Back to Journals » Journal of Pain Research » Volume 13
Prediction of Postoperative Pain and Opioid Consumption Using Intraoperative Surgical Pleth Index After Surgical Incision: An Observational Study
Authors Jung K, Park MH , Kim DK, Kim BJ
Received 22 May 2020
Accepted for publication 29 September 2020
Published 6 November 2020 Volume 2020:13 Pages 2815—2824
DOI https://doi.org/10.2147/JPR.S264101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Kangha Jung, Mi Hye Park, Duk Kyung Kim, Byung Jun Kim
Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
Correspondence: Mi Hye Park
Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 51 Ilwon-Ro, Gangnam-Gu, Seoul 06351, South Korea
Tel +82-2-3410-5258
Fax +82-2-3410-6626
Email [email protected]
Background: We evaluated whether the surgical pleth index (SPI) following surgical incision was related to postoperative pain and opioid consumption.
Methods: This prospective observational study was performed in 50 patients undergoing laparotomy under sevoflurane-based general anesthesia. We recorded the highest SPI during surgical incision. The postoperative pain with a numerical rating scale (NRS) and opioid consumption during postoperative 24 h were compared in patients who showed SPI over 50 (Group H) or 20– 50 (Group L). The relationship between postoperative opioid consumption and SPI values (pre-incision, post-incision SPI value, change of SPI value, and post-incision SPI minus pre-incision SPI) was evaluated using receiver-operating characteristic analysis.
Results: The mean of the highest SPI value during surgical incision was 56 (SD, 12; range, 26– 85). Twenty-nine (63%) patients were included in Group H and 17 (37%) patients in Group L. There was a significant difference in NRS during recovery room stay and on postoperative 24 h in two groups (5 [5, 6] vs 7 [6, 8], p=0.007 and 3.5 [3, 5] vs 5 [5,6], p=0.006, Group L vs Group H). Group H used higher fentanyl via patient control analgesia during postoperative 24 h (573 (253) μg vs 817 (305) μg, p=0.008). A change of pre-incision and post-incision SPI value of 23, which showed the highest sensitivity (67%) and specificity (68%), was defined post hoc as the cut-off for fentanyl consumption during postoperative 24 h ≥ 1000 μg.
Conclusion: Our finding suggests that the SPI response to nociceptive stimuli during surgery is closely related to the degree of patient postoperative pain and opioid requirements. This information may be used to provide proper intraoperative analgesia and individual postoperative pain management.
Keywords: opioid, postoperative pain, stress response, surgical incision, surgical pleth index
Introduction
Postoperative pain intensity has not been significantly reduced in the past two decades despite considerable clinical progress in anesthetic and surgical procedures.1–3 One to two-thirds of patients suffer from moderate to severe postoperative pain, which is associated with adverse outcomes, including delirium, pulmonary and cardiac complications, and the development of persistent pain after surgery.4 Thus, preoperative identification of high pain responders may be helpful to prevent severe postoperative pain as well as these side effects.
Several studies have focused on prediction of acute postoperative pain.3,5–9 Female, younger age, preoperative pain, surgical nerve damage, and extensive surgical procedures are known risk factors for postoperative severe pain. But the prediction of severe postoperative pain is ambiguous, even the maximum postoperative pain intensity varied after same surgery presumed slight extent of postoperative pain.5,10 Also, we found there was considerable interindividual variation in pain intensity and opioid consumption through patient-controlled analgesia (PCA) even with similar surgical procedures after liver resection.11 These results highlight the need for individual evaluation of pain sensitivity and individually optimized pain management.
Previous studies attempting to assess pain perception were performed to evaluate individual pain thresholds and evaluated to serve as predictors of postoperative pain.5,7,12 The results suggested a link between preoperative pain thresholds and postoperative pain.
