Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Prediction of Risk Factors for the Evolution of Traumatic Subdural Effusion into Chronic Subdural Hematoma
Authors Chen S , Peng H, Shao X , Yao L, Liu J, Tian J, Sun L, Dai Y, Jiang X, Cheng L
Received 13 January 2020
Accepted for publication 29 March 2020
Published 9 April 2020 Volume 2020:16 Pages 943—948
DOI https://doi.org/10.2147/NDT.S245857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Sansong Chen,1 Hui Peng,2 Xuefei Shao,1 Lin Yao,1 Jie Liu,1 Jiongping Tian,1 Lean Sun,1 Yi Dai,1 Xiaochun Jiang,1,* Limin Cheng3,*
1Department of Neurosurgery, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital), Wuhu 241001, Anhui, People’s Republic of China; 2Administration Office of Hospital Admission and Discharge, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital), Wuhu 241001, Anhui, People’s Republic of China; 3Morphology Experiment & Training Center, School of Preclinical Medicine, Wannan Medical College, Wuhu 241002, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaochun Jiang
Department of Neurosurgery, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital), Wuhu 241001, Anhui, People’s Republic of China
Email [email protected]
Limin Cheng
Morphology Experiment & Training Center, School of Preclinical Medicine, Wannan Medical College, Wuhu 241002, Anhui, People’s Republic of China
Email [email protected]
Purpose: To explore the risk factors of the evolution of traumatic subdural effusion (TSE) into chronic subdural hematoma (CSDH).
Materials and Methods: The 70 patients’ gender, age, location of effusion, unilateral and bilateral, Glasgow coma score (GCS) at admission, presence or absence of brain contusion, the time of effusion appeared, daily amount of mannitol, mannitol number of days used, with or without atorvastatin calcium tablets, with or without antiplatelet aggregation drugs, with or without anticoagulant drugs, with or without abnormalities in blood coagulation routines, computed tomography (CT) layer height, the thickness, and CT value of the first effusion were analyzed by single factor. Logistic multivariate regression analysis was performed on the statistically significant indicators. Power of the regression model was evaluated using receiver operating characteristic (ROC) curve.
Results: Univariate analysis showed that the presence or absence of brain contusion, the time of effusion appeared, atorvastatin calcium tablets use or not, the CT value of the effusion, and TSE into CSDH evolution varied significantly compared to the non-evolved group (P< 0.05). Logistic multivariate regression analysis showed that combined brain contusion (odds ratio (OR)=16.247, 95% confidence interval (CI), 1.831– 144.157, P = 0.012), early onset of effusion (OR = 0.573, 95% CI: 0.349– 0.941, P = 0.028), atorvastatin calcium tablets not used after effusion (OR = 60.028, 95% CI: 6.103– 590.399, P = 0.0001), and high CT value (OR = 1.285, 95% CI: 1.067– 1.547, P = 0.008) were risk factors for the evolution of TSE into CSDH. The ROC model suggested that the prediction of these risk factors had high diagnostic accuracy.
Conclusion: TSE patients with brain contusion, early onset of effusion, without the usage of atorvastatin calcium tablets after effusion, and high CT value of the first effusion are at a risk of evolving into CSDH.
Keywords: TSE, CSDH, evolution, risk factors
Introduction
Traumatic subdural effusion (TSE) was first reported by Mayo et al in 1894. In the past, due to the lack of examination methods and the characteristic clinical symptoms, the incidence was considered low. However, with the development of modern imaging medicine and the improvement of living standards, the incidence is reported as 1.16–10%,1 which was further elevated based on the CT findings. Nonetheless, due to the lack of specific symptoms or concomitant brain contusion, TSE is easily ignored by clinicians or masked by other conditions. A large number of studies have shown that some cases chronically evolve into subdural hematomas and might require surgical treatment. So, what clinical characteristics of TSE patients are likely to evolve into CSDH? This retrospective study analyzed the clinical data of 70 patients, who were confirmed with TSE in the Yijishan Hospital of Wannan Medical College. Also, the risk factors for TSE that evolve into CSDH were explored, providing a reference for future clinical prevention and treatment.
