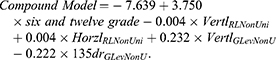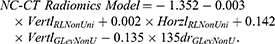Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Prediction of Hepatocellular Carcinoma Response to Transcatheter Arterial Chemoembolization: A Real-World Study Based on Non-Contrast Computed Tomography Radiomics and General Image Features
Authors Guo Z, Zhong N, Xu X, Zhang Y, Luo X, Zhu H, Zhang X, Wu D, Qiu Y, Tu F
Received 17 April 2021
Accepted for publication 22 June 2021
Published 9 July 2021 Volume 2021:8 Pages 773—782
DOI https://doi.org/10.2147/JHC.S316117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ahmed Kaseb
Zheng Guo,1,2,* Nanying Zhong,3,* Xueming Xu,4 Yu Zhang,4 Xiaoning Luo,4 Huabin Zhu,3 Xiufang Zhang,3 Di Wu,5 Yingwei Qiu,6,7 Fuping Tu4
1Department of Oncology, Ganzhou Key Laboratory of Gastrointestinal Carcinomas, First Affiliated Hospital of Gannan Medical University, Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China; 2Department of Hematology and Oncology, International Cancer Center, Shenzhen Key Laboratory of Precision Medicine for Hematological Malignancies, Shenzhen University General Hospital, Shenzhen University Clinical Medical Academy, Shenzhen University Health Science Center, Shenzhen, Guangdong, People’s Republic of China; 3First School of Clinical Medicine, Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China; 4Department of Oncology, First Affiliated Hospital of Gannan Medical University, Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China; 5Department of Imaging, First Affiliated Hospital of Gannan Medical University, Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China; 6Department of Radiology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 7Department of Radiology, Huazhong University of Science and Technology Union Shenzhen Hospital, Shenzhen, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingwei Qiu; Fuping Tu Email [email protected]; [email protected]
Objective: To construct a predictive model of short-term response and overall survival for transcatheter arterial chemoembolization (TACE) treatment in hepatocellular carcinoma (HCC) patients based on non-contrast computed tomography (NC-CT) radiomics and clinical features.
Methods: Ninety-four HCC patients who underwent CT scanning 1 week before the first TACE treatment were retrospectively recruited and divided randomly into a training group (n = 47) and a validation group (n = 47). NC-CT radiomics data were extracted using MaZda software, and the compound model was calculated from radiomics and clinical features by logistic regression. The performance of the different models was compared by examining the area under the receiver operating characteristic curve (AUC). The prediction of prognosis was evaluated using survival analysis.
Results: Thirty NC-CT radiomic features were extracted and analyzed. The compound model was formed using four NC-CT run-length matrix (RLM) features and general image features, which included the maximum diameter (cm) of the tumor and the number of tumors (n). The AUCs of the model for TACE response were 0.840 and 0.815, whereas the AUCs of the six-and-twelve grade were 0.754 and 0.750 in the training and validation groups, respectively. HCC patients were divided into two groups using the cutoff value of the model: a group in which the TACE-response led to good survival and a group in which TACE-nonresponse caused poor prognosis.
Conclusion: Radiomic features from NC-CT predicted TACE-response. The compound model generated by NC-CT radiomics and clinical features is effective and directly predicts TACE-response and overall survival. The model may be used repeatedly and is easy to operate.
Keywords: hepatocellular carcinoma, transcatheter arterial chemoembolization, computed tomography, radiomics, texture
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers of the digestive system and is especially prevalent in China. More than 80% of HCC patients were diagnosed with advanced-stage cancer without opportunity for surgery, leading to the second-highest cancer-related mortality in China.1 Transcatheter arterial chemoembolization (TACE) is one of the most recommended choices for advanced HCC patients without extrahepatic metastasis, because of its use in a broad range of indications and a 30–70% objective response rate (ORR),2 according to the guidelines of the American Association for the Study of Liver Diseases (AASLD), the European Society for Medical Oncology (ESMO), and the Chinese Society of Clinical Oncology (CSCO).3,4 It is difficult to accurately predict the treatment response before TACE due to the high heterogeneity of HCC5,6 and the wide application of TACE. At present, the treatment options are usually based on the clinical stage of HCC3,7 and the imaging features of the tumor.8 The hepatoma arterial-embolization prognostic (HAP) score is calculated using albumin, bilirubin, alpha-fetoprotein (AFP), or the size of the dominant tumor, and has been verified as a prognostic system for HCC.9 Recently, a novel model based on general image information called six-and-twelve grade (equal to the maximum diameter (cm) of the tumor plus the number of tumors) was developed, and its accuracy for predicting the effectiveness of TACE using survival probability was 70–75%.10 A pre-TACE prediction model based on six-and-twelve grade was also published for survival prediction.11 The six-and-twelve grade was recommended as a pre-TACE expected survival model in the 2020 CSCO guidelines.