The minimum alveolar concentration (MAC) of sevoflurane needed to block the adrenergic response (BAR) to superficial incision in 50% of patients (MAC-BAR) was reported as 2.8–4% end-tidal sevoflurane.13,14 Analgesic properties that have been described for volatiles are based on response to painful stimulation.15 The surgical pleth index (SPI), based on the sum of photoplethysmographic pulse wave amplitude and the normalized heartbeat interval, has been shown to correlate with surgical stress intensity.15 The range of SPI value between 20 and 50 for guiding opioid titration during general anesthesia has been recommended in clinical practice.16,17 Also, SPI values at the end of surgery were closely related to the degree of postoperative pain.11,18,19
Thus, we tried to evaluate the level of stress response measured with SPI (SPI > 50 group vs SPI ≤ 50 group) and the steep increasing of SPI at equal MAC levels of end-tidal 3% sevoflurane during surgical incision13,20 and compare the relationship with postoperative pain score and opioid consumption. We hypothesized the stress response to surgical incision based on SPI enables evaluation of pain sensitivity and that it can be used to predict postoperative pain and opioid consumption.
Materials and Methods
Study Design
Ethical approval for this prospective observational study (SMC 2018–11-118) was provided by the Institutional Review Board, Samsung Medical Center, Seoul, South Korea. We registered clinical trial (ClinicalTrials.gov identifier NCT 03761433) and performed between November 2018 and May 2019 at Samsung Medical Centre, Seoul, South Korea. Patients aged 20 to 80 years with ASA physical status I–II who received laparotomy for stomach resection due to stomach cancer under sevoflurane-based general anesthesia were enrolled in this study. All patients were informed about the study by corresponding author (MHP), provided written consent, and completed the assessment of psychological factors on the day of surgery. The questionnaire assessed dichotomous questions (below average/above average) about use of medications, preoperative anxiety, expected amount of postoperative pain medication needed, and existing preoperative pain at any site. A single co-author (DKK) performed all anesthesia, but the intraoperative data were recorded by nurses who were not included in this study. A co-author (BJK) who was blinded to the intraoperative parameters collected data on postoperative results. The first author (KHJ) performed the analysis. Exclusion criteria included arrhythmia, creatinine > 2.0 mg/dL, and refusal of participant. Patients taking β-blocker/calcium channel blocker were excluded from the study. During the study, patients were dropped out if vasoactive drugs were administrated 5 mins before recording the SPI value.
Anesthesia and Pain Management
After arrival in the operating room, patients were monitored with electrocardiogram, non-invasive arterial pressure measurements, and pulse oximetry. Anesthetic induction was performed using 2 mg/kg propofol and 1 mg/kg rocuronium with sevoflurane. An endotracheal tube was inserted in all patients. Mechanical ventilation was initiated with a mixture of O2 and air with FiO2 0.5 and adjusted to maintain end-tidal CO2 pressure of 35 and 40 mmHg. Ventilator settings were maintained with tidal volume of 8 mL/kg of ideal body weight at PEEP 5 cm H2O, and volume-controlled ventilation with an inspiratory pause of 30% and inspiration to expiration ratio of 1:2. The bispectral index (BIS; Model A-2000, Aspect Medical Systems, Norwood, MA) was maintained between 40 and 60 during operation preparation (usually 20 mins). The 3% end-tidal concentration of sevoflurane was stably maintained for at least 1 min before surgical incision. Neuromuscular blockade was maintained train-of-four (TOF) count 0. If mean blood pressure or heart rate decreased to a level that necessitated administration of a vasoactive agent within 5 mins before skin incision (mean blood pressure < 50 mmHg, heart rate < 45 bpm), the patient was withdrawn from the study. The midline incision was started with sharp scalpel to make a skin and subcutaneous incision and controlled superficial bleeding with electrocautery. We monitored the parameters 1 min before and up until applying retractors (which usually took 5 min). All data were recorded continuously and were exported to the computer provided with the S/5 Collect software (GE healthcare, Helsinki, Finland). And the highest SPI level and measured the highest heart rate and blood pressure during surgical incision. When the abdominal incision was completed and retractors were applied, then the inspired sevoflurane concentration was titrated to maintain the BIS at 40–60 and systolic blood pressure at 90–140 mmHg. If the SPI level was over 50 and the systolic blood pressure was over 150 mmHg, we administrated fentanyl 50 μg during surgery. Hydromorphone 0.01 mg/kg was administrated upon initiation of peritoneum closure. Intravenous patient-controlled analgesia (IV-PCA, GemStar; Hospira Inc., Lake Forest, IL, USA) was started at this injection time. An infusion of fentanyl (10 or 15 μg/h) was administered with a subsequent 10 μg bolus and 15 min lockout. All patients were not received regional anesthesia for pain management.
