Back to Journals » Cancer Management and Research » Volume 12
Prediction of Pathologic Complete Response by Ultrasonography and Magnetic Resonance Imaging After Neoadjuvant Chemotherapy in Patients with Breast Cancer
Authors Zhang K, Li J, Zhu Q, Chang C
Received 25 January 2020
Accepted for publication 25 March 2020
Published 16 April 2020 Volume 2020:12 Pages 2603—2612
DOI https://doi.org/10.2147/CMAR.S247279
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Kai Zhang,* Jiawei Li,* Qian Zhu, Cai Chang
Department of Medical Ultrasound, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cai Chang
Fudan University Shanghai Cancer Center, No. 270, Dong’an Road, Shanghai, Xuhui District 200032, People’s Republic of China
Tel +86 180 1731 2605
Fax +86 21 6417 4774
Email [email protected]
Purpose: To compare the diagnostic performance for pathologic complete response (pCR) in breast cancer after neoadjuvant chemotherapy (NAC) between ultrasound (US) and magnetic resonance imaging (MRI).
Patients and Methods: A total of 1,219 breast cancer patients with 1,232 tumors who accepted US and/or MRI examination after NAC and before breast surgery were included. The diagnostic performance of US, MRI, and US plus MRI in predicting pCR was compared.
Results: The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of US for pCR were 36.2%, 90.2%, 71.0%, 67.3%, and 71.9%, respectively, while for MRI they were 44.4%, 92.9%, 75.6%, 77.7%, and 75.0%, respectively. The combination of US and MRI had increased specificity (98.0%) and PPV (86.8%), decreased sensitivity (22.5%) and NPV (68.8%), but similar accuracy (70.5%) in comparison with US or MRI alone. The prediction of pCR by imaging differed in different histological, molecular subtypes and primary tumor size.
Conclusion: Neither US nor MRI could predict a pCR with sufficient accuracy. The combination of US and MRI could not predict a pCR reliably either. The explanation of imaging for pCR should take into account histological, molecular subtypes, and primary tumor size.
Keywords: breast neoplasms, neoadjuvant therapy, pathologic complete response, ultrasonography, magnetic resonance imaging
Introduction
Neoadjuvant chemotherapy (NAC) is initially recommended as the standard care for locally advanced inoperable breast cancer. In recent decades, lots of studies have indicated that preoperative chemotherapy in patients with operable breast cancer was equally effective as postoperative chemotherapy in terms of disease free and overall survival.1–4 Therefore, nowadays NAC is widely used in patients with breast cancer.5,6 The traditional roles of NAC include downstaging,2 eradicating possible micrometastatic lesions,7 and detecting in vivo responsiveness to chosen chemotherapeutic agents.8 In addition, several studies have demonstrated the association between pathologic complete response (pCR) after NAC and improved survival in certain patients. A pooled analysis by Cortazar et al9 proved that patients who attained pCR (eradication of tumor from both breast and lymph nodes) had improved survival, especially in aggressive tumor subtypes (triple-negative and HER2-positive, hormone-receptor-negative). The meta-analysis presented by Broglio et al10 indicated that pCR in HER2-positive breast cancer was associated with substantially longer times to recurrence and death. The result of a recent study by LeVasseur et al11 also supports the above findings.
As complete resection of residual tumor is a recommended care after NAC,12 it is significant to preoperatively delineate the residual disease with high accuracy in order to prevent incomplete resection. The most often used methods to determine tumor extent after NAC in patients with breast cancer include mammography, ultrasound (US), and magnetic resonance imaging (MRI), among which MRI was commonly recognized as the most effective way.13 However, the accuracy of MRI in predicting pCR in breast cancer after NAC varied in different researches.14–17 Furthermore, the accuracy between MRI and US is uncertain,18 though the latter is often an alternative tool to evaluate tumor response. Therefore, the purpose of this retrospective study was to compare the diagnostic performance of US, MRI, and the combination of US and MRI in predicting pCR in primary breast cancer after NAC. Meanwhile, factors that affect their predicting power were also analyzed.
