Back to Journals » International Journal of General Medicine » Volume 15
Prediction of Patient Hepatic Encephalopathy Risk with Freiburg Index of Post-TIPS Survival Score Following Transjugular Intrahepatic Portosystemic Shunts: A Retrospective Study
Authors Cai W, Zheng B, Lin X, Wu W, Chen C
Received 27 January 2022
Accepted for publication 4 April 2022
Published 13 April 2022 Volume 2022:15 Pages 4007—4016
DOI https://doi.org/10.2147/IJGM.S359918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Weimin Cai,1 Beishi Zheng,2 Xinran Lin,1 Wei Wu,1 Chao Chen1
1Department of Gastroenterology and Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 2Department of Internal Medicine, Woodhull Medical Center, Brooklyn, NY, 11206, USA
Correspondence: Chao Chen, Department of Gastroenterology and Hepatology, the First Affiliated Hospital of Wenzhou Medical University, No. 2, Fuxue Lane, Wenzhou, 325000, People’s Republic of China, Tel +86 18857838243, Fax +86 576 87755312, Email [email protected]
Background: Hepatic encephalopathy is a complication of portal hypertension. The Freiburg index of transjugular portosystemic shunt (TIPS) and patient outcomes have recently been described. This retrospective study was conducted at a single center in China and included 241 patients with portal hypertension who underwent TIPS implantation to evaluate the Freiburg index of post-TIPS survival score (FIPS) to predict hepatic encephalopathy.
Methods: A single-center retrospective study including 241 patients who underwent TIPS operation between April 2015 and July 2019 was conducted. Clinical demographics and relevant clinical parameters within 24h after admission were collected. The prediction performances of FIPS, Child–Pugh and the model for end-stage liver disease (MELD) scores were compared by decision curve analysis and receiver operating characteristic (ROC) curve analysis. In addition, multivariate analyses were performed to identify independent predictors.
Results: Eighty-three out of 241 patients (34.4%) finally developed post-TIPS hepatic encephalopathy. The area under the ROC curve of FIPS was 0.744 (95% confidence interval: 0.684– 0.798). FIPS was identified as an independent risk factor for post-TIPS hepatic encephalopathy (hazard ratio: 2.23, 95% confidence interval: 1.71– 2.90, p< 0.001). Moreover, we further grouped the FIPS scores into two categories (FIPS ≤-0.97, low-risk; FIPS >-0.97, high risk) to improve its applicability. Patients with high FIPS scores had a significantly higher incidence of hepatic encephalopathy than patients with low FIPS scores (P< 0.05).
Conclusion: This study showed that FIPS could be used to evaluate the risk of hepatic encephalopathy in this patient group with improved predictive performance when compared with the Child–Pugh and MELD scores.
Keywords: transjugular intrahepatic portosystemic shunts, hepatic encephalopathy, Freiburg index of post-TIPS survival, model for end-stage liver disease
Introduction
Over the last few decades, the transjugular intrahepatic portosystemic shunt (TIPS) procedure has been widely used to treat severe portal hypertension complications, such as esophageal/gastric variceal bleeding and refractory ascites.1,2 The development of hepatic encephalopathy after procedure is one of the most prevalent postoperative complications, which considerably affects the quality of life and the prognosis of patients.3 Several clinical studies reported that the occurrence of post-TIPS hepatic encephalopathy was approximately 29–45%, which was much higher than the expected rate.4,5 However, there is still no effective method to prevent the severe complication after TIPS.6 Therefore, screening patients with a high risk of post-TIPS hepatic encephalopathy and placing such patients under close monitoring followed by early treatment is an integral part of post-TIPS hepatic encephalopathy treatment.