After surgery, 200 mg sugammadex was injected to reverse the muscle relaxant. After evaluation of orientation regarding person and place, the pain score was assessed with a numerical rating scale (NRS) at rest in the post-anesthesia care unit (PACU). Patients with complaints of NRS ≥ 4 or with request of analgesics were treated with intravenous hydromorphone 0.01 mg/kg. Pain intensity was reassessed and treated every 10 min.
Postoperative pain was controlled by rescue analgesics administered by hospital personnel in addition to IV-PCA. Patients with complaints of NRS ≥ 4 or with request of analgesics in the ward were treated with intravenous pethidine 50 mg. The complete history of continuous infusion, bolus infusion, and bolus demand for the IV-PCA device was downloaded after surgery.
Statistical Analysis
The primary endpoint was to compare the highest pain score in PACU according to the level of nociception response measured with SPI at equal MAC levels of end-tidal 3% sevoflurane during surgical incision. The sample size calculation was based on a pilot study of 20 patients. We observed a 2:1 ratio in the proportion of Group H: Group L. The mean (SD) postoperative pain score was 7 (2.2) for Group H and 5 (2.0) for Group L. The initial sample size calculation of 42 patients was estimated using a two-sided t-test with an ɑ-error of 5% and a power of 80%.
All data were tested for normal distribution using the Kolmogorov–Smirnov test. We compared the patients who showed SPI levels 50 or not. Values were provided as mean (SD) or median [interquartile], as appropriate. Demographic data, perioperative data, and clinical outcomes were examined with the Chi-square test or Fisher’s exact test for categorical variables and with independent t-test or Mann–Whitney U-test for continuous variables, as appropriate.
The relationship between postoperative opioid consumption and SPI values (pre-incision, post-incision SPI value), and change in SPI value (ΔSPI= post-incision SPI minus pre-incision SPI) was evaluated. Receiver-operating characteristic (ROC) analysis was used to calculate the sensitivity and specificity of the SPI value to distinguish different states of the upper 25% of high opioid consumption. The prediction of the upper 25% of high opioid consumption by SPI values was also compared using a ROC curve. Cut-off values used for calculation of sensitivity and specificity were calculated as ‘best fit’ (highest combined sensitivity and specificity).
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and SPSS (version 24, Chicago, IL, USA). A two-sided alpha of 0.05 was used for all statistical tests.
Results
Between November 2018 and May 2019, 51 patients were assessed for eligibility; among them, 1 patient declined participation and 50 patients enrolled in this study. Two patients were administrated ephedrine because of mean blood pressure < 50 mmHg before surgical incision and were withdrawn from the study. Forty-eight patients evaluated the response to surgical incision, but 2 patients were excluded from the study because of peritoneal seeding or missing data regarding postoperative IV-PCA use. Flow charts of the patient and study protocol are presented in Figure 1. Two patients temporarily were stopped using IV-PCA due to oversedation, but they used IV-PCA with oxygen supply under close monitoring.
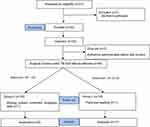 |
Figure 1 Flow diagram of patient selection and study protocol. |
Finally, the data of 46 patients were analyzed. The mean highest SPI value during surgical incision was 56 (SD 12) with a range of 26 to 85. Twenty-nine (63%) patients showed the highest SPI level over 50 during surgical incision (Group H) under end-tidal 3% sevoflurane without opioid use. The highest SPI value of the other 17 (27%) patients remained within 20–50 (Group L). There were no significant differences in demographic and surgical data presented in the two groups (Table 1). Figure 2 shows the trend for SPI values throughout the perioperative period in both groups.