Patients and Methods
Patients Enrollment
Consecutive patients were selected from the digital medical data system of our hospital by limiting the search terms to “breast cancer” AND “chemotherapy” AND “surgery” during November 2013 and March 2018. Then patients were screened on the basis of the following criteria. The inclusion criteria included: 1) initially pathological diagnosis of breast cancer, 2) receiving NAC and subsequent mastectomy or breast-conserving surgery, and 3) evaluating tumor response after NAC and before surgery with US or MRI. Exclusion criteria included: 1) open surgical biopsy for breast cancer diagnosis, 2) breast cancer with skin involvement and metastasis, 3) accompanied with other malignant tumors, and 4) more than 30 days between US or MRI assessment immediately after NAC and surgery.
Pathological Examination and Assessment
The diagnosis of breast cancer for all patients was based on percutaneous core needle biopsy. The expression status of estrogen receptor (ER), progesterone receptor (PR), and HER2 were determined with immunohistochemical (IHC) staining. Hormone receptor (HR) positivity was defined as >1% of cells staining for ER and/or PR.19 Otherwise tumors were defined as HR negative. HER2 was scored according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guideline recommendations for HER2 testing in breast cancer.20 Tumors with HER2 scores of 3+ were considered positive, scores of 1+ or 0 considered negative. In tumors with 2+ scores, fluorescence in situ hybridization was used to determine HER2 amplification. Based on the IHC results tumors were classified into four molecular subtypes: HR+/HER2+, HR-/HER2+, HR+/HER2-, and HR-/HER2- (triple negative). The AJCC manual 8 was the reference for clinical stages of tumors. For patients who suffered from multifocal breast cancer, only the largest lesion was analyzed. Tumors of bilateral breast cancer were considered separately. Two definitions of pCR were applied and analyzed in this study: complete disappearance of invasive carcinoma in primary tumor area at microscopic study, regardless of the presence of carcinoma in situ (ypT0/is), and complete disappearance of any carcinoma in primary tumor area (ypT0). Nodal status was not considered for this study.
Chemotherapy Regimens
NAC regimens were made according to the local standard based on the latest National Comprehensive Cancer Network (NCCN) guidelines. The regimens included CEF (cyclophosphamide 600 mg/m2 on day 1, epirubicin 60 mg/m2 on day 1, and 5-fluorouracil 600 mg/m2 on day 1 every 3 weeks), PC (paclitaxel 80 mg/m2 and carboplatin AUC 2 mg min/mL on days 1, 8, and 15 of a 28-day cycle), PE (paclitaxel 80 mg/m2 on days 1, 8, and 15, epirubicin 60 mg/m2 on day 1 every 3 weeks) and TEC (Taxotere 75 mg/m2, epirubicin 60 mg/m2, cyclophosphamide 600 mg/m2 on day 1 of a 21-day cycle) for a median of six cycles (range=1–8 cycles). For HER2 positive tumors, Trastuzumab was added as a 4 mg/kg loading dose followed by 2 mg/kg weekly combined with chemotherapy. US and/or MRI were used to assess the tumor extent before, during every other cycle of, and after NAC. After the last imaging examination, patients had mastectomy or breast-conserving surgery based on the physical examination, imaging findings, and discussion between the surgeon and patient.
US Examination and Assessment
US examinations were conducted by experienced doctors specialized in ultrasonic diagnosis. All examinations were performed with Logic E9 (GE Healthcare, Kretz, Zipf, Austria), IU22 (Philips Medical Systems, Bothell, WA), Aixplorer (Supersonic Imaging, Aix-en-Provence, France), Aplio 500 (Toshiba medical system, Japan), and Mylab90 (Esaote, Genoa, Italy) equipped with a 5–14-MHz linear-array transducer. Patients with no signs of residual disease and parenchymal distortion in US after completing NAC in the primary tumor area were considered as US complete response (uCR). Otherwise the case was considered as non-uCR.
MRI Examination and Assessment
Dynamic contrast-enhanced MRI was performed with a 1.5-T Dedicated spiral breast MRI System (Aurora Imaging Technology, Aurora Systems, Inc., Canada) with a breast coil. The following sequences were acquired while patients were in a prone position: a precontrast axial T2-weighted fat-suppressed sequence (TR 6,680 ms, TE 29 ms, thickness 3 mm) and axial T1-weighted fat-suppressed sequences (TR 4.8 ms, TE 29 ms, thickness 1.1 mm, FOV 360 mm, matrix 360×360×128) before and after a bolus of a gadolinium-based contrast agent (gadopentetate dimeglumine, 0.1 mmol/kg) was injected at a rate of 2 mL/s. Postcontrast images were obtained at 90, 180, 270, and 360 seconds after the injection.