Materials and Methods
Ethical Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Wannan Medical College (Yijishan Hospital). As this study featured a retrospective design, the Ethics Committee judged it as minimal risk research and the participants could not be located. All study data of the participants were kept confidentially by the investigators according to the guideline of the Ethics.
General Information
From January 2017 to December 2017, 70 patients, who were admitted to the Department of Neurosurgery of the First Affiliated Hospital of Wannan Medical College, were diagnosed with TSE and had returning data. The cohort comprised of 59 males and 11 females, aged 17–88 (median: 66.5) years. There were 42 cases of bilateral effusion and 28 cases of unilateral effusion. All patients had a history of head trauma. The Glasgow coma score (GCS) was 6–15 (average: 13.23 ± 2.72) points on admission. The selected patients met the diagnostic criteria for TSEs as proposed by John et al.:2,3 (1) The effusion appeared within 10 days of head trauma; (2) Similar uniform low-density area and width > 3mm; (3) CT value of lesion area < 20 Hu; (4) CT did not show any enhanced capsule. Patients with intracranial hematoma requiring surgery or those with other diseases requiring special treatment after admission are excluded. Subsequently, 17 patients were assigned to the evolved group and 53 to the non-evolved group based on their evolution into CSDH.
The Standards of TSE Evolves to CSDH
(1) CT scan within 10 days after traumatic subdural effusion and subdural hematoma appeared 3 weeks after traumatic brain injury; (2) CT scan of the skull showed slightly low or low-density shadow, CT value > 20Hu; (3) MRI showed marked changes in the signal in both T1 and T2 weighted images, and the envelope was enhanced.4,5
Data Collection
All patients underwent more than 3 CT examinations during hospitalization. The concomitant brain contusion, the location of the first unilateral and bilateral effusion, the time of occurrence, the CT value of the effusion, the maximum CT thickness of the effusion, and CT layer height were recorded. Among these parameters, the CT layer height of the effusion refers to the layer thickness of the CT scan × the number of layers of the effusion. The layer thickness of the conventional CT scan in our hospital was 6 mm. The maximum CT thickness of the effusion refers to the maximum vertical distance of the skull plate from the brain surface, which is directly measured by the picture archiving and communication systems (PACS). The CT value of the effusion was directly measured by the PACS. Also, the daily amount of mannitol and the number of days during the treatment, whether or not atorvastatin calcium tablets were administered orally, whether antiplatelet aggregation drugs and anticoagulants were used, and whether the blood coagulation routine was abnormal was evaluated. All patients were followed up for 1 year. CT examination was performed to understand the absorption, progression, and evolution of effusion.
Statistical Analysis
SPSS18.0 was used to analyze the data, and the measurement data consistent with the normal distribution were expressed as (mean ± standard deviation). The measurement data for non-normal distributions were expressed as medians, and non-parametric tests were applied. Enumeration data were assessed by the χ2 test. Logistic regression multivariate analysis of variance was used to explore the related risk factors. P < 0.05 indicated statistically significant difference.
Results
A total of 70 TSE patients included 28 with unilateral effusion, 42 with bilateral effusion, 42 with frontal effusion, and 28 with frontotemporal effusion. Of these, 17 patients (24.3%) evolved into CSDH: 9 evolved into hematomas on the left, 4 on the right, and 4 on both sides. The maximum thickness of subdural effusion detected by was 3–17.4 (average: 5.144 ± 1.98) mm. The CT layer height of the effusion was 12–72 (median: 30) mm. The effusion CT value was 3–18 (average: 8.06 ± 2.29) Hu. The time of effusion appearance was 1–10 (average: 4.24 ± 2.64) days after injury. TSE evolved into CSDH in 22–185 (median: of 51) days.
Single-Factor Analysis Affecting the Evolution of TSE to CSDH
The univariate analysis showed that compared to the non-evolved group, the presence or absence of combined brain contusion, the time of effusion appearance, the use or not of atorvastatin calcium tablets after the effusion, and the CT value of the effusion were statistically significant (P < 0.05). The other factors did not significantly between the two groups (Table 1).