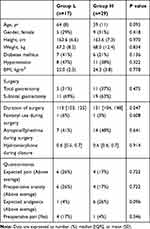 |
Table 1 Demographic and Surgical Characteristics |
The pre-incision and post-incision of surgery SPI values were higher in Group H than in Group L, but the pre-incision and post-incisional blood pressure, heart rate, and BIS were not different between the two groups (Table 2). The change of SPI value (ΔSPI=post-incision SPI minus pre-incision SPI) following surgical incision was significant in Group H (16 (8) vs 28 (9), p < 0.001, Group L vs Group H).
 |
Table 2 Pre-Induction, Pre-Incision and Post-Incisional Surgical Pleth Index, Blood Pressure, Heart Rate, and Bispectral Index |
There was a significant difference in pain score during PACU stay and on postoperative 24 h in Group L and Group H (5 [5, 6] vs 7 [6,8], p=0.007 and 3.5 [3,5] vs 5 [5,6], p=0.006, respectively). Patients in Group H used larger amounts of opioid (hydromorphone) during their PACU stays (0.6 (0.4) mg vs 0.9 (0.4) mg, p=0.024, Group L vs Group H). The details of opioid consumption were Group H used higher fentanyl via PCA and pethidine after pain assessment by hospital personnel than Group L during the first postoperative 24 h (573 (253) µg vs 817 (305) µg, p=0.008 and 97 (65) mg vs 154 (83) mg, p=0.024; Group L vs Group H, respectively).
The change of pre-incision and post-incision SPI value to surgical incision (∆SPI) was useful to predict and distinguish different states of high postoperative IV-PCA fentanyl consumption (≥1000 µg, 25% quantile of high IV-PCA consumption during the first postoperative 24 h) (ROC analysis, Area under curve=0.71) (Figure 3). The ∆SPI of 23, which showed the highest sensitivity (67%) and specificity (68%), was defined post hoc as the cut-off for PCA consumption during postoperative 24 h ≥1000 µg.
 |
Figure 3 Receiver operating characteristics for SPI during surgery to distinguish different states of postoperative fentanyl consumption (≥1000 μg, 25% quantile of high opioid consumption). |
The relation with SPI values and questionnaire about preoperative pain, anxiety, expecting postoperative pain, and analgesic consumption is shown in Table 3. The patients who expressed ‘expected more analgesics themselves than others’ showed higher SPI following incision than who did not. These patients used larger amount of rescue opioid following pain assessment by hospital personnel or request themselves in PACU (hydromorphone) and ward (pethidine) (121 (71) mg vs 193 (93) mg, p=0.023 vs 76 (39) mg vs 110 (29) mg, p=0.034, respectively). However, the amount of IV-PCA consumption was not significantly different between those patients (707 (314) µg vs 833 (272) µg, p=0.327, below average vs above average). The patients who had preoperative pain at any site showed higher SPI values than those who did not, but there was no difference in IV-PCA consumption (pain vs non-pain 620 (258) µg vs 1130 (309) µg (p=0.106)). Other questionnaires were not associated with SPI values following surgical incision and opioid consumption.
 |
Table 3 The Brief Interview for Assessing Preoperative Anxiety and Psychological Status |
Discussion
We tried to evaluate the hypothesis that individual pain sensitivity varies and is related to postoperative pain intensity. This study was assessed individual pain sensitivity according to stress response based on SPI monitoring of surgical incision under end-tidal 3% sevoflurane without opioid infusion. The SPI values of 20–50 are usually considered as a nociception–antinociception balance state during general anesthesia, so we divided the two groups as SPI > 50 or SPI 20–50 following surgical incision. Patients who showed SPI values > 50 showed worse pain intensity and greater opioid consumption during the first postoperative 24 h. The ∆SPI of 23 was the best cut-off predictor for the upper 25% of patients with IV-PCA opioid consumption.
