MMP-2 can be inhibited by artesunate, leading to remarkable suppression of the migration and invasion of RA-fibroblast-like synoviocytes (FLS).30 MMP-2 also plays a negative regulatory role in osteoporosis by MMP-2/B7-H3.31 The expression of FGFR1 is regulated by the SHH-Gli signaling pathway, which subsequently leads to the abnormal proliferation of RA-synovial fibroblast (RA-SF). Blocking the SHH-Gli pathway can inhibit RA-SF cells proliferation and increase apoptosis to treat RA.32 Besides, inhibition of FGFR1 and ERK1/2 signaling rescues the anti-osteogenic effects.33 The up-regulation of MCL-1 promotes the anti-apoptotic ability of FLS through the activation of the PI3K/Akt and STAT3 pathways to aggravate RA.34 Therefore, they can represent therapeutic targets for RA and osteoporosis. Overall, experimental results were consistent with these studies in vitro and in vivo suggesting that EGFR, MAP2K1, MMP2, FGFR1, and MCL1 all can directly or indirectly participate in the pathogenesis, pathological process, and related treatment of RA and osteoporosis. So EGFR, MAP2K1, MMP2, FGFR1, and MCL1 are potential targets of CA for RA and osteoporosis treatment. CA may exert anti-RA and anti-osteoporosis effects by inhibiting their expressions. However, the validated targets used in experimental verification are based on the network pharmacology prediction and may be deviated from the actual targets. In addition, our results can only prove that CA down-regulated the expression of these five targets, and further research is needed on how CA regulates downstream pathways to exert anti-osteoporosis and anti-RA effects. Thus, our study just suggested there was a possibility that CA might act on osteoporosis and RA associated KEGG pathways (enriched with these five genes) such as nitrogen metabolism, estrogen signaling pathway, Rap1 signaling pathway, and PI3K/Akt signaling pathway to exert anti-osteoporosis and anti-RA effect. In summary, experimental verification preliminarily proved that our prediction based on network pharmacology on how CA exerted its anti-osteoporosis and anti-RA effects may be correct and credible.
Our results were derived from network pharmacology-based prediction. In order to improve the accuracy of prediction, SwissTarget and TargetNet were used to predict and overlap the targets of CA for RA and osteoporosis. The prediction of SwissTarget was based on a combination of 2D and 3D similarity with a library of 370ʹ000 known actives on more than 3000 proteins from three different species. Its success rate that at least one of the experimentally known targets can be found among the predicted top-15 was 72%.23 Besides, the TargetNet can predict the activity of the user’s molecule across 623 human proteins by establishing the high-quality QSAR model for each human protein. Its AUC scores were 75%-100%.22 Although using two methods to predict and overlap the targets can increase the credibility of the results, some prediction errors and missing will occur due to the incomplete coverage of the library/QSAR model and the accuracy of prediction methods. So there may exist some deviations due to that important real targets are not predicted or are not among the 26 overlapping genes when target prediction and overlapping are performed at the beginning. Therefore, targets other than our predicted results should not be ignored in future studies. In this study, our intention was to initially construct a drug-target-pathway network of CA in osteoporosis and RA and to briefly verify the accuracy of this network by qPCR experiments. However, we did not further explore the pivotal targets that play a key role in this network. In future research, we will conduct the network analysis for screening the pivotal targets and explore precise mechanism on how CA exerts anti-osteoporosis and anti-RA effects. Besides, for further verification, more specific and accurate experimental verification still needs us to carry out in future studies in addition to qPCR. Nevertheless, our results still provide a relatively credible prediction about the pharmacological mechanism of how CA treats osteoporosis and RA, which is useful for further developing new CA-based drugs in RA and applications of network pharmacology in drug discovery.