Images were reviewed by two dedicated radiologists, who were blinded to the results of US and surgical data. MRI complete response (mCR) was defined as no enhanced tumor visible on any serial images of dynamic contrast-enhanced T1-weighted images. If MRI showed any amount of enhancement in primary tumor area, the case was diagnosed as non-mCR in this study.
Statistical Analysis
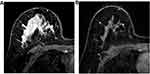
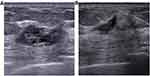
Quantitative data was presented as median with the first and third quartile (Q1, Q3) and compared with Kruskal–Wallis test. Qualitative data was shown as frequencies with percentages. True positive (TP) was defined as a complete response on both US or MRI and pathology, false positive (FP) as a complete response on US or MRI (Figure 1), but presentation of a residual tumor on pathology, true negative (TN) as presentation of a residual tumor on both US or MRI and pathology, and false negative (FN) as presentation of a residual tumor on US or MRI, but complete response on pathology (Figure 2). For the combination of US and MRI, cases were considered as clinical complete response (cCR) only when both of them presented complete response. Other forms of combinations were considered non-cCR. The following formulas were used to calculate the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of US and MRI in predicting pCR in breast after NAC. Sensitivity was equal to the percentage result of TP/(TP+FN), specificity the result of TN/(TN+FP), accuracy the result of (TP+TN)/total, PPV the result of TP/(TP+FP), and NPV the result of TN/(TN+FN). The 95% confidence intervals were estimated according to the normal approximation method for sensitivity, specificity, accuracy, PPV, and NPV. The difference of proportions between groups were compared with z test or χ2 test, as appropriate. A two-sided P-value was calculated for each comparison and P<0.05 was considered statistically significant. The significance threshold for a difference was adjusted by Bonferroni method when multiple comparisons were conducted. All the statistical analyses were performed by SPSS 19 (IBM, Armonk, NY) and EXCEL within Microsoft Office 2016.
Results
Patient Characteristics
A total of 1219 eligible patients with 1232 tumors (13 patients suffered from bilateral breast cancer) were screened out from the 2367consecutive patients which were retrieved from the data system (Figure 3). Among them, 1150 patients with 1162 tumors (12 bilateral breast cancers) had US assessment (US group) after NAC and before surgery, 1021 patients with 1031tumors (10 bilateral breast cancers) had MRI assessment (MRI group), while 953 patients with 962 tumors (9 bilateral breast cancers) had both US and MRI assessment (US+MRI group). The clinicopathologic characteristics of patients were comparable among these three groups, as well as the pCR rates regardless of its definitions (Table 1). The median ages for all the three groups were about 50 years. All patients were female, except one male patient who was allocated to unknown menstrual status in each group. The median maximum sizes of tumors before treatment were 32 mm, 33 mm and 33 mm in the US, MRI and US+MRI group, respectively. In this order, patients who had incorporated trastuzumab into their NAC regimens accounted for 94.1% (443/471), 94.5% (396/419) and 94.7% (377/398) of total patients with HER2 positive in each group. The median intervals between the last imaging examination and surgery were seven (4, 11) and eight (6, 13) days for US and MRI group, respectively. In US+MRI group, the median intervals were 7.5 (5, 12) days for US and eight (6, 13) days for MRI, and median interval between the last US and MRI examination was one day. The numbers of tumors that had a pCR (ypT0) were 351 (30.2%), 309 (30.0%) and 296 (30.8%) in US, MRI and US+MRI group respectively. Residual carcinoma in situ (ypTis) was found in 63 (5.4% of 1162) tumors in the US group, 60 (5.8% of 1031) tumors in MRI group, and 55 (5.7% of 962) tumors in US+MRI group. Thus, in terms of the alternative definition of pCR (ypT0/is), the pCR rates of these groups increased to 35.6% (414 of 1162), 35.8% (369 of 1031) and 36.5% (351 of 962) respectively. However, the pCR rate was always highest in HR-/HER2+ subtype followed by triple negative subtype, while the HR+/HER2- subtype had the lowest pCR rate no matter what definition of pCR was applied (Supplemental Table S1–4).