Opioids are widely used because they are effective for relieving severe postoperative pain and familiar because they have been used for a long tradition.2,21 However, opioid-related adverse events including nausea, vomiting, constipation, oversedation and respiratory depression are associated with prolonged hospital stay and life threatening. Thus, it is necessary to identify and predict patients with severe acute postoperative pain.
Experimental testing assessment of pain sensitivity has been performed by different stimulation modalities such as thermal, mechanical, ischemic, or electrical stimulation.8,12 These preoperative measurements of experimental pain by quantitative sensory testing showed that it was related to serve as a predictor of postoperative pain.7,12,22 However, threshold testing was examined with clinical signs including subjective pain scoring, somatic (movement), and autonomic (heart rate increase, blood pressure increase, or sweating) responses which have traditionally been used to judge adequacy of analgesia. In a previous study, surgical incision was considered a similar nociceptive factor with experimental testing.9 Surgical incision results are consisted with mixed mechanical, electrical, and heat stimuli as these stimuli are sequentially performed for several minutes.
Also, surgical incision as a routine perioperative procedure required no additional procedure and time. Persson et al reported postoperative pain intensity associated with peripheral venous cannulation and propofol infusion could easily be evaluated at bedside before surgery without specific equipment.5,6 However, other study in cesarean section showed assessing site of pain threshold was related to results predicting acute post-cesarean section pain and analgesic requirements.8 Considering the nature and site of the stimuli, we thought that surgical incision was more closely related to postoperative pain than experimental testing and other stimuli.
A previous study of intraoperative SPI values with respect to postoperative pain was evaluated at the period of emergence. Ledowski et al proposed a value of SPI > 30 measured during the last 10 min of surgery, before arousal, to predict major pain in the PACU, with a positive predictive value = 89.7% and negative predictive value = 50%.18 But the value of SPI measured just before tracheal extubation was not predictive of major pain in the PACU.19 This is not surprising because the SPI values for the arousal phase may be significantly interfered with by the intubated endotracheal tube.
There was a report that SPI values should be maintained <50 and a fast increase of SPI > 10 should be avoided.23 Gruenewald et al reported that under age-adjusted 0.7 MAC sevoflurane without muscle relaxation, ∆SPI of 10 was found to be the threshold for movement, and, if SPI increased by 10 or more during surgical stimulation, inadequate analgesia may be assumed.15 However, previous studies approached the nociception response during general anesthesia using movement, but the absence of a sympathetic response could not be relied on as a good indicator of a lack of somatic response.20 The ∆SPI in our study was higher than that study (16 (8) vs 28 (9), p < 0.001), Group L vs Group H, but there were no cases of movement. Because we evaluated the change of SPI during skin incisions under maintaining end tidal 3% sevoflurane and full muscle relaxation in TOF count 0/4. The threshold of ∆SPI for nociception-antinociception balance could not be identified. Because there was no ideal way of the assessment of nociception-antinociception level during general anesthesia. The most frequent utilised response to surgical stress is an increase in sympathetic activity or the corresponding decrease in parasympathetic tone. The monitoring of SPI values promised a more accurate reflection of nociception than the traditionally used vital signs, blood pressure and heart rate.24 In our study, blood pressure, heart rate and SPI increased during incision, but only SPI values showed significantly different in two groups.
We did not use opioids during surgical incision because we thought that the opioid response was individual and that was not mandatory.25,26 The sevoflurane requirement for blunting sympathetic responses after surgical incision (MACBAR) with no additional opioids was reported as 2.8% [95% confidence interval: 2.5–3.0%].13 Sandin et al reported at 1.0 MAC of sevoflurane, the BIS values were 26–42 before pain stimulation, but BIS, heart rate, and blood pressure increased significantly during pain stimulation of experimental testing. But, these reactions were suppressed at 1.5 MAC.14 Previous studies have shown a similar effect of opioids on the MAC reduction of different inhaled anesthetics, where the fentanyl doses produce a 50% MAC reduction of sevoflurane.20,27 Thus, we hypothesized the patients who showed SPI over 50 were greater sensitivity in surgical stimuli.
