Back to Journals » International Journal of General Medicine » Volume 12
Prediction Of Serum Digoxin Concentration Using Estimated Glomerular Filtration Rate In Thai Population
Authors Sae-lim O , Doungngern T , Jaisue S, Cheewatanakornkul S , Arunmanakul P, Anutrakulchai S , Kanjanavanit R, Wongpoowarak W
Received 5 June 2019
Accepted for publication 5 November 2019
Published 2 December 2019 Volume 2019:12 Pages 455—463
DOI https://doi.org/10.2147/IJGM.S218393
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Orawan Sae-lim,1 Thitima Doungngern,1 Siriluk Jaisue,2 Sirichai Cheewatanakornkul,3 Poukwan Arunmanakul,4 Sirirat Anutrakulchai,5 Rungsrit Kanjanavanit,6 Wibul Wongpoowarak7
1Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Songkhla, Thailand; 2Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand; 3Division of Cardiology, Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand; 4Department of Pharmaceutical Care, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand; 5Division of Nephrology, Department of Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; 6Division of Cardiology, Department of Internal Medicine, Faculty of Medicine, Chaing Mai University, Chiang Mai, Thailand; 7Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Songkhla, Thailand
Correspondence: Thitima Doungngern
Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Prince of Songkla University Songkhla, 90112, Thailand
Tel/Fax +6674428222
Email [email protected]
Purpose: Serum digoxin concentration (SDC) monitoring may be unavailable in some healthcare settings. Predicted SDC comes into play in the efficacy and toxicity monitoring of digoxin. Renal function is the important parameter for predicting SDC. This study was conducted to compare measured and predicted SDC when using creatinine clearance (CrCl) from Cockcroft–Gault (CG) equation and estimated glomerular filtration rate (eGFR) calculated from CKD-Epidemiology Collaboration (CKD-EPI), re-expressed Modification of Diet in Renal Disease (Re-MDRD4), Thai-MDRD4, and Thai-eGFR equations in Sheiner’s and Konishi’s pharmacokinetic models.
Patients and methods: In this retrospective study, patients with cardiovascular disease with a steady-state of SDC within 0.5–2.0 mcg/L were enrolled. CrCl and studied eGFR adjusted for body surface area (BSA) were used in the models to determine the predicted SDC. The discrepancies of the measured and the predicted SDC were analyzed and compared.
Results: One hundred and twenty-four patients ranging in age from 22 to 88 years (median 60 years, IQR 50.2, 69.2) were studied. Their serum creatinine ranged from 0.40 to 1.80 mg/dL (median 0.90 mg/dL, IQR 0.79, 1.10). The mean±SD of measured SDC was 1.12±0.34 mcg/L. In the Sheiner’s model, the mean predicted SDC was calculated by using the CG and the BSA adjusted CKD-EPI equations and was not different when compared with the measured levels (1.10±0.36 mcg/L (p=0.669) and 1.08±0.42 mcg/L (p=0.374), respectively). The CG, CKD-EPI, and Re-MDRD4 equations were a better fit for patients with creatinine ≥0.9 mg/dL for prediction with minimal errors. In the Konishi’s model, the predicted SDC using the CG and the studied eGFR equation was lower than the measured SDC (p<0.05).
Conclusion: In Sheiner’s model, the CG and the BSA adjusted CKD-EPI equations should be used for predicting SDC, especially in patients with serum creatinine ≥0.9 mg/dL. The other studied eGFRs underestimated SDC in both Sheiner’s and Konishi’s model.