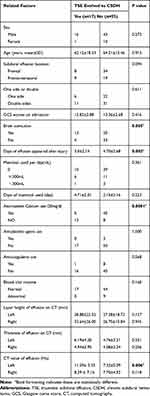 |
Table 1 Clinical Characteristics of the 70 TSE Patients and Single-Factor Analysis of the Related Risk Factors |
Multifactorial Analysis of the Evolution of TSE to CSDH
Factors that were statistically different in univariate analysis, such as a combined brain contusion, the time of effusion appearance, consumption of atorvastatin calcium tablets after the effusion, and the CT value of the effusion as independent variables, evolved into CSDH. The presence or absence of CSDH was used as the binary classification dependent variable. Furthermore, logistic multivariate regression analysis showed that the combined brain contusion (OR = 16.247, 95% CI: 1.831–144.157, P = 0.012), no consumption of atorvastatin calcium tablets (OR = 60.028, 95% CI: 6.103–590.399, P = 0.0001) and high CT value of the first effusion (OR = 1.285, 95% CI: 1.067–1.547, P = 0.008) were independent risk factors for the evolution of TSE into CSDH. The time of the effusion appearance (OR = 0.573, 95% CI: 0.349–0.941, P = 0.028) was a protective factor, indicating that earlier the effusion appears, easier it is to evolve into CSDH (Table 2).
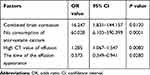 |
Table 2 Logistic Multivariate Regression Analysis of Risk Factors for TSE to CSDH Evolution |
ROC Model of the Independent Risk Factors
The independent risk factors obtained by logistic multivariate regression analysis were included in the ROC model. The area under the curve, standard error, 95% CI, P-value, and a cutoff value of the independent risk factors are shown in Table 3. Except for the area under the brain contusion curve (0.694), the area under the other curves was >0.70, suggesting that the prediction model has high diagnostic accuracy (Figure 1). The cutoff value of the time of effusion appearance after the injury was 3.5 days, and the cutoff CT value of the effusion was 11.5 Hu, suggesting that if the time of effusion appearance after the injury was <3.5 days and the CT value of the effusion was >11.5 Hu, the condition of the patients could potentially evolve into CSDH.
 |
Table 3 ROC Model of the Independent Risk Factors |
Discussion
TSE was first proposed by Mayo in 1894 but did not attract clinicians’ attention until 1979 when Yamada et al reported three cases in which TSE evolved to CSDH.6 Since then, related reports have gradually increased, but the evolution rates of their respective reports have also differed by 8–47%.7–9 This evolution might be attributed to the following reasons: the increase in subdural effusion that leads to an increase in the space between the arachnoid and the dura mater, which in turn, leads to bleeding after the bridge vein is pulled; after a long period of subdural effusion, an envelope is formed; bleeding after neovascularization in the membrane; fibrin dissolves and increases in the effusion, leading to bleeding due to coagulation dysfunction; changes in the properties of the effusion, small molecule inflammatory substances cause degradation of lymphocyte aggregation after degradation, leading to the formation of hematomas due to leakage of new capillaries.5,10,11 In clinical practice, we observed that most of the subdural effusion could be self-absorbed, some progressed, and a small part of the effusion evolved into CSDH. Thus, the clinical characteristics of TSE patients that are likely to evolve into CSDH are worth investigating. In this retrospective study, we analyzed the clinical data of 70 TSE patients with return visit information and found that 17 evolved into CSDH. Thus, the analysis of relevant factors to summarize the risk factors for TSE into CSDH would aid in early detection, intervention, and treatment in the clinical setting in the future.