Previous studies have revealed that prior history of hepatic encephalopathy, advanced age, Child–Pugh and MELD scores were risk factors for post-TIPS hepatic encephalopathy.7,8 However, the conventional methods used to predict post-TIPS hepatic encephalopathy have limitations. The accuracy of Child–Pugh score is still under debate because it is calculated based on subjective variables, such as ascites status and degree of hepatic encephalopathy, which influence its accuracy.9 The MELD score is widely used to predict the mortality of patients with end-stage liver disease. This scoring scheme incorporates three objective laboratory parameters including creatinine, total bilirubin (TBil), and international normalized ratio (INR).10 Although the MELD score prevents the occurrence of subjective bias in the determination of the ascites status and degree of HE,11 it exhibits nearly 18% within-patient variation over time.12 Therefore, the conventional prognostic scores that have been previously discussed are not ideal methods for predicting post-TIPS HE.13,14
Recently, Bettinger et al developed a novel objective score model referred to as the Freiburg index of post-TIPS survival (FIPS) to predict survival rates in those patients who underwent TIPS.15 Furthermore, Stockhoff et al16 validated the accuracy of the FIPS score in patients with advanced cirrhosis without TIPS creation. FIPS has recently been used as a new tool to predict post-TIPS hepatic encephalopathy at our center and it has exhibited a considerably high accuracy without subjective effects. Therefore, this retrospective study was conducted at a single center in China and included 241 patients with portal hypertension who underwent TIPS implantation to evaluate the FIPS to predict hepatic encephalopathy.
Materials and Methods
Patients Selection
Wenzhou Medical University's First Affiliated Hospital’s ethics committee has approved this study, and the retrospective study was performed according to the Declaration of Helsinki. Informed consent was waived because of the retrospective design of this study. The patient selection process based on the inclusion and exclusion criteria used in the present study is illustrated in Figure 1. From April 2015 to July 2019, a total of 268 consecutive patients who underwent TIPS at the Wenzhou Medical University First Affiliated Hospital were retrospectively enrolled in the present study. Patients were selected based on the following inclusion criteria: 1) over the age of 18 years and no more than 80 years; 2) patients diagnosed with portal hypertension; and 3) underwent TIPS implantation for the first time. The exclusion criteria were set: 1) with hepatocellular carcinoma or other malignant tumors; 2) individuals with low serum albumin levels caused by malnutrition, etc; 3) increased bilirubin production caused by biliary tract diseases, hemolytic diseases, etc; 4) kidney or liver dysfunction caused by diseases other than cirrhosis; 5) accepted liver transplant within 1 year after TIPS.
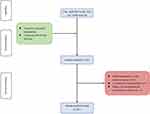 |
Figure 1 Flowchart shows patients inclusion and exclusion. |
Data Collection
Clinical data of patients including medical history, physical examination and laboratory results, such as complete blood count, prothrombin time, liver function tests, and basic metabolic panel within 24h after admission were reviewed. The traditional liver function reserve scores and FIPS were calculated based on the collected data. Ascites and HE were assessed and graded in line with the West-Haven hepatic encephalopathy criteria.17 Participants were consistently followed up at 4 weeks, 12 weeks, 24 weeks, and 48 weeks after placement of TIPS by telephone calls, outpatient clinic or hospital visits. Follow-up of patients ended at 12 months after TIPS; the last follow-up date was counted as the end if patient died within 1 year. The scoring formulas used to calculate patient scores were as follows:
MELD score = 11.2*ln (INR) + 3.78*ln (TBil mg/dL) + 9.57*ln (creatinine mg/dL) + 6.418
FIPS score = 1.43*log10 (TBil mg/dL) – 1.71/ (creatinine mg/dL) + 0.02* (age years) − 0.02* (albumin g/L).15
Statistical Analyses
Statistical analyses were performed using IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA), R version 4.0.1 (The R Foundation for Statistical Computing, Vienna, Austria), and MedCalc (Mariakerke, Ostend, Belgium). Statistical results were regarded as significantly different when P-value was less than 0.05. Continuous variables are presented as mean ± standard deviation (SD) if they were normally distributed. Otherwise, the variables were expressed as median with interquartile ranges (IQR). Qualitative variables were presented as numbers (percentages) and assessed using the χ2-test or Fisher’s exact test. Continuous variables were compared using t-test or the Mann–Whitney U-test. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify the significant and independent predictors of post-TIPS hepatic encephalopathy. The area under receiver operating curve (AUROC), which was used to evaluate the predictive performance of scoring models, was used to compare the accuracy of FIPS and other score models. The best cutoff point of the models was identified. A higher value of AUROC represented a more accurate prognostic stratification. The calibration curve was used to compare the predicted probability of incidence and the actual incidence. Decision curve analysis (DCA) was used to evaluate the clinical significance of the scoring models for improved decision-making.19–21