 |
Table 1 The Clinicopathologic Characteristics of Patients in the Three Groups |
 |
Figure 3 Patient screening flow. NAC, neoadjuvant chemotherapy; MT, malignant tumor; pCR, pathologic complete response, that is absence of invasive carcinoma in primary cancer area (ypT0/is). |
Prediction of pCR in Terms of Different Definitions
Of the 223 tumors that displayed as uCR in the US group, 134 tumors had a pCR (ypT0) and 151 tumors had a pCR (ypT0/is) (TP). Of the 211 tumors that exhibited mCR in the MRI group, 146 tumors had a pCR (ypT0) and 165 tumors had a pCR (ypT0/is) (TP). In the US+MRI group, 192 tumors showed uCR and 201 tumors were diagnosed as mCR. Only 91 tumors that presented as both uCR and mCR were considered as cCR, in which 73 tumors had a pCR (ypT0) and 79 tumors had a pCR (ypT0/is) (TP). Of the 351 tumors that had a pCR (ypT0/is) in the US+MRI group, 35 tumors showed uCR and non-mCR, while 37 tumors showed non-uCR and mCR. Under the definition of pCR (ypT0/is), the sensitivity, specificity, accuracy, PPV, and NPV were 36.5%, 90.4%, 71.2%, 67.7%, and 72.0% for US, 44.7%, 93.1%, 75.8%, 78.2%, and 75.1% for MRI, and 20.5%, 98.0%, 69.8%, 85.7%, and 68.2% for US+MRI, respectively (Table 2). For US, the sensitivity (P=0.627), specificity (P=0.382), accuracy (P=0.178), and PPV (P=0.097) were similar between the two definitions of pCR, whereas it had slightly increased NPV (P=0.015) under the alternative definition (Table 2). For MRI, there was no difference in sensitivity (P=0.510), specificity (P=0.160), and accuracy (P=0.251), except the PPV (P=0.036) and NPV (P=0.015), between the two definitions (Table 2). For US+MRI, the sensitivity (P=0.586), specificity (P=0.302), and PPV (P=0.167) were comparable between the two definitions, while it had slightly increased accuracy (P=0.036) and NPV (P=0.011) under the alternative definition (Table 2).
 |
Table 2 The Diagnostic Performance of US, MRI, and US+MRI Under Different Definitions of pCR |
Comparison of Prediction of pCR by US, MRI, and US+MRI
There was a difference among US, MRI, and US+MRI in sensitivity (P<0.001), specificity (P<0.001), accuracy (P=0.018), PPV (P<0.001), and NPV (P=0.017) (Table 3). Specifically, US+MRI had the highest specificity, but lowest sensitivity in comparison with the other two modalities (P<0.001 for all comparisons) (Table 4). MRI had the highest accuracy among the three modalities (P=0.016 for comparison with US, P=0.011 for comparison with US+MRI). In addition, US+MRI had higher PPV than US (P<0.001), but lower NPV than MRI (P=0.004).
 |
Table 3 Comparison of Prediction of pCR by US, MRI, and US+MRI |
 |
Table 4 Multiple Comparisons of Prediction of pCR by US, MRI, and US+MRI (Significance Threshold, P<0.017) |
Factors Affecting the Prediction of pCR
Factors including histological types, primary tumor size, and molecular subtypes that would affect the prediction of pCR by imaging modalities were analyzed. The diagnostic performance of US varied in different histological types, molecular subtypes, and primary tumor size (Table 5). To be specific, the accuracy and NPV were significantly lower in invasive ductal carcinoma (IDC) than non-IDC (69.8% vs 80.4%, P=0.007 for accuracy, 69.5% vs 86.9%, P<0.001 for NPV), while PPV was significantly higher in IDC than non-IDC (70.2% vs 26.7%, P<0.001). The sensitivity (P=0.070) and specificity (P=0.669) were comparable between IDC and non-IDC. Similar to pathology, the molecular subtypes influenced the accuracy (P<0.001), PPV (P=0.001), and NPV (P<0.001), but not the sensitivity (P=0.687) or specificity (P=0.321). Among the four molecular subtypes, accuracy (82.4%) and NPV (87.7%) were significantly higher in the HR+/HER2- subtype than any other subtypes (P<0.001 for all comparisons) (Table 6). The PPV in HR-/HER2+ subtype was significantly higher than any other subtypes except the triple negative one (Table 6). Besides, the sensitivity and PPV were significantly higher in smaller tumors than larger ones (42.7% vs 23.0%, P<0.001 for sensitivity, 73.4% vs 56.7%, P=0.020 for PPV). Tumor size had no effect on the specificity (P=0.122), accuracy (P=0.496), and NPV (P=0.103). As for MRI, the aforementioned factors had similar influences on the diagnostic performance in predicting pCR (Supplemental Table S5, S6). Nevertheless, the diagnostic performance of US+MRI in predicting pCR was not affected by histological types, primary tumor size, or molecular subtypes (Supplemental Table S7).