Radiomics is a form of texture analysis and computes a set of features from clinical images to characterize an individual’s disease.12 Heterogeneity makes it difficult to predict the treatment response and prognosis for malignant tumors before treatment, but the high-dimensional quantitative radiomic features extracted from pretreatment imaging have been successfully applied to predict survival and treatment options for non–small-cell lung cancer and rectal cancer.13,14 In 2020, it has been reported that ultrasound or magnetic resonance imaging (MRI) radiomics might be an effective predictor for short-term HCC prognosis after TACE.15–17 Deep learning technology for CT images can also improve prediction of therapeutic response to TACE.18 This study was the first to use a single image of non-contrast computed tomography (NC-CT) radiomics combined with features selected from clinical characteristics, laboratory data, and general CT imaging to generate a new compound model for the prediction of TACE response. Moreover, the probability of overall survival was also investigated to evaluate this new compound model.
Materials and Methods
Study Population
This retrospective study was approved and exempted from written informed consent by the Ethical Review Committee of First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, China. In total, data from 1084 HCC patients were collected at the First Affiliated Hospital of Gannan Medical University between January 2018 and May 2020. The inclusion criteria were as follows: 1) patients diagnosed with HCC and treated in accordance with the Guidelines for Diagnosis and Treatment of BCLC (Barcelona Clinic Liver Cancer) Stage, and Child–Pugh class A or B;7 2) no surgical indications, or patients refused to perform the operation; 3) patients where TACE was the first treatment administered during the course of disease, or recurrence was demonstrated on the first digital subtraction angiography (DSA) post-surgery and TACE was performed at that time; 4) patients where emergency TACE was undertaken to stop bleeding and reduce the pain caused by the tumor or arteriovenous fistula; and 5) patients where a CT scan was performed both within 1 week before TACE and within 8 weeks after TACE at our center. The exclusion criteria were as follows: 1) Child–Pugh class C; 2) patients where ablation, radiotherapy, or systemic therapy were used before first follow-up with computed tomography (CT) scan; 3) patients where no CT scan was performed in our center 1 week before TACE and 8 weeks after TACE; or 4) patients who had HCC combined with other malignant tumors.
TACE Procedure
TACE was performed by two interventional radiologists with more than 10 years of experience using an angiography X-ray system (UNIQ FD20, Philips). Twenty-nine candidates in BCLC stage A were included for the small residual volume of normal liver, recurrence post-surgery, bad location of the tumor, and/or refusal of patient to operation. The interventional microcatheter was used to selectively insert into the tumor-feeding artery through the hepatic artery using digital subtraction angiography (DSA) imaging. Superior mesenteric artery angiography was performed to find out whether there was a collateral blood supply at the same time. Chemoembolization was performed with iodized oil (5–20 mL) mixed with platinum (10–40 mg) or epirubicin (10–40 mg). After chemoembolization, the feeding vessels of the tumor were blocked, and the tumor staining from angiography disappeared. Gelatin sponge particles were added to block the residual blood flow from tumor blood supply vessels. Four to 8 weeks after the first TACE treatment, liver enhanced CT, AFP, liver and kidney function tests and blood routine were examined to evaluate the effect of treatment.
Clinical Features and TACE Response Assessment
General and laboratory data before the TACE procedure was performed were collected, including age, sex, hepatitis B virus infection status, AFP, Child–Pugh class, BCLC stage, tumor size, and number. Six-and-twelve grade was defined as the maximum diameter (cm) of the tumor plus the number of tumors. The pre-TACE expected survival model was calculated as reported.10,11 Sequential therapy information post-TACE, including re-TACE, ablation, and systemic therapy, were collected at the same time. All CT images were performed in our hospital, in accordance with the liver protocol using two devices (SOMATOM Definition Flash, Siemens AG; Lightspeed VCT, GE Medical Systems). Iohexol was used as the contrast agent and the contrast images were collected at 20, 55, and 100 seconds after injection as artery phase (AP), venous phase (VP), and delay phase (DP), respectively. The number of tumors and maximum tumor diameter were assessed. CT images with contrast pre-TACE (within 1 week) and post-TACE (within 8 weeks) were collected. Modified response evaluation criteria (mRECIST) in solid tumors19 were referenced as response assessment post-TACE. The smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started was taken as reference: the disappearance of any intra-tumoral arterial enhancement in all target lesions was defined as completed response (CR); at least a 30% decrease in the sum of diameters of viable target lesions was defined as partial response (PR); an increase of at least 20% in the sum of the diameters of viable target lesions indicated progressive disease (PD); and any cases that did not qualify for either PR or PD were considered stable disease (SD). The follow-up time was up to July 2020 to obtain the overall survival (OS) time of these patients.