Keywords: predicted concentration, digoxin, creatinine clearance, estimated glomerular filtration rate equation, Cockcroft–Gault equation
Introduction
The Cockcroft–Gault (CG) equation provides an estimation of creatinine clearance (CrCl) in a patient with stable renal function.1 CrCl is commonly used for medication dosage adjustment in patients with renal impairment.2–4 The estimated glomerular filtration rate (eGFR), on the other hand, is widely used to monitor and classify the stage of renal function.2,4 Several eGFR equations have been proposed, for example, the Modification of Diet in Renal Disease (MDRD) equation and the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation. In addition, the Thai-MDRD4 and Thai-eGFR equations have been developed and validated for the Thai population.5 Currently, the validity of eGFR-based drug dosing recommendation is limited.6
Digoxin is excreted in the kidney and has a narrow therapeutic index of 0.5–2.0 mcg/L.7 It is approved for treating heart failure and atrial fibrillation.8,9 The Digitalis Investigation Group found that a serum digoxin concentration (SDC) greater than 0.9 mcg/L increased the mortality rate in patients with reduced ejection fraction heart failure.10 A higher SDC may be required to control ventricular rate in patients with atrial fibrillation. However, a recent study in patients with atrial fibrillation revealed an increase in risk of death if SDC is more than 1.2 mcg/L.11 Thus, closely monitoring SDC is required to optimize therapeutic effects and avoid toxicity. However, SDC monitoring may not be available in all healthcare settings. The prediction of SDC using the digoxin pharmacokinetic model may come into play in efficacy and toxicity monitoring. Several pharmacokinetic models were developed to predict SDC; for example, Sheiner et al proposed the equation for predicting serum digoxin clearance in patients with and without heart failure.7 Konishi et al developed a simple equation to predict SDC in patients with heart failure,12 and it has recently been studied in Thai population.13 The important parameter of these pharmacokinetic models is the renal function, where the CrCl estimated by the CG equation is recommended.7 The eGFRs, on the other hand, have not been studied for predicting SDC.7,14 Therefore, this study was conducted to compare the measured SDC with the predicted SDC when using the CrCl and eGFR calculated from various equations including CKD-EPI, re-expressed 4 variable MDRD (Re-MDRD4), Thai-MDRD4 and Thai-eGFR in the Konishi’s and Sheiner’s pharmacokinetic models.
Materials And Methods
Study Design And Setting
A multicenter, retrospective medical chart review study was conducted in three university-based hospitals in Thailand including Songklanagarind Hospital, Srinagarind Hospital, and Maharaj Nakorn Chaingmai Hospital. The Institution Review Board (IRB) of each institute approved the study protocol; the number of the Institution Review Board from all three hospitals were 58-011-19-9, HE-581138, and NONE-2558-02832, respectively. Due to the retrospective nature of the study, the patient consent was waived by the IRBs. This study was conducted in accordance with the Declaration of Helsinki. We confirmed that the data were maintained with confidentiality.
Patient Selection And Data Collection
The study population was patients age 18 years or older who had SDC measured from January 2012 to December 2015. Patients were included in the study if they were taking digoxin tablets orally in a dose that has remained unchanged for at least 14 days, and had obtained SDC for at least 6 hrs but not more than 24 hrs after the last digoxin dose. Exclusion criteria were having SDC less than 0.5 mcg/L or greater than 2.0 mcg/L, being diagnosed with hypothyroidism or hyperthyroidism, acute kidney injury, requiring renal replacement therapy, and having received medications that effect SDC (eg, amiodarone, verapamil, quinidine, rifampicin) within 1 month. Patient information was retrospectively reviewed from a computer-based patient recording system. Clinical-related data including age, gender, weight, height, serum creatinine, serum albumin, medical history, and concurrent medication use were collected. The body weight, height and serum creatinine were collected on the same day or within 3 months of SDC monitoring. Digoxin-specific information collected in this study include the dosage regimen of digoxin, the timing of digoxin administration, and the schedule of obtaining SDC levels.