After applying a retractor on the abdominal wall, most patients maintained SPI 20–50 during surgery. Only 2 patients were administrated fentanyl 50 µg during surgery because they showed the SPI > 50 with a systolic blood pressure >150 mmHg. However, we could not convince that other patients were nociception-antinociception balance status without opioid during surgery. Because the bradycardia with/without hypotension was frequently observed, and 40% of patients were given ephedrine or atropine after applying a retractor to the abdominal wall. The vagally mediated reflex bradycardia occurs frequently during gastrectomy, the frequency was higher than in our previous study.28 The absence of opiate analgesia at the time of tracheal intubation and surgical incision could have triggered a major stimulation of the parasympathetic response secondary to acute pain stimulation. We assumed the transient high concentration of sevoflurane and inappropriate pain management caused this effect.
In our study, brief preoperative interviews showed interesting results. We evaluated the psychologic factors effect on SPI values. SPI was high before induction with an average of 53 (18) in awake state. Sympathetic tone in conscious subjects is highly volatile and influenced not only by pain, but a diversity of factors, such as anxiety, noise level or drugs. However, we observed the patients who had preoperative pain in any site showed higher SPI values than who were not. Also, patients who expected using more analgesics than average showed higher SPI values during surgical incision and larger change of SPI. Pain is a multifaceted phenomenon that consists of physiological, emotional, and behavioral components. Preoperative period is an especially stressful situation that evokes both physiologic and emotional reactions.29–31 Thus, we designed this study was performed surgical incision under general anesthesia to exclude these factors. But we found interesting results about the relation of questionnaire about psychologic factors and SPI. In a previous study, SPI values of awake patients with postoperative pain, SPI differentiated pain intensities of ≤5 and >5 on the NRS (0–10), whereas it was unable to differentiate lower pain intensities of ≤3 and >3.32 A further, large-scale study needs to evaluate the association of psychological factors and SPI.
There were several limitations in the present study. First, we managed to standardize the perioperative procedures so that all study patients received the same inhalational agent concentration and anesthetic drugs according to body weight. The MAC-BAR of halogenated anesthetic agents has a wide range of variations. The same concentration applied in every patient did not reflect the same blockade of adrenergic response. Especially, age was the greatest influencing factor of MAC, and MAC also could be changed in cancer patients.33 There was no difference in demographic characteristics in the two groups, and most of the patients were between 50 and 70 years old. Our patients did not receive neoadjuvant chemotherapy, and most patients were diagnosed with cancer during health screenings. Second, hypertension and peripheral vascular status could affect on SPI monitoring. The cardiovascular autonomic control was influenced by medications such as β-receptor blockers and calcium channel blockers.34 We excluded patients who received those medications. Third, we did not use opioids because the individual response to opioids is ambiguous. Insufficient pain control during surgical incision might cause a more stressful status and worse postoperative pain. Finally, mixed opioids were administrated in PACU and ward. We should admit the use of opioid with their accumulated experience because the nurses who were not included in this study assessed the pain intensity and administrated opioids.
Our finding was SPI values were different according to the stimulation of the surgical incision undergoing laparotomy under equi-sevoflurane concentration, and there was large interpatient variation of postoperative pain intensity and opioid consumption. Individual SPI values to surgical incision may provide valuable additional information for individual prediction of postoperative pain and opioid consumption. This method is needed to confirm in varied surgery and anesthetic management.
Conclusions
The surgical pleth index to nociceptive stimuli during surgery is closely related to the degree of postoperative pain and opioid requirements. This information may be used to provide proper analgesia during surgery and individual postoperative pain management.