Conclusion
Collectively, the present study revealed a systematic and visual overview of the probable molecular mechanisms and signaling pathways of how CA exerted its anti-RA and anti-osteoporosis effects. CA can be considered as a good drug candidate for osteoporosis and RA. Consistent with the prediction, experimental results also proved that the prediction based on network pharmacology methods may be accurate and credible.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81671592) and the Science and Technology Department of Jilin Province (20190201140JC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lacativa PGS, Farias M. Osteoporosis and inflammation. Arq Bras Endocrinol Metabol. 2010;54(2):123–132. doi:10.1590/S0004-27302010000200007
2. Tang C-H. Research of Pathogenesis and Novel Therapeutics in Arthritis. Int J Mol Sci. 2019;20(7):1646. doi:10.3390/ijms20071646
3. Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–1874. doi:10.1016/S0140-6736(07)60784-3
4. Shen B, New Golden A. Age of Natural Products Drug Discovery. Cell. 2015;163(6):1297–1300. doi:10.1016/j.cell.2015.11.031
5. Peng W, Han T, Xin WB, et al. Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med Chem Res. 2011;20(2):146–151. doi:10.1007/s00044-010-9311-8
6. Li X, Yang X, Cai Y, et al. Proanthocyanidins from Grape Seeds Modulate the NF-κB Signal Transduction Pathways in Rats with TNBS-Induced Ulcerative Colitis. Molecules. 2011;16(8):6721–6731. doi:10.3390/molecules16086721
7. Jiang Q, Yi M, Guo Q, et al. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-κB pathway. Int Immunopharmacol. 2015;29(2):370–376. doi:10.1016/j.intimp.2015.10.027
8. Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. doi:10.1016/s1875-5364(13)60037-0
9. Wang X, Zhang M, Zhang D, et al. Structural elucidation and anti-osteoporosis activities of polysaccharides obtained from Curculigo orchioides. Carbohydr Polym. 2019;203:292–301. doi:10.1016/j.carbpol.2018.09.059
10. Ding H, Gao G, Zhang L, et al. The protective effects of curculigoside A on adjuvant-induced arthritis by inhibiting NF-кB/NLRP3 activation in rats. Int Immunopharmacol. 2016;30:43–49. doi:10.1016/j.intimp.2015.11.026
11. Wang L, He Y-J, Han T, et al. Metabolites of curculigoside in rats and their antiosteoporotic activities in osteoblastic MC3T3-E1 cells. Fitoterapia. 2017;117:109–117. doi:10.1016/j.fitote.2017.01.009
12. Tan S, Xu J, Lai A, et al. Curculigoside exerts significant anti‑arthritic effects in vivo and in vitro via regulation of the JAK/STAT/NF‑κB signaling pathway. Mol Med Report. 2019;19(3):2057–2064. doi:10.3892/mmr.2019.9854
13. Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1111. doi:10.1038/nbt1007-1110
14. Wang Z, Liu J, Yu Y, Chen Y, Wang Y. Modular pharmacology: the next paradigm in drug discovery.. Expert Opin Drug Discov. 2012;7(8):667–677. doi:10.1517/17460441.2012.692673
15. Zeng L, Yang K, Liu H, Zhang G. A network pharmacology approach to investigate the pharmacological effects of Guizhi Fuling Wan on uterine fibroids. Exp Ther Med. 2017;14(5):4697–4710. doi:10.3892/etm.2017.5170