 |
Table 5 Factors Affecting the Prediction of pCR by US |
 |
Table 6 Multiple Comparisons of Prediction of pCR by US Between Different Subtypes (Significance Threshold, P <0.008) |
Discussion
Numerous studies on the diagnostic performance of imaging to evaluate the primary tumor response to NAC in breast cancer have been published. However, the definitions of pCR varied from the absence of residual invasive cancer with or without cancer in situ (ypT0/is)14,17,21,24 to the absence of residual invasive and noninvasive cancer (ypT0)15,16,25 in the primary tumor area. What is more, some studies defined the presence of a pCR as positivity,16,17,21 whereas others considered the presence of residual tumor as positivity14,15,22,23,25 when they analyzed the diagnostic performance of imaging with sensitivity, specificity, accuracy, PPV, and NPV, and so on. A recent study has reported that there was little difference between different pCR definitions in the diagnostic performance of MRI.17 However, our present study found that different definitions of pCR led to differences in some diagnostic indicators for US, MRI, and US+MRI. Thus, these discrepancies should be noted when it comes to comparisons between these studies.
Some previous studies have supported that MRI was superior to other methods such as physical examination, mammography, and US in evaluating tumor response in the neoadjuvant setting.13,26,27 Nevertheless, US is used to assess tumor response to NAC in breast cancer as often as MRI, if not more, due to its convenience and less contraindication. And later researches did not achieve a consistent conclusion on which one of US and MRI could be better to assess tumor response to NAC in breast cancer. Nakahara et al28 reported that correlation of US to pathological tumor size was lower than that of MRI in triple negative breast cancer. But Vriens et al29 concluded that US was at least as good as MRI in predicting tumor size after NAC in breast cancer. A recent study also suggested that contrast enhanced US was as effective as MRI (75% accuracy for both) in predicting pCR based on a group of 15 patients.30 In this study, we found that US and MRI were comparable in predicting pCR in terms of sensitivity, specificity, and NPV, though MRI had slightly higher accuracy and PPV than US. It should be noted that US+MRI had extremely high specificity and PPV, which were comparable with that reported in a recent study that showed MRI combined with second-look US was useful in predicting pCR with specificity and PPV as high as 97.3% and 86.8%.31 However, the sensitivity was much lower in our study (22.5%) than in the above research (66.6%). It is also noteworthy that all the three imaging modalities had relatively high specificity and low sensitivity in predicting pCR, which may be a result of the generally conservative attitude of doctors that would tend to overestimate the residual disease after NAC. The relatively high specificity and low sensitivity suggest that all three measures could detect most residual tumors, but were less sensitive to pCR. The moderate accuracy of all three imaging modalities indicates that they are not reliable enough to predict pCR in a clinical setting, as was concluded in previous studies.22,25,32
As is indicated in other studies,15,17,21,28,33 this study showed that the diagnostic performance of US or MRI varied between different histological and molecular subtypes. This may be correlated with the different pCR rates between these groups.34,35 In other words, HR-/HER2+ and triple negative tumors had higher PPVs with higher pCR rates, whereas HR+/HER2- tumors had higher NPV and accuracy with a lower pCR rate. Also, maximum primary tumor size before NAC also had a significant impact on the diagnostic performance of US and MRI. Interestingly, these factors did not affect the diagnostic performance of US+MRI.
Several limitations of this study should be noted. First of all, the nature of retrospective design and single center research might be an inevitable defect. Second, though we had MRI reviewed by two experienced doctors, the US assessment of this study was based on the US reports from the medical records and therefore to a great extent relied on personal experience. Finally, the non-IDC group included in situ carcinoma and other invasive cancer except for IDC. But we could not analyze them separately because of the small number.