Univariate analysis revealed that the subdural effusion appeared earlier in the evolution group than the non-evolution group, with an average of 3.0 ± 2.14 days. Logistic multivariate regression analysis showed that the OR value of the time of the effusion appearance was 0.573, which was <1. It indicates that the earlier the effusion appears, the easier it is to evolve into CSDH. The cutoff value of 3.5 days, suggested that the time of effusion appearance after the injury was <3.5 days and could potentially evolve into CSDH. In addition, the univariate and multivariate analysis suggested that combined brain contusion was an independent risk factor for TSE to evolve into CSDH (P < 0.05). In summary, the reason may be that the rupture of the arachnoid after injury, ie, the “one-way valve” effect is obvious. The subdural effusion increased significantly in the short term, causing brain tissue displacement. Caused by the bridge vein stretch bleeding or long-term effusion formation of the envelope, bleeding after the formation of new blood vessels forms a subdural hematoma. The brain contusion patients with venous bleeding on the surface of the brain and flowing into the subdural alters the properties and promotes the evolution of the effusion. In addition, due to the rupture of the pia mater and arachnoid in the brain contusion patients, the cerebrospinal fluid flowed into the subdural space. In light of the glymphatic theory,12,13 the inflow of cerebrospinal fluid into the deep part of the brain along the periarterial space was reduced, so that aquaporin-4 (AQP4)-mediated outflow of cerebrospinal fluid decreased. Then, the ability of pushing the brain interstitial fluid to flow into the venous space was weakened, which greatly weakened the bulk flow ability and the glymphatics’ clearance ability.14–16 The proteins in the CSF, cell debris, proteins and peptides produced by the brain contusion were accumulated in a short time in the subdural space which chelated inflammatory cells to aggregate and produce inflammatory mediators, thereby promoting neovascularization and bleeding, leading to the formation of CSDH.
Consistent with the conclusions of other researchers,17 the current univariate analysis showed that those with high CT values of effusion were more likely to evolve into CSDH, and the difference was statistically significant (P = 0.006). Logistic multivariate regression analysis also showed that the high CT value was an independent risk factor for TSE to evolve into CSDH (P = 0.008), and the OR value was 1.285. The cutoff value of 11.5 Hu suggested that if the CT value of the first effusion was ≥11.5 Hu, it is likely to evolve into CSDH. We know that the CT value of the effusion represents the blood and protein content of the effusion. A high CT value indicates a high blood content and protein content in the effusion, which can produce several inflammatory mediators after degradation, and the inflammatory chemotactic factors can promote the formation of new envelopes and blood vessels. The microvascular hemorrhage of the new blood vessels alters the properties of the effusion, which gradually evolves into CSDH.
Interestingly, both univariate and multivariate analysis showed that no usage of atorvastatin calcium tablets after effusion is a risk factor for TSE evolving into CSDH, and the differences were statistically significant. Cao et al also reported that atorvastatin calcium tablets could reduce the evolution of TSE into CSDH.5 Another study demonstrated that atorvastatin calcium tablets can not only inhibit the synthesis of cholesterol, but also significantly inhibit the inflammatory response, block inflammatory factors, such as IL-8, and induce inflammatory cell infiltration and vascular proliferation, thereby effectively promoting the absorption of TSE and reducing the evolution to CSDH.18,19 In addition, another study also demonstrated that MMP-2 and MMP-9 played an important role in promoting the formation of CSDH in the outer membrane of hematomas.20 Statins can reduce the expression of MMP-9 mRNA and protein, which may inhibit the evolution of TSE to CSDH by inhibiting the local inflammation of subdural effusion.21,22 In this retrospective analysis, some patients administered atorvastatin calcium tablets due to dyslipidemia or combined cardiovascular and cerebrovascular diseases. The results showed that atorvastatin calcium tablets exert a preventive effect on the evolution of TSE into CSDH.
In summary, the current study found that traumatic subdural effusion combined with brain contusion, early onset of effusion, high CT value of the effusion, and no use atorvastatin calcium tablets after effusion were risk factors for the evolution of TSE into CSDH, especially in patients with subdural effusion that appeared within 3.5 days after injury, and the first CT value of the effusion was >11.5 Hu. These patients need to be under observation, close follow-up, and dynamic review of CT. After the progress or evolution of effusion is found, early intervention and treatment are needed. For patients who are not contraindicated with oral atorvastatin calcium tablets, administration of atorvastatin calcium tablets after effusions may play a role in promoting the absorption of effusions and reducing the evolution into CSDH. Nevertheless, this study had some limitations due to small sample retrospective studies. Thus, additional samples and multicenter prospective studies would be needed to substantiate these findings in the future.
Acknowledgments
This work was supported by youth project of Natural Science Foundation of Anhui Province (No.1908085QH362) and Collegiate Major Natural Science Research Project of Anhui Province Education Department (No.KJ2019A0406).
Disclosure
The authors do not have any actual or potential conflicts of interest with other people or organizations.