16. Tian JS, Meng Y, Wu YF, et al. A novel insight into the underlying mechanism of Baihe Dihuang Tang improving the state of psychological suboptimal health subjects obtained from plasma metabolic profiles and network analysis. J Pharm Biomed Anal. 2019;169:99–110. doi:10.1016/j.jpba.2019.02.041
17. Shi XQ, Yue SJ, Tang YP, et al. A network pharmacology approach to investigate the blood enriching mechanism of Danggui buxue Decoction. J Ethnopharmacol. 2019;235:227–242. doi:10.1016/j.jep.2019.01.027
18. Wang N, Zhao G, Zhang Y, et al. A Network Pharmacology Approach to Determine the Active Components and Potential Targets of Curculigo Orchioides in the Treatment of Osteoporosis. Med Sci Monit. 2017;23:5113–5122. doi:10.12659/MSM.904264
19. Wang J, Li Y, Yang Y, et al. A New Strategy for Deleting Animal drugs from Traditional Chinese Medicines based on Modified Yimusake Formula. Sci Rep. 2017;7(1):1504. doi:10.1038/s41598-017-01613-7
20. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6(1):13. doi:10.1186/1758-2946-6-13
21. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi:10.1038/srep42717
22. Yao ZJ, Dong J, Che YJ, et al. TargetNet: a web service for predicting potential drug-target interaction profiling via multi-target SAR models. J Comput Aided Mol Des. 2016;30(5):413–424. doi:10.1007/s10822-016-9915-2
23. Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357–W364. doi:10.1093/nar/gkz382
24. Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(WebServer issue):W214–220. doi:10.1093/nar/gkq537
25. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi:10.1038/nprot.2008.211
26. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:1–3. doi:10.1016/S0169-409X(00)00129-0
27. Wang L, Song G, Zheng Y, et al. miR-573 is a negative regulator in the pathogenesis of rheumatoid arthritis. Cell Mol Immunol. 2016;13(6):839–849. doi:10.1038/cmi.2015.63
28. Kim J-E, Son JE, Jung SK, et al. Cocoa polyphenols suppress TNF-α-induced vascular endothelial growth factor expression by inhibiting phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase kinase-1 (MEK1) activities in mouse epidermal cells. Br J Nutr. 2010;104(7):957–964. doi:10.1017/S0007114510001704
29. Yu T, Wu Q, You X, et al. Tomatidine Alleviates Osteoporosis by Downregulation of p53. Med Sci Monit. 2020;26:e923996. doi:10.12659/MSM.923996
30. Ma J-D, Jing J, Wang J-W, et al. A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res Ther. 2019;21(1):153. doi:10.1186/s13075-019-1935-6
31. Feng P, Zhang H, Zhang Z, et al. The interaction of MMP-2/B7-H3 in human osteoporosis. Clin Immunol. 2016;162:118–124. doi:10.1016/j.clim.2015.11.009
32. Qin S, Sun D, Li H, et al. The Effect of SHH-Gli Signaling Pathway on the Synovial Fibroblast Proliferation in Rheumatoid Arthritis. Inflammation. 2016;39(2):503–512. doi:10.1007/s10753-015-0273-3
33. Simann M, Le Blanc S, Schneider V, et al. Canonical FGFs Prevent Osteogenic Lineage Commitment and Differentiation of Human Bone Marrow Stromal Cells Via ERK1/2 Signaling. J Cell Biochem. 2017;118(2):263–275. doi:10.1002/jcb.25631
34. Jiao Y, Ding H, Huang S, et al. Bcl-XL and Mcl-1 upregulation by calreticulin promotes apoptosis resistance of fibroblast-like synoviocytes via activation of PI3K/Akt and STAT3 pathways in rheumatoid arthritis. Clin Exp Rheumatol. 2018;36(5):841–849.