The AxSYM® Digoxin III assay (Abbott Laboratories, IL, USA), using the microparticle enzyme immunoassay (MEIA) technique, was used to measure SDC levels. The enzymatic creatinine assay (Roche, Basel, Switzerland) was used to measure serum creatinine levels. All study settings used the same techniques for analysis. The estimated digoxin clearance of each patient calculated as follows:
(1)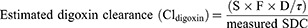
S corresponds to the active fraction of the administered form (digoxin = 1 because digoxin is not administered as a salt), F is the bioavailability of digoxin (digoxin tablet = 0.7), D is the digoxin daily dose (mcg/day) and τ is the dosing interval.7
Estimated Renal Function
Creatinine clearance was estimated from the patient’s serum creatinine using CG equation. The eGFR was estimated by using the CKD-EPI equation, the Re-MDRD4 equation, the Thai-MDRD4 equation, and the Thai-eGFR equation as follows:
CG equation1
(2)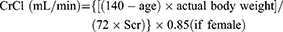
CKD-EPI equation15
(3)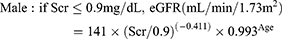
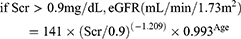
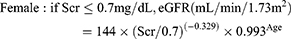
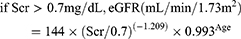
Re-MDRD4 equation16
(4)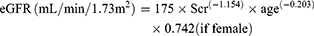
Thai-MDRD4 equation5
(5)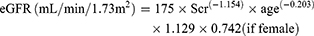
Thai-eGFR equation5
(6)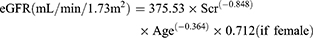
Scr corresponds to the patient’s serum creatinine. The eGFRs computed from equation 3–6 were adjusted for body surface area to convert eGFR to mL/min.
Predicted Serum Digoxin Concentration
Konishi’s Model12
The predicted SDC at steady-state (Css) of Konishi’s digoxin pharmacokinetic model was determined as follows:12
(7)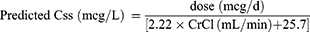
The CrCl, calculated from the CG equation, was replaced by the adjusted eGFRs calculated from equations 3–6 in the model.
Sheiner’s Model7
The predicted digoxin clearance (Cldigoxin) in patient with and without heart failure calculated from equation 8 and 9, respectively.7 The CrCl was replaced by the eGFR calculated from Equations 3–6. After that the predicted patient’s SDC at a steady-state was calculated from Equation 10.
(8) (9) (10)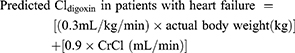

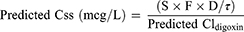
S corresponds to the active fraction of the digoxin (1), F is the bioavailability of digoxin tablet (0.7), D is the digoxin daily dose (mcg/day) and τ is the dosing interval.7
Statistical Analysis
The difference of the measured and the predicted SDC were analyzed using a paired t-test with a two-sided significance level of p<0.05. An unpaired t-test was used to compare the two different patient groups. Degree of agreement of the measured values and the predicted values was assessed by the mean of difference between the measured values with the predicted values (mean prediction error, ME) and the mean absolute deviation between the measured and predicted values (mean absolute prediction error, MAE). The modified Bland–Altman plot was used to show the difference between the measured SDC and the ME when the studied eGFR equations were used for prediction.
Results
A total of 124 patients were enrolled in this study. Sixty-four patients (51.6%) were 60 years or older and had a median serum creatinine of 0.9 mg/dL (Table 1). Forty-seven patients (37.9%) were underweight (body mass index <20 kg/m2) and 24 patients (19.4%) were overweight (body mass index >25 kg/m2). Among 55 patients who were diagnosed with heart failure, 23 of these patients (41.8%) had acute decompensated heart failure when SDC was measured.
 |
Table 1 Baseline Characteristics Of Patients Included In The Study (n = 124) |
Estimated Renal Function
The adjusted eGFR for body surface area estimated by the studied equations were significantly higher than the CrCl estimated by the CG equation (Table 2).
 |
Table 2 Comparison Of Creatinine Clearance And Adjusted eGFR For Body Surface Area (n = 124) |
Measured Serum Digoxin Concentration And Estimated Digoxin Clearance
The mean measured SDCs of patients with heart failure (n = 55) and without heart failure (n = 69) were 1.13 ± 0.35 and 1.11 ± 0.34 mcg/L, respectively. The mean estimated digoxin clearance of all patients was 92.92 ± 40.41 L/day. Patients with heart failure had a slightly lower estimated digoxin clearance than those without heart failure (86.96 ± 36.07 vs 97.66 ± 43.24 L/day, p = 0.141 using unpaired t-test).