Conclusion
The diagnostic performance of US, MRI, and US+MRI to predict pCR was different in different definitions of pCR. The US and MRI had comparable diagnostic performance, but neither of them could predict pCR with sufficient accuracy. The combination of US and MRI had increased specificity and PPV but decreased sensitivity and NPV compared to US or MRI alone. Still it could not predict pCR with sufficient accuracy. So, other assistant examination, such as vacuum-assisted biopsy mentioned in a recent study,31 may be combined with imaging to increase accuracy. The histological types, molecular subtypes and primary tumor size had a significant impact on the diagnostic performance of imaging for pCR. Thus, the explanation of imaging results should take into account these factors.
Abbreviations
BCS, breast conservative surgery; cCR, clinical complete response; ER, estrogen receptor; FN, false negative; FP, false positive; HR, hormone receptor; IDC, invasive ductal carcinoma; IHC, immunohistochemical; NAC, neoadjuvant chemotherapy; NPV, negative predictive value; mCR, MRI complete response; MRI, magnetic resonance imaging; MST, mastectomy; pCR, pathologic complete response; PPV, positive predictive value; PR, progesterone receptor; Q1/3, the first/third quartile; TN, true negative; TP, true positive; uCR, US complete response; US, ultrasound.
Data Sharing Statement
Please contact the corresponding author for data requests.
Author Contributions
Cai Chang and Kai Zhang conceived and designed the study. Kai Zhang and Jiawei Li wrote the manuscript. All authors contributed to data analysis, drafting and reviewing of the article, provided final approval for the publication of the final version, and agreed to be accountable for all aspects of the work.
Ethics and Consent Statement
This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center. Informed consent was waived due to its retrospective design and no identifiable information was disclosed.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81627804, 81830058).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J National Cancer Inst Monogr. 2001;2001(30):96–102. doi:10.1093/oxfordjournals.jncimonographs.a003469
2. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from national surgical adjuvant breast and bowel project B-18. J clin oncol. 1997;15(7):2483–2493. doi:10.1200/JCO.1997.15.7.2483
3. Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95(4):681–695. doi:10.1002/cncr.10741
4. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J clin oncol. 2008;26(5):778–785. doi:10.1200/JCO.2007.15.0235
5. Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94(10):1189–1200. doi:10.1002/bjs.5894
6. Kaufmann M, von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927–1934. doi:10.1093/annonc/mdm201
7. Kim R, Osaki A, Toge T. Current and future roles of neoadjuvant chemotherapy in operable breast cancer. Clin Breast Cancer. 2005;6(3):223–232. (). doi:10.3816/CBC.2005.n.024
8. Chollet P, Amat S, Cure H, et al. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002;86(7):1041–1046. doi:10.1038/sj.bjc.6600210
9. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi:10.1016/S0140-6736(13)62422-8
10. Broglio KR, Quintana M, Foster M, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA oncol. 2016;2(6):751–760. doi:10.1001/jamaoncol.2015.6113
11. LeVasseur N, Sun J, Gondara L, et al. Impact of pathologic complete response on survival after neoadjuvant chemotherapy in early-stage breast cancer: a population-based analysis. J Cancer Res Clin Oncol. 2020;146(2):529–536. doi:10.1007/s00432-019-03083-y
12. Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the st. gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017;28(8):1700–1712. doi:10.1093/annonc/mdx308
13. Marinovich ML, Macaskill P, Irwig L, et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: individual patient data meta-analysis. BMC Cancer. 2015;15(1):662. doi:10.1186/s12885-015-1664-4
14. van Ramshorst MS, Loo CE, Groen EJ, et al. MRI predicts pathologic complete response in HER2-positive breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat. 2017;164(1):99–106. doi:10.1007/s10549-017-4254-0
15. De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119(10):1776–1783. doi:10.1002/cncr.27995