Results
A total of 241 patients were included in the present study based on the inclusion and exclusion criteria. All patients in the present study received an expanded polytetrafluoroethylene (ePTFE)-covered stent with a diameter of 6–8 mm. A summary of demographic and clinical characteristics of patients is presented in Table 1. Briefly, the dataset included 176 (73.0%) males and 65 (23.0%) females. The age of the patients ranged from 24 to 77 years, and the mean age of diagnosis was 52.95 years with a standard deviation of 10.36. The proportion of patients who developed post-TIPS hepatic encephalopathy during the follow-up period was 83 (34.4%). With regard to etiology, 129 patients had viral cirrhosis, 53 patients had a clinical history of alcoholic cirrhosis, 32 cases were diagnosed with both hepatitis and alcohol-related cirrhosis, and the causes of remaining 27 patients consisted of autoimmune hepatitis cirrhosis, primary biliary cirrhosis, and Budd–Chiari syndrome. In terms of TIPS indication, 218 patients were variceal bleeding, 9 patients were refractory ascites, while the indication of the remaining 14 patients included pleural fluid (n = 4) and portal vein thrombosis (n = 10). Patients with Child–Pugh grade B patients accounted for the majority (n = 126, 52.3%). The median Child–Pugh and MELD scores after hospital admission were 7 and 10.87, respectively, while the mean FIPS score was −1.14. The median follow-up interval was 12 months (2 to 365 days). A total of 83 (34.4%) patients finally developed post-TIPS HE during the follow-up period. The number of patients diagnosed with grade 1, 2, 3, and 4 HE were 30, 46, 12, and 3, respectively. After the creation of TIPS, 19 patients (7.9%) died of various causes within 1 year. Seven of the patients had post-TIPS hepatic encephalopathy. Detailed information is presented in Table 2.
 |
Table 1 Characteristics of the Study Population, Stratified by Hepatic Encephalopathy Event |
 |
Table 2 Laboratory Parameters and Clinical Scores of the Study Population, Stratified by Hepatic Encephalopathy Event |
Univariate and Multivariate Analyses of Variables Associated with Post-TIPS Hepatic Encephalopathy
To find the independent prognostic risk factors in patients with TIPS, we carried out univariate and multivariate Cox regression analyses. A total of nine significantly different parameters were selected through univariate analysis (P<0.05). The parameters included age, serum albumin, total bilirubin, red blood cell count, platelet count, diabetes mellitus, Child–Pugh, MELD and FIPS scores. Subsequently, FIPS was identified as an independent predictor of post-TIPS hepatic encephalopathy within 1 year, which was statistically significant (P<0.05). The results of multivariate Cox regression analyses revealed that FIPS (P<0.01) was significantly independent predictor for the incidence of post-TIPS hepatic encephalopathy (Table 3).
 |
Table 3 Univariate and Multivariate Cox Proportional Hazards Model of the Association Between Clinical Parameters and Hepatic Encephalopathy Occurrence Rate (in a Year) |
Performances of Child–Pugh, Model for End-Stage Liver Disease, and FIPS Scores in the Prediction of Post-Transjugular Intrahepatic Portosystemic Shunt Hepatic Encephalopathy
The ROC curves were used to evaluate the ability of FIPS, MELD, and Child–Pugh scores to predict hepatic encephalopathy occurrence after 1-year follow-up. The AUROCs of the scoring models before TIPS, including MELD, Child–Pugh, and FIPS were 0.655, 0.643, 0.744, respectively (Figure 2). The optimal cutoff values of MELD, Child–Pugh, and FIPS were 12.22, 8, −0.97, respectively. The sensitivity, specificity, positive and negative likelihood ratios, and positive and negative predictive values were 0.699, 0.710, 2.40, 0.43, 0.56, and 0.82, respectively, when the best cutoff point for FIPS (FIPS = −0.97) was used to predict the occurrence of post-TIPS hepatic encephalopathy within 1-year of follow-up. The details of the scoring models were presented in Table 4. Besides, as Figure 3 shows, the black straight solid line in the graph represented no-TIPS creation, and the gray line measured the net benefit of recommending TIPS to each of patients regardless of hepatic encephalopathy risk. Thus, DCA results indicated that FIPS had a relatively high net benefit across a wider and reasonable range of threshold probabilities in the prediction of post-TIPS within 1 year of follow-up. The figure illustrated that FIPS was more appropriate selecting patients.