Abbreviations
BAR, block the adrenergic response; BIS, bispectral index; MAC, minimum alveolar concentration; PACU, post-anesthesia care unit; ROC, receiver-operating characteristic; SPI, surgical pleth index, SD, standard deviation; TOF, train-of-four.
Data Sharing Statement
The data used to support the findings of this study are included in the article.
Ethics Approval and Informed Consent
Ethical approval for this prospective observational study (SMC 2018-11-118) was provided by the Institutional Review Board, Samsung Medical Center, Seoul, South Korea. We registered clinical trial (ClinicalTrials.gov identifier NCT 03761433). The trial was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. doi:10.1097/ALN.0b013e31828866b3
2. Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol. 2016;33(3):160–171. doi:10.1097/eja.0000000000000366
3. Persson AK, Dyrehag LE, Akeson J. Prediction of postoperative pain from electrical pain thresholds after laparoscopic cholecystectomy. Clin J Pain. 2017;33(2):126–131. doi:10.1097/ajp.0000000000000394
4. Baca Q, Marti F, Poblete B, Gaudilliere B, Aghaeepour N, Angst MS. Predicting acute pain after surgery: a multivariate analysis. Ann Surg. 2019. doi:10.1097/sla.0000000000003400
5. Persson AKM, Akeson J. Prediction of acute postoperative pain from assessment of pain associated with venous cannulation. Pain Pract. 2019;19(2):158–167. doi:10.1111/papr.12729
6. Persson AK, Pettersson FD, Dyrehag LE, Akeson J. Prediction of postoperative pain from assessment of pain induced by venous cannulation and propofol infusion. Acta Anaesthesiol Scand. 2016;60(2):166–176. doi:10.1111/aas.12634
7. Granot M. Can we predict persistent postoperative pain by testing preoperative experimental pain? Curr Opin Anaesthesiol. 2009;22(3):425–430. doi:10.1097/ACO.0b013e32832a40e1
8. Pan PH, Coghill R, Houle TT, et al. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology. 2006;104(3):417–425. doi:10.1097/00000542-200603000-00007
9. Abrishami A, Chan J, Chung F, Wong J. Preoperative pain sensitivity and its correlation with postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2011;114(2):445–457. doi:10.1097/ALN.0b013e3181f85ed2
10. Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. 2014;120(5):1237–1245. doi:10.1097/aln.0000000000000108
11. Park M, Kim BJ, Kim GS. Prediction of postoperative pain and analgesic requirements using surgical pleth index: a observational study. J Clin Monit Comput. 2020;34(3):583–587. doi:10.1007/s10877-019-00338-4
12. Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100(1):
13. Albertin A, Casati A, Bergonzi P, Fano G, Torri G. Effects of two target-controlled concentrations (1 and 3 ng/mL) of remifentanil on MAC(BAR) of sevoflurane. Anesthesiology. 2004;100(2):255–259. doi:10.1097/00000542-200402000-00012
14. Sandin M, Thorn SE, Dahlqvist A, Wattwil L, Axelsson K, Wattwil M. Effects of pain stimulation on bispectral index, heart rate and blood pressure at different minimal alveolar concentration values of sevoflurane. Acta Anaesthesiol Scand. 2008;52(3):420–426. doi:10.1111/j.1399-6576.2007.01569.x
15. Gruenewald M, Meybohm P, Ilies C, et al. Influence of different remifentanil concentrations on the performance of the surgical stress index to detect a standardized painful stimulus during sevoflurane anaesthesia. Br J Anaesth. 2009;103(4):586–593. doi:10.1093/bja/aep206
16. Chen X, Thee C, Gruenewald M, et al. Comparison of surgical stress index-guided analgesia with standard clinical practice during routine general anesthesia: a pilot study. Anesthesiology. 2010;112(5):1175–1183. doi:10.1097/ALN.0b013e3181d3d641