35. Liu Z, Guo F, Wang Y, et al. BATMAN-TCM: a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci Rep. 2016;6(1):21146. doi:10.1038/srep21146
36. Xu H-Y, Zhang Y-Q, Liu Z-M, et al. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47(D1):D976–D982. doi:10.1093/nar/gky987
37. Zhang R-Z, Yu S-J, Bai H, Ning K. TCM-Mesh: the database and analytical system for network pharmacology analysis for TCM preparations. Sci Rep. 2017;7(1):2821. doi:10.1038/s41598-017-03039-7
38. Cheng F, Kovács IA, Barabási A-L. Network-based prediction of drug combinations. Nat Commun. 2019;10(1):1197. doi:10.1038/s41467-019-09186-x
39. Kibble M, Saarinen N, Tang J, Wennerberg K, Mäkelä S, Aittokallio T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat Prod Rep. 2015;32(8):1249–1266. doi:10.1039/c5np00005j
40. Ye H, Wei J, Tang K, Feuers R, Hong H. Drug Repositioning Through Network Pharmacology. Curr Top Med Chem. 2016;16(30):3646–3656. doi:10.2174/1568026616666160530181328
41. Jing C, Sun Z, Xie X, et al. Network pharmacology-based identification of the key mechanism of Qinghuo Rougan Formula acting on uveitis. Biomed Pharmacother. 2019;120:109381. doi:10.1016/j.biopha.2019.109381
42. Ren L, Zheng X, Liu J, et al. Network pharmacology study of traditional Chinese medicines for stroke treatment and effective constituents screening. J Ethnopharmacol. 2019;242:112044. doi:10.1016/j.jep.2019.112044
43. Poornima P, Kumar JD, Zhao Q, Blunder M, Efferth T. Network pharmacology of cancer: from understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacol Res. 2016;111:290–302. doi:10.1016/j.phrs.2016.06.018
44. Hu R-F, Sun X-B. Design of new traditional Chinese medicine herbal formulae for treatment of type 2 diabetes mellitus based on network pharmacology. Chin J Nat Med. 2017;15(6):436–441. doi:10.1016/S1875-5364(17)30065-1
45. Xue J, Shi Y, Li C, Song H. Network pharmacology-based prediction of the active ingredients, potential targets, and signaling pathways in compound Lian-Ge granules for treatment of diabetes. J Cell Biochem. 2019;120(4):6431–6440. doi:10.1002/jcb.27933
46. Hua Y-L, Ma Q, Yuan Z-W, et al. A novel approach based on metabolomics coupled with network pharmacology to explain the effect mechanisms of Danggui Buxue Tang in anaemia. Chin J Nat Med. 2019;17(4):275–290. doi:10.1016/S1875-5364(19)30031-7
47. Guo W, Huang J, Wang N, et al. Integrating Network Pharmacology and Pharmacological Evaluation for Deciphering the Action Mechanism of Herbal Formula Zuojin Pill in Suppressing Hepatocellular Carcinoma. Front Pharmacol. 2019;10:1185. doi:10.3389/fphar.2019.01185
48. Tao W, Xu X, Wang X, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013;145:1. doi:10.1016/j.jep.2012.09.051
49. Zuo J, Wang X, Liu Y, et al. Integrating Network Pharmacology and Metabolomics Study on Anti-rheumatic Mechanisms and Antagonistic Effects Against Methotrexate-Induced Toxicity of Qing-Luo-Yin. Front Pharmacol. 2018;9:1472. doi:10.3389/fphar.2018.01472
50. McNiff P, Svensson L, Pazoles CJ, Gabel CA. Tenidap modulates cytoplasmic pH and inhibits anion transport in vitro. I. Mechanism and evidence of functional significance. J Immunol. 1994;153(5):2180–2193.
51. Gosselt HR, van Zelst BD, de Rotte MCFJ, Hazes JMW, de Jonge R, Heil SG. Higher baseline global leukocyte DNA methylation is associated with MTX non-response in early RA patients. Arthritis Res Ther. 2019;21(1):157. doi:10.1186/s13075-019-1936-5
52. Alonso-Aperte E, Varela-Moreiras G. Drugs-nutrient interactions: a potential problem during adolescence. Eur J Clin Nutr. 2000;54(Suppl 1):S69–S74. doi:10.1038/sj.ejcn.1600989
53. Noronha-Matos JB, Correia-de-Sá P. Mesenchymal Stem Cells Ageing: targeting the “Purinome” to Promote Osteogenic Differentiation and Bone Repair. J Cell Physiol. 2016;231(9):1852–1861. doi:10.1002/jcp.25303