Predicted Serum Digoxin Concentration
Konishi’s Model
The mean predicted SDC of all patients calculated by using the CG and the studied eGFR equations was significantly lower than the measured level (Table 3). In patients with heart failure, the predicted SDC was also under-prediction.
 |
Table 3 Predicted Serum Digoxin Concentration Using The Konishi’s Model (n = 124) |
Sheiner’s Model
The mean predicted SDC calculated by using the CG and the adjusted CKD-EPI equations was not different as compared with the measured values. Whereas the predicted SDC using the Re-MDRD4, the Thai-MDRD4, and the Thai-eGFR equations was significantly lower than the measured values (Table 4). The mean prediction SDC error was lowest when the CG equation was used for prediction but the mean absolute prediction error was not different from the other equations (Table 5). The prediction error was slightly lower in the group of Thai-eGFR.
 |
Table 4 Predicted Digoxin Clearance And Serum Digoxin Concentration Using The Sheiner’s Model (n = 124) |
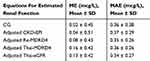 |
Table 5 Predictive Performance Of The Studied Equations To Estimated Serum Digoxin Concentration Using The Sheiner’s Model (n = 124) |
Figure 1 shows the distribution of prediction errors using the Sheiner’s model. The prediction error was low if the measured levels were less than 1.0 mcg/L. Predicted SDC using the Thai-MDRD4 equation and the Thai-eGFR equation had the highest prediction error. Figure 2 shows the percent prediction errors. The majority of predicted SDC were underpredicted. If we divided the patients into two groups according to serum creatinine levels, the CG equation, the CKD-EPI equation, and the Re-MDRD4 equation were better fit for the group with serum creatinine ≥0.9 mg/dL to predict SDC with minimal prediction errors as compared to the measured values (Table 6). However, in the group that had serum creatinine less than 0.9 mg/dL only the CG equation could be used for prediction.
 |
Table 6 Predicted Serum Digoxin Concentration Divided By Serum Creatinine Using The Sheiner’s Model (n = 124) |
Discussion
Renal function was a key parameter for prediction of serum digoxin concentration in the Konishi’s and Sheiner’s model.7,12 Both models recommended using creatinine clearance estimated from the CG equation. The CG equation was developed from the study of creatinine excretion in patients with an average weight of 72 kg and stable renal function.1 While the studied eGFRs were developed from the clearance of exogenous filtration markers such as 125I-iothalamate and 99mTc-DTPA.5,15,16 The Re-MDRD4 equation was developed from patients with chronic kidney disease,16 therefore, it may be not fit for patients with normal renal function.15 The CKD-EPI equation was developed from patients with or without chronic kidney disease, so that it was more accurate than the Re-MDRD4 equation to predict renal function, especially in those patients with GFR > 60 mL/min/1.73 m2.15 Thus, the CKD-EPI equation became accepted among healthcare providers and used for classification and staging of chronic kidney disease.17 However, the Re-MDRD4 equation and CKD-EPI equation were reported to overestimate the renal function as compared with the CG equation.3,18 Similar to our study, the studied eGFR, adjusted for body surface area, were significantly overestimated as compared with the CrCl estimated by the CG equation. The Thai-MDRD4 equation and Thai-eGFR equation were proposed for use in Thai population with chronic kidney disease. To our knowledge, both equations had not been studied for drug dosage adjustment. This study revealed that the Thai-MDRD4 equation and Thai-eGFR equation should not be used for prediction of SDC with the Konishi’s and the Sheiner’s model.