References
1. Xu F. The research progress of traumatic subdural effusion. Int J Neurol Neurosurg. 2011;38:182–185.
2. John JN, Dila C. Traumatic subdural hydroma in adults. Neurosurgery. 1981;9:621–625.
3. Liu YG, Jia T, Liu M. Typing and clinical characteristics of traumatic subdural effusion. Chi J Surg. 2003;41(10):763–765.
4. Liu YG, Zhu SG, Jiang YQ. Evolution of traumatic subdural hydroma into chronic subdural hematoma. Chi J Surg. 2002;40(5):360–362.
5. Cao DM, Qi WT, Wu YK. Atorvastatin clinical study on reducing the conversion of traumatic subdural effusion into chronic subdural hematoma. Chi J Crit Care Med (Electronic Edition). 2018;11(3):197–199.
6. Yamada H, Nihei H, Watanabe T, et al. Chronic subdural hematoma occurring consequently to the posttraumatic subdural hygroma–on the pathogenesis of the chronic subdural hematoma (author’s transl). No To Shinkei. 1979;31(2):115–121.
7. Ohno K, Suzuki R, Masaoka H, et al. Role of traumatic subdural fluid collection in developing process of chronic subdural hematoma. Bull Tokyo Med Dent Univ. 1986;33(3):99–106.
8. Lee KS, Bae WK, Park YT, et al. The pathogenesis and fate of traumatic subdural hygroma. Br J Neurosurg. 1994;8(5):551–558. doi:10.3109/02688699409002947
9. Wang Y, Wang C, Liu Y. Chronic subdural haematoma evolving from traumatic subdural hydroma. Brain Inj. 2015;29(4):462–465. doi:10.3109/02699052.2014.990513
10. Feng JF, Jiang JY, Bao YH, et al. Traumatic subdural effusion evolves into chronic subdural hematoma: two stages of the same inflammatory reaction? Med Hypotheses. 2008;70:1147–1149. doi:10.1016/j.mehy.2007.11.014
11. Xu GS, Li MH, Li YY, et al. Subdural effusion is the basis of chronic subdural hematoma formation foundation. Jiangxi Med J. 2010;45:114–116.
12. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. doi:10.1126/scitranslmed.3003748
13. Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40(12):2583–2599. doi:10.1007/s11064-015-1581-6
14. Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–16193. doi:10.1523/JNEUROSCI.3020-14.2014
15. Gaberal T, Gakuba C, Goulay R, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45(10):3092–3096. doi:10.1161/STROKEAHA.114.006617
16. Jullienne A, Obenaus A, Ichkova A, et al. Chronic cerebrovascular dysfunction after traumatic brain injury. J Neurosci Res. 2016;94(7):609–622. doi:10.1002/jnr.23732
17. Guo ZY, Liu CX, Zhou R, et al. Discussion on factors related to the transformation of traumatic subdural effusion into chronic subdural hematoma. Ner Inj Func Reconstruc. 2016;11(2):122–124.
18. Wang Y, Chang H, Zou J, et al. The effect of atorvastatin on mRNA levels of inflammatory genes expression in human peripheral blood lymphocytes by DNA microarray. Bio Pharma. 2011;65(2):118–122. doi:10.1016/j.biopha.2010.12.005
19. Wang D, Li T, Tian Y, et al. Effects of atorvastatin on chronic subdural hematoma: a preliminary report from three medical centers. J Neurol Sci. 2014;336(1–2):237–242. doi:10.1016/j.jns.2013.11.005
20. Wang ZF, Liao DG. Role of matrix metalloproteinases in the transformation of subdural effusion into chronic subdural hematoma. J of Sout Medi Univ. 2010;30(5):1188–1189.
21. Wang YL, Hu SJ, Wang H, et al. Effects of different doses of rosuvastatin on serum high-sensitivity C-reactive protein and matrix metalloproteinase-9 in patients with acute coronary syndrome. Chi J Crit Care Med (Electronic Edition). 2014;7(3):195–198.
22. Ferretti G, Bacchetti T, Banach M, et al. Impact of statin therapy on plasma MMP-3, MMP-9, and TIMP-1 concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Angi. 2017;68(10):850–862. doi:10.1177/0003319716688301
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