54. Su X, Floyd DH, Hughes A, et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 2012;122(10):3579–3592. doi:10.1172/JCI38576
55. Smallwood MJ, Nissim A, Knight AR, Whiteman M, Haigh R, Winyard PG. Oxidative stress in autoimmune rheumatic diseases. Free Radic Biol Med. 2018;125. doi:10.1016/j.freeradbiomed.2018.05.086
56. Khojah HM, Ahmed S, Abdel-Rahman MS, Hamza A-B. Reactive oxygen and nitrogen species in patients with rheumatoid arthritis as potential biomarkers for disease activity and the role of antioxidants. Free Radic Biol Med. 2016;97:285–291. doi:10.1016/j.freeradbiomed.2016.06.020
57. Sapir-Koren R, Livshits G. Postmenopausal osteoporosis in rheumatoid arthritis: the estrogen deficiency-immune mechanisms link. Bone. 2017;103:102–115. doi:10.1016/j.bone.2017.06.020
58. Chrzanowska-Wodnicka M. Distinct functions for Rap1 signaling in vascular morphogenesis and dysfunction. Exp Cell Res. 2013;319(15):2350–2359. doi:10.1016/j.yexcr.2013.07.022
59. Jeevaratnam K, Salvage SC, Li M, Huang CLH. Regulatory actions of 3ʹ,5ʹ-cyclic adenosine monophosphate on osteoclast function: possible roles of Epac-mediated signaling. Ann N Y Acad Sci. 2018;1433(1):18–28. doi:10.1111/nyas.13861
60. Leech MT, Morand EF. Fibroblasts and synovial immunity. Curr Opin Pharmacol. 2013;13(4):565–569. doi:10.1016/j.coph.2013.04.001
61. Zhang HG, Wang Y, Xie JF, et al. Regulation of tumor necrosis factor alpha-mediated apoptosis of rheumatoid arthritis synovial fibroblasts by the protein kinase Akt. Arthritis Rheum. 2001;44(7):1555–1567. doi:10.1002/1529-0131(200107)44:7<1555::AID-ART279>3.0.CO;2-M
62. Song B, Li X-F, Yao Y, et al. BMP9 inhibits the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via the PI3K/AKT signaling pathway. Int Immunopharmacol. 2019;74:105685. doi:10.1016/j.intimp.2019.105685
63. Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283(38):25900–25912. doi:10.1074/jbc.M707692200
64. Hu J, Mao Z, He S, et al. Icariin protects against glucocorticoid induced osteoporosis, increases the expression of the bone enhancer DEC1 and modulates the PI3K/Akt/GSK3β/β-catenin integrated signaling pathway. Biochem Pharmacol. 2017;136:109–121. doi:10.1016/j.bcp.2017.04.010
65. Liu W, Zhang Y, Zhu W, et al. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front Immunol. 2018;9:2228. doi:10.3389/fimmu.2018.02228
66. Zhang N, Xu C, Li N, et al. Folate receptor-targeted mixed polysialic acid micelles for combating rheumatoid arthritis: in vitro and in vivo evaluation. Drug Deliv. 2018;25(1):1182–1191. doi:10.1080/10717544.2018.1472677
67. Siouti E, Andreakos E. The many facets of macrophages in rheumatoid arthritis. Biochem Pharmacol. 2019;165:152–169. doi:10.1016/j.bcp.2019.03.029
68. Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(8):472–485. doi:10.1038/nrrheum.2016.91
69. Liu Z, Song L, Wang Y, et al. A novel fusion protein attenuates collagen-induced arthritis by targeting interleukin 17A and tumor necrosis factor α. Int J Pharm. 2018;547(1–2):72–82. doi:10.1016/j.ijpharm.2018.05.058
70. Boutet M-A, Najm A, Bart G, et al. IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro. Ann Rheum Dis. 2017;76(7):1304–1312.
71. Pan Y-J, Wang W-H, Huang T-Y, et al. Quetiapine ameliorates collagen-induced arthritis in mice via the suppression of the AKT and ERK signaling pathways. Inflamm Res. 2018;67(10):847–861. doi:10.1007/s00011-018-1176-1
72. Han E-J, Kim HY, Lee N, et al. Suppression of NFAT5-mediated Inflammation and Chronic Arthritis by Novel κB-binding Inhibitors. EBioMedicine. 2017;18:261–273. doi:10.1016/j.ebiom.2017.03.039
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.