 |
Table 4 Diagnostic Accuracy of Different Scoring Systems in Predicting Post-Transjugular Intrahepatic Portosystemic Shunt Hepatic Encephalopathy Within 1-Year at the Optimal Cutoff Point |
Relationship Between FIPS Grade and Post-TIPS Hepatic Encephalopathy
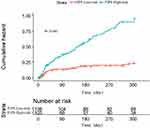
The optimal cutoff point as determined by the ROC curve was used to increase the practicality of the FIPS by dividing the scores into two categories: low-risk and high-risk (FIPS ≤−0.97, low-risk; FIPS >−0.97, high risk), and the cumulative risk curve was plotted in Figure 4. Moreover, as the Figure 5 displays, the calibration curve of FIPS HE’s incidence was also plotted. The calibration curve indicated an agreement between FIPS score prediction and the actual observed post-TIPS hepatic encephalopathy.
Discussion
The present retrospective study evaluated the performance of FIPS as a novel and objective predictive indicator for post-TIPS hepatic encephalopathy in patients who underwent TIPS within 1 year for the first time. As one of the major complications of TIPS, hepatic encephalopathy impaired the long-term quality of life of the patients, leads to readmission, and a considerable economic burden on patients.22 The traditional methods, such as Child–Pugh and MELD scores, predict the incidence of post-TIPS hepatic encephalopathy by evaluating liver function, although the methods are associated with certain limitations. Therefore, FIPS was imported as a novel index and has been demonstrated to be effective in predicting mortality after TIPS procedure.15 In total, 241 patients were enrolled in our study for data analysis to investigate prediction capacity of FIPS. From our study, FIPS exhibited a better performance in the prediction of post-TIPS hepatic encephalopathy than traditional methods.
FIPS was first reported by Bettinger et al15 and has since been used to predict the survival prognosis of TIPS. FIPS comprises four objective clinical parameters, including total serum bilirubin, serum creatinine, age, and albumin. Several researchers have found that albumin5,23,24 and bilirubin23,25 are associated with hepatic encephalopathy. Yin et al26 suggested that serum creatinine was a significant risk factor for cognitive impairment in patients with cirrhosis. However, the results of the present study were not consistent with the findings of the previous study, which could be attributed to the following reasons: 1) a few patients with refractory ascites and hepatorenal syndrome were included and 2) patients with chronic kidney dysfunction were excluded in the present study. Age has been identified as a strong predictor of poor prognosis in patients who have undergone TIPS.15,25,27 Aging patients are more vulnerable to toxic metabolites, such as ammonia. Previous studies demonstrated that the occurrence of post-TIPS hepatic encephalopathy was theoretically related to the severity of liver dysfunction before TIPS, age, renal function among others.26–29 However, the risk factors of post-TIPS hepatic encephalopathy in recent studies have largely focused on traditional liver functional reserve models such as MELD25,30 and Child–Pugh scores.23,31 In addition, traditional scores were assessed at a time when TIPS technique was less developed when compared to the technology used today. Current studies indicated that both of them did not perform well in predicting post-TIPS hepatic encephalopathy.13,14 In patients with renal dysfunction, the MELD scale may not provide an accurate prognosis.32 Additionally, patients who undergo TIPS procedure often have low MELD scores, which is consistent with the findings of the present study. Therefore, the discrimination accuracy of the MELD score for predicting post-TIPS hepatic encephalopathy is limited. FIPS score is not influenced by subjective variables such as ascites status and degree of hepatic encephalopathy when compared to the Child–Pugh score. The objective parameters of the Child–Pugh score and their cutoff values were empirically selected and have not been fully validated.33 Therefore, Bettinger et al15 proposed FIPS, which was significantly related to survival of patients who underwent TIPS implantation. The FIPS is not only suitable in end-stage cirrhosis but also not affected by subjective factors, thereby presenting a broad application prospect. In the present study, we observed that 34.4% participants finally developed hepatic encephalopathy within 1 year after TIPS procedures, which was in line with previous observations.34 When comparing, we found that the AUROC of the FIPS was 0.744 (95% CI 0.684–0.798), which was superior to those of the above two conventional models (P<0.05) with regard to prognostic accuracy and it serves as an alternative risk prediction tool for clinical physicians. Afterward, we investigated the net benefit of the three models, indicating that FIPS had the best net benefit in a reasonable range. In this study, after adopting a cut-off value (FIPS = −0.97) by ROC curves, we could identify two distinct risk groups of patients with different hepatic encephalopathy incidence through Kaplan–Meier analysis. FIPS, as a novel prediction model initially based on risk factors for post-TIPS survival, FIPS could be used to identify patients at high risk of developing post-TIPS hepatic encephalopathy.