17. Struys MM, Vanpeteghem C, Huiku M, Uutela K, Blyaert NB, Mortier EP. Changes in a surgical stress index in response to standardized pain stimuli during propofol-remifentanil infusion. Br J Anaesth. 2007;99(3):359–367. doi:10.1093/bja/aem173
18. Ledowski T, Burke J, Hruby J. Surgical pleth index: prediction of postoperative pain and influence of arousal. Br J Anaesth. 2016;117(3):371–374. doi:10.1093/bja/aew226
19. Ledowski T, Schneider M, Gruenewald M, Goyal RK, Teo SR, Hruby J. Surgical pleth index: prospective validation of the score to predict moderate-to-severe postoperative pain. Br J Anaesth. 2019;123(2):e328–e332. doi:10.1016/j.bja.2018.10.066
20. Katoh T, Kobayashi S, Suzuki A, Iwamoto T, Bito H, Ikeda K. The effect of fentanyl on sevoflurane requirements for somatic and sympathetic responses to surgical incision. Anesthesiology. 1999;90(2):398–405. doi:10.1097/00000542-199902000-00012
21. Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377(9784):2215–2225. doi:10.1016/s0140-6736(11)60245-6
22. Duan G, Guo S, Zhang Y, et al. Effects of epidemiological factors and pressure pain measurements in predicting postoperative pain: a prospective survey of 1,002 Chinese patients. Pain Physician. 2017;20(6):E903–e914.
23. Bapteste L, Szostek AS, Chassard D, Desgranges FP, Bouvet L. Can intraoperative surgical pleth index values be predictive of acute postoperative pain? Anaesth Crit Care Pain Med. 2019;38(4):391–392. doi:10.1016/j.accpm.2018.05.004
24. Ledowski T. Objective monitoring of nociception: a review of current commercial solutions. Br J Anaesth. 2019;123(2):e312–e321. doi:10.1016/j.bja.2019.03.024
25. Sia AT, Lim Y, Lim EC, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109(3):520–526. doi:10.1097/ALN.0b013e318182af21
26. Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105(2):334–337. doi:10.1097/00000542-200608000-00016
27. Katoh T, Ikeda K. The effects of fentanyl on sevoflurane requirements for loss of consciousness and skin incision. Anesthesiology. 1998;88(1):18–24. doi:10.1097/00000542-199801000-00006
28. Kim DK, Ahn HJ, Lee SW, Choi JW. Analysis of factors related to vagally mediated reflex bradycardia during gastrectomy. J Anesth. 2015;29(6):874–880. doi:10.1007/s00540-015-2053-5
29. Sobol-Kwapinska M, Babel P, Plotek W, Stelcer B. Psychological correlates of acute postsurgical pain: a systematic review and meta-analysis. Eur J Pain. 2016;20(10):1573–1586. doi:10.1002/ejp.886
30. De Cosmo G, Congedo E, Lai C, Primieri P, Dottarelli A, Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient-controlled analgesia. Clin J Pain. 2008;24(5):399–405. doi:10.1097/AJP.0b013e3181671a08
31. Zavras N, Tsamoudaki S, Ntomi V, Yiannopoulos I, Christianakis E, Pikoulis E. Predictive factors of postoperative pain and postoperative anxiety in children undergoing elective circumcision: a prospective cohort study. Korean J Pain. 2015;28(4):244–253. doi:10.3344/kjp.2015.28.4.244
32. Ledowski T, Ang B, Schmarbeck T, Rhodes J. Monitoring of sympathetic tone to assess postoperative pain: skin conductance vs surgical stress index. Anaesthesia. 2009;64(7):727–731. doi:10.1111/j.1365-2044.2008.05834.x
33. Du W, Li C, Wang H, et al. Effect of neoadjuvant chemotherapy on sevoflurane MAC-BAR value of patients undergoing radical stomach carcinoma surgery. Int J Clin Exp Med. 2015;8(4):5649–5657.
34. Ledowski T. Objective monitoring of nociception: a review of current commercial solutions. Br J Anaesth. 2019;123(2):e312–e321. doi:10.1016/j.bja.2019.03.024
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