Our study found that the Konishi’s model significantly underpredicted SDC by approximately 1.3-fold as compared to the measured SDC (mean error of 0.25 ± 0.44 mcg/L) when the CG equation was used for prediction. The Konishi’s model was developed for predicting SDC in patients with stable heart failure. Patients with acute illness were excluded from the model.12 Our study, on the other hand, did include patients with both stable and unstable heart failure conditions. We also included patients who had been taking digoxin for treatment of atrial fibrillation and pulmonary hypertension into the study. The subgroup analysis of our results in patients with stable heart failure (n = 38) continued to find the underpredicted SDC (measured SDC 1.17 ± 0.14 vs predicted SDC by using CG equation 0.76 ± 0.26 mcg/L, p < 0.001). In contrast, the recent study of Jiratham-Opas et al who found that using the CG equation by Konishi’s model was better in predicting SDC in Thai patients. This study reported the sensitivity and specificity of Konishi’s model to be 72.7% and 80.6%, respectively. The Konishi’s model was appropriate to predict SDC, especially in patients with reduced ejection fraction heart failure (correlation coefficient of 0.80). However, 63.2% of the measured SDC in that study was subtherapeutic (< 0.5 mcg/L),13 that was not included in our study.
The Sheiner’s model, on the other hand, was found to be more suitable to predict SDC in our study. The CrCl estimated by the CG equation was the best fit to predict SDC since it showed the minimum prediction error. Our result was similar to the study of Vazquez–Hernandez et al who found the predicted SDC using the CG and the Re-MDRD4 equation with the Sheiner’s model had a deviation of more than 15% in patients with heart failure.19 Besides the CrCl, the adjusted CKD-EPI equation for body surface area could be used for prediction of SDC, especially in those patients with serum creatinine ≥0.9 mg/dL. The Re-MDRD4 equation could also be used for prediction if serum creatinine was ≥0.9 mg/dL. However, it was not recommended if serum creatinine < 0.9 mg/dL. Serum creatinine generation could be reduced in the setting of low muscle mass,20,21 therefore it could overpredict renal function. If CrCl increases, SDC may decrease. This could explain our finding of underprediction of SDC in patients with serum creatinine < 0.9 mg/dL.
The CrCl estimated by the CG equation is commonly recommended for drug dosage adjustment.22 The eGFR, especially estimated by the CKD-EPI equation, usually reports in clinical setting for classifying chronic kidney disease and staging of renal function. The eGFR presents in the unit of mL/min/1.73 m2 whereas the CrCl presents in mL/min. The use of the adjusted CKD-EPI equation for body surface area (change to mL/min) had a great rate of concordance with the CG equation reported by Khanal et al23 and Okparavero et al.24 Similar to our study, the CKD-EPI with adjustment for body surface area was appropriate for prediction of SDC, especially in the patients with serum creatinine ≥0.9 mg/dL.
There were some limitations in this study which may affect the results. The study was performed retrospectively; therefore, the compliance to digoxin therapy could not be verified. The results could be applied to patients who had serum digoxin concentration within the therapeutic range of 0.5–2.0 mcg/L. Finally, even though we did not obtain the trough SDC (before the next dose), the median time of blood drawn was 23 hrs (ranging from 8 to 24 hrs). Therefore, we believe that the time difference could have little effect on SDC in our study.
Conclusion
The CrCl estimated by the CG equation continues to be the key parameter for predicting SDC with the Sheiner’s pharmacokinetic model. The adjusted CKD-EPI equation for body surface area can also be used for the prediction of SDC, especially in patients with serum creatinine ≥0.9 mg/dL. However, the CKD-EPI equation should be used with caution when predicting serum digoxin concentration when serum creatinine <0.9 mg/dL. The Thai-MDRD4 equation and the Thai-eGFR equation should not be used for prediction of serum digoxin concentration.