However, some limitations remain to be focused on in this study. First, the current study was a single institution study, and the follow-up sample size was not enough. As expected, there were demographic differences among patients enrolled in the present study and FIPS’ initial population, such as human race, etiology of cirrhosis among others. Second, patient compliance with post-TIPS therapy varied, which may influence the predictive value of FIPS. Third, improvement of the technical aspect of TIPS also played a pivotal role in post-TIPS prognosis. To minimize bias, we collected data of consecutive patients from 2015 to 2019. Finally, most of patients in our study were indicated for variceal bleeding; therefore, the applicability of FIPS to patients with refractory ascites requires further investigation.
Conclusion
This study showed that the FIPS could be used to evaluate the risk of hepatic encephalopathy in this patient group with improved predictive performance when compared with the Child–Pugh and MELD scores. FIPS could be a novel, objective and practical auxiliary tool for selecting patients, stent-grafts diameters before TIPS procedure. Much work remains to do to refine and validate the scoring model.
Ethics Approval
This retrospective study was approved by the Wenzhou Medical University's First Affiliated Hospital Ethics Committee. All procedures were performed according to the Declaration of Helsinki. The relevant data are anonymous, therefore informed consent was waived.
Funding
The study was supported from the Wenzhou Science & Technology Bureau of China (Y20190613). The funding body did not take part in the study designing, data collecting, analyzing and the manuscript writing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. de Franchis R. Developing consensus in portal hypertension. J Hepatol. 1996;25:390–394. doi:10.1016/S0168-8278(96)80127-9
2. Boyer TD, Haskal ZJ. The role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. 2010;51:306. doi:10.1002/hep.23383
3. Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034–1041.e1031. doi:10.1016/j.cgh.2012.05.016
4. Hernández-Gea V, Procopet B, Giráldez Á, et al. Preemptive-TIPS improves outcome in high-risk variceal bleeding: an Observational Study. Hepatology. 2019;69:282–293. doi:10.1002/hep.30182
5. Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738–2746. doi:10.1111/j.1572-0241.2008.02102.x
6. Riggio O, Masini A, Efrati C, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled study. J Hepatol. 2005;42:674–679. doi:10.1016/j.jhep.2004.12.028
7. Flud CR, Duarte-Rojo A. Prognostic implications of minimal/covert hepatic encephalopathy: large-scale Validation Cohort Studies. J Clin Exp Hepatol. 2019;9:112–116. doi:10.1016/j.jceh.2018.04.009
8. Bai M, Qi X, Yang Z, et al. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systematic review. J Gastroenterol Hepatol. 2011;26:943–951. doi:10.1111/j.1440-1746.2011.06663.x
9. Kaplan DE, Dai F, Aytaman A, et al. Development and performance of an algorithm to estimate the Child-Turcotte-Pugh score from a national electronic healthcare database. Clin Gastroenterol Hepatol. 2015;13:
10. Hoekstra LT, de Graaf W, Nibourg GA, et al. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27–36. doi:10.1097/SLA.0b013e31825d5d47
11. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi:10.1053/jhep.2001.22172
12. Gaba RC, Shah KD, Couture PM, et al. Within-patient temporal variance in MELD score and impact on survival prediction after TIPS creation. Ann Hepatol. 2013;12:797–802. doi:10.1016/S1665-2681(19)31322-5
13. Wang Z, Wu YF, Yue ZD, et al. Comparative study of indocyanine green-R15, Child-Pugh score, and model for end-stage liver disease score for prediction of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. World J Gastroenterol. 2021;27:416–427. doi:10.3748/wjg.v27.i5.416
14. Lin X, Gao F, Wu X, Cai W, Chen X, Huang Z. Efficacy of albumin-bilirubin score to predict hepatic encephalopathy in patients underwent transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2020;33(6):862–871.