16. Weber JJ, Jochelson MS, Eaton A, et al. MRI and prediction of pathologic complete response in the breast and axilla after neoadjuvant chemotherapy for breast cancer. J Am Coll Surg. 2017;225(6):740–746. doi:10.1016/j.jamcollsurg.2017.08.027
17. Gampenrieder SP, Peer A, Weismann C, et al. Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathologic complete response (pCR). Breast Cancer Res. 2019;21(1):19. doi:10.1186/s13058-018-1091-y
18. Marinovich ML, Houssami N, Macaskill P, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105(5):321–333. doi:10.1093/jnci/djs528
19. Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–197. doi:10.1200/JOP.777003
20. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: american Society Of Clinical Oncology/College Of American Pathologists clinical practice guideline update. J clin oncol. 2013;31(31):3997–4013. doi:10.1200/JCO.2013.50.9984
21. Fukuda T, Horii R, Gomi N, et al. Accuracy of magnetic resonance imaging for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy: association with breast cancer subtype. SpringerPlus. 2016;5(1):152. doi:10.1186/s40064-016-1800-x
22. Croshaw R, Shapiro-Wright H, Svensson E, Erb K, Julian T. Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients. Ann Surg Oncol. 2011;18(11):3160–3163. doi:10.1245/s10434-011-1919-5
23. Kim Y, Sim SH, Park B, et al. Magnetic Resonance Imaging (MRI) assessment of residual breast cancer after neoadjuvant chemotherapy: relevance to tumor subtypes and MRI interpretation threshold. Clin Breast Cancer. 2018;18(6):459–467. doi:10.1016/j.clbc.2018.05.009
24. Ko ES, Han BK, Kim RB, et al. Analysis of factors that influence the accuracy of magnetic resonance imaging for predicting response after neoadjuvant chemotherapy in locally advanced breast cancer. Ann Surg Oncol. 2013;20(8):2562–2568. doi:10.1245/s10434-013-2925-6
25. Baumgartner A, Tausch C, Hosch S, et al. Ultrasound-based prediction of pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2018;39:19–23. doi:10.1016/j.breast.2018.02.028
26. Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy–results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263(3):663–672.
27. Marinovich ML, Macaskill P, Irwig L, et al. Meta-analysis of agreement between MRI and pathologic breast tumour size after neoadjuvant chemotherapy. Br J Cancer. 2013;109(6):1528–1536. doi:10.1038/bjc.2013.473
28. Nakahara H, Yasuda Y, Machida E, et al. MR and US imaging for breast cancer patients who underwent conservation surgery after neoadjuvant chemotherapy: comparison of triple negative breast cancer and other intrinsic subtypes. Breast Cancer. 2011;18(3):152–160. doi:10.1148/radiol.12110748
29. Vriens BE, de Vries B, Lobbes MB, et al. Ultrasound is at least as good as magnetic resonance imaging in predicting tumour size post-neoadjuvant chemotherapy in breast cancer. Eur J Cancer. 2016;52:67–76. doi:10.1016/j.ejca.2015.10.010
30. Lee SC, Grant E, Sheth P, et al. Accuracy of contrast-enhanced ultrasound compared with magnetic resonance imaging in assessing the tumor response after neoadjuvant chemotherapy for breast cancer. J Ultrasound Med. 2017;36(5):901–911. doi:10.7863/ultra.16.05060
31. Hayashi N, Tsunoda H, Namura M, et al. Magnetic resonance imaging combined with second-look ultrasonography in predicting pathologic complete response after neoadjuvant chemotherapy in primary breast cancer patients. Clin Breast Cancer. 2018;19(1):71–77.
32. Schaefgen B, Mati M, Sinn HP, et al. Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol. 2016;23(3):789–795. doi:10.1245/s10434-015-4918-0
33. Loo CE, Straver ME, Rodenhuis S, et al. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J clin oncol. 2011;29(6):660–666. doi:10.1200/JCO.2010.31.1258
34. Sasanpour P, Sandoughdaran S, Mosavi-Jarrahi A, Malekzadeh M. Predictors of pathological complete response to neoadjuvant chemotherapy in iranian breast cancer patients. Asian Pac j Cancer Prev. 2018;19(9):2423–2427. doi:10.22034/APJCP.2018.19.9.2423
35. Matsuda N, Wang X, Lim B, et al. Safety and efficacy of panitumumab plus neoadjuvant chemotherapy in patients with primary HER2-negative inflammatory breast cancer. JAMA oncol. 2018;4(9):1207–1213. doi:10.1001/jamaoncol.2018.1436
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.