Acknowledgments
This study was funded by a research grant of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand. The authors would like to thank Ms Arnurai Jitsurong and other technicians for acquisition of data and Ms Maria Suzanne Mullet for reviewing the manuscript.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi:10.1159/000180580
2. Hudson JQ, Nyman HA. Use of estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20(5):482–491. doi:10.1097/MNH.0b013e328348c11f
3. Nyman HA, Dowling TC, Hudson JQ, Peter WL, Joy MS, Nolin TD. Comparative evaluation of the Cockcroft-Gault Equation and the Modification of Diet in Renal Disease (MDRD) study equation for drug dosing: an opinion of the Nephrology Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2011;31(11):1130–1144. doi:10.1592/phco.31.11.1130
4. Park EJ, Wu K, Mi Z, et al. A systematic comparison of cockcroft-gault and modification of diet in renal disease equations for classification of kidney dysfunction and dosage adjustment. Ann Pharmacother. 2012;46(9):1174–1187. doi:10.1345/aph.1Q757
5. Praditpornsilpa K, Townamchai N, Chaiwatanarat T, et al. The need for robust validation for MDRD-based glomerular filtration rate estimation in various CKD populations. Nephrol Dial Transplant. 2011;26(9):2780–2785. doi:10.1093/ndt/gfq815
6. Hudson JQ, Nolin TD. Pragmatic use of kidney function estimates for drug dosing: the tide is turning. Adv Chronic Kidney Dis. 2018;25(1):14–20. doi:10.1053/j.ackd.2017.10.003
7. Winter M. Digoxin. In: Winter M, editor. Basic Clinical Pharmacokinetics.
8. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132.
9. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327. doi:10.1161/CIR.0b013e31829e8776
10. Digitalis Investigation G. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525–533. doi:10.1056/NEJM199702203360801
11. Lopes RD, Rordorf R, De Ferrari GM, et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71(10):1063–1074. doi:10.1016/j.jacc.2017.12.060
12. Konishi H, Shimizu S, Chiba M, Minouchi T, Koida M, Yamaji A. Predictive performance of serum digoxin concentration in patients with congestive heart failure by a hyperbolic model based on creatinine clearance. J Clin Pharm Ther. 2002;27(4):257–265. doi:10.1046/j.1365-2710.2002.00418.x
13. Jiratham-Opas J, Kanjanavanit R, Wongcharoen W, et al. Can available mathematical models predict serum digoxin levels in Thai patients? J Clin Pharm Ther. 2018;43(3):377–384. doi.org/10.1111/jcpt.12667
14. Gheorghiade M, Adams KF
15. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
16. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi:10.7326/0003-4819-130-6-199903160-00002
17. Kidney Disease: improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(1):5–14.
18. Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–1009. doi:10.2215/CJN.06870909
19. Vazquez-Hernandez M, Bouzas L, Tutor JC. Glomerular filtration rate estimation using the Cockcroft-Gault and modification of diet in renal disease formulas for digoxin dose adjustment in patients with heart failure. Ups J Med Sci. 2009;114(3):154–159. doi:10.1080/03009730903191853
20. Smythe M, Hoffman J, Kizy K, Dmuchowski C. Estimating creatinine clearance in elderly patients with low serum creatinine concentrations. Am J Hosp Pharm. 1994;51(2):198–204.
21. Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. 2016;8(5):E305–311. doi: 10.21037/jtd.2016.03.62
22. McConachie SM, Hanni CM, Raub JN, Kucemba M, Wilhelm SM. National assessment of renal estimation for drug dosing among pharmacists. J Am Coll Clin Pharm. 2018;2(3):204–213. doi:10.1002/jac5.1057
23. Khanal A, Peterson GM, Jose MD, Castelino RL. Comparison of equations for dosing of medications in renal impairment. Nephrology. 2017;22(6):470–477. doi: 10.1111/nep.12834
24. Okparavero AA, Tighiouart H, Krishnasami Z, et al. Use of glomerular filtration rate estimating equations for drug dosing in HIV-positive patients. Antivir Ther. 2013;18(6):793–802. doi:10.3851/IMP2676
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