15. Bettinger D, Sturm L, Pfaff L, et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J Hepatol. 2021;74(6):1362–1372.
16. Stockhoff L, Schneider H, Tergast TL, Cornberg M, Maasoumy B. Freiburg index of post-TIPS survival (FIPS) a valid prognostic score in patients with cirrhosis but also an advisor against TIPS? J Hepatol. 2021;75(2):487–489. doi:10.1016/j.jhep.2021.02.031
17. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi:10.1002/hep.27210
18. Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42(Suppl):S100–S107. doi:10.1016/j.jhep.2004.11.015
19. Van Calster B, Wynants L, Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74:796–804. doi:10.1016/j.eururo.2018.08.038
20. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. doi:10.1136/bmj.i6
21. Holmberg L, Vickers A. Evaluation of prediction models for decision-making: beyond calibration and discrimination. PLoS Med. 2013;10:e1001491. doi:10.1371/journal.pmed.1001491
22. Tapper EB. Predicting overt hepatic encephalopathy for the population with cirrhosis. Hepatology. 2019;70:403–409. doi:10.1002/hep.30533
23. Bale A, Pai CG, Shetty S, Balaraju G, Shetty A. Prevalence of and factors associated with minimal hepatic encephalopathy in patients with cirrhosis of liver. J Clin Exp Hepatol. 2018;8:156–161. doi:10.1016/j.jceh.2017.06.005
24. Rowley MW, Choi M, Chen S, Hirsch K, Seetharam AB. Refractory hepatic encephalopathy after elective transjugular intrahepatic portosystemic shunt: risk factors and outcomes with revision. Cardiovasc Intervent Radiol. 2018;41:1765–1772. doi:10.1007/s00270-018-1992-2
25. Fonio P, Discalzi A, Calandri M, et al. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol Med. 2017;122:713–721. doi:10.1007/s11547-017-0770-6
26. Yin X, Zhang F, Guo H, et al. A nomogram to predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients. Sci Rep. 2020;10:9381. doi:10.1038/s41598-020-65227-2
27. Nardelli S, Gioia S, Pasquale C, et al. Cognitive impairment predicts the occurrence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2016;111:523–528. doi:10.1038/ajg.2016.29
28. Routhu M, Safka V, Routhu SK, et al. Observational cohort study of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS). Ann Hepatol. 2017;16:140–148. doi:10.5604/16652681.1226932
29. Kalaitzakis E, Björnsson E. Renal function and cognitive impairment in patients with liver cirrhosis. Scand J Gastroenterol. 2007;42:1238–1244. doi:10.1080/00365520701373983
30. Casadaban LC, Parvinian A, Minocha J, et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60:1059–1066. doi:10.1007/s10620-014-3391-0
31. Luo X, Zhao M, Wang X, et al. Long-term patency and clinical outcome of the transjugular intrahepatic portosystemic shunt using the expanded polytetrafluoroethylene stent-graft. PLoS One. 2019;14:e0212658. doi:10.1371/journal.pone.0212658
32. Alessandria C, Gaia S, Marzano A, Venon WD, Fadda M, Rizzetto M. Application of the model for end-stage liver disease score for transjugular intrahepatic portosystemic shunt in cirrhotic patients with refractory ascites and renal impairment. Eur J Gastroenterol Hepatol. 2004;16:607–612. doi:10.1097/00042737-200406000-00015
33. Wong M, Busuttil RW. Surgery in patients with portal hypertension. Clin Liver Dis. 2019;23:755–780. doi:10.1016/j.cld.2019.07.003
34. Mandiga P, Foris LA, Bollu PC. Hepatic encephalopathy. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.