Extraction of Radiomic Features
Normalized non-contrast CT (NC-CT) images before TACE in DICOM format (Digital Imaging and Communications in Medicine) were used to extract features via an open access and free software, MaZda, which has proven to be efficient and reliable for quantitative image analysis.20–22 The process of region of interest (ROI) drawing and features extraction is presented in Supplementary Materials and Results (Figure S1). The indicated NC-CT layer of the maximum diameter of the tumor was selected to outline the ROI in each patient and was divided into two groups: a green region was used to define the TACE-response group, including CR and PR patients, while patients with a red region were included in the TACE-nonresponse group, containing PD and SD patients. The ROIs were drawn by two experts on primary HCCs to avoid blood vessels and margin of lesion. Over 300 features were generated, and 30 features were collected in a training group using Fisher coefficient, probability of classification error, correlation coefficient (POE + ACC), and mutual information (MI) calculation.
Statistical Analysis
χ2 was used to detect the difference in clinical features between the training and validation groups. Linear discriminant analysis of B11 was used to discriminate the radiomic features. A logistic risk model and Lasso regression were performed to identify significantly affected features that predict TACE response in the training group. The receiver operating characteristic (ROC) curve was drawn to evaluate the sensitivity and specificity of different predictive models. Patients underwent follow-up until July 2020; eight cases were lost to follow-up. Potential correlation of the models with OS was assessed by Kaplan–Meier analysis. Cox regression was used to evaluate the different risk factors to OS. All of the data were analyzed using SPSS 22.0 version (SPSS, Inc., Chicago, IL, USA). P values less than 0.05 indicated a statistically significant difference.
Results
Study Procedure and Characteristics of Patients
The procedure of this study is shown in Figure 1. Ninety-four HCC patients with TACE treatment were enrolled in this study and randomized into training (n = 47) and validation (n = 47) datasets. The training group was used to establish the TACE predictive model based on CT imaging and clinical features, whereas the validation group was used to verify the accuracy of the model. A comparison of the baseline characteristics between the two datasets is shown in Table 1. The clinical features, including sex, age, HBV infection, AFP, Child–Pugh class, maximum size of tumor, number of tumors, BCLC stage, six-and-twelve grade,10 and pre-TACE prediction grade,11 in the two cohorts showed no significant difference. The estimated average OSs were 21.04 months (95% CI: 16.97–25.10 months) and 20.04 months (95% CI: 16.34–23.74 months) in the training and validation groups, respectively, which is consistent with the six-and-twelve model study10 and indicates no difference between the two sets (P = 0.954). Moreover, the number for each sequential treatment post-TACE is shown in Table 1. The ratios of sequential combination therapies after TACE in these two groups showed no difference, excluding the effect of treatment on survival and making the analysis of survival difference in our model more reliable.
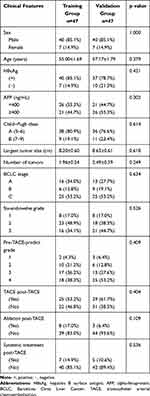 |
Table 1 The Difference in Clinical Features Between Training and Validation Groups |
Role of Clinical Features in Predicting TACE Response
The ORR for TACE across the entire cohort was 55.3%. We first analyzed clinical features, such as general patient characteristics, laboratory data, and general CT features, including six-and-twelve grade, in the training dataset to address the critical features involved in predicting TACE response. As shown in Table 2, only six-and-twelve grade calculated from general CT images was defined as an independent risk factor for TACE response in our cohort (P = 0.041). The odds ratio was 68.749, indicating that the higher the six-and-twelve grade, the poorer the response to TACE treatment in HCC patients. Other factors, including age, HBV infection, AFP, Child–Pugh class, maximum size of tumor, number of tumors, BCLC stage, and pre-TACE prediction grade, presented no significant effects in predicting TACE response individually (P > 0.05), which indicates that tumor burden evaluated by six-and-twelve grade provided the most effective prediction.
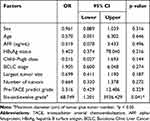 |
Table 2 Logistic Analysis of Clinical Features for Evaluation of TACE Response in the Training Group |
Construction of New Classifier Model
NC-CT radiomic features were extracted using the MaZda software to generate a new compound model for predicting TACE response. Thirty features were included from Image Histogram, Image Gradient, run-length matrix (RLM), and Wavelet transform. Linear discriminant analysis was performed to classify the ROI in the training cohort using these features. The Fisher coefficient was 12.6 and linear separability was 0.75, which indicates that these NC-CT radiomic features might play classifying roles in predicting TACE response.
To optimize the radiomic features, logistic regression analysis was used to screen the most valuable features of NC-CT radiomics (we repeated feature selection using least absolute shrinkage and selection operator (Lasso) regression and details can be found in the Supplementary Materials and Results (Figure S2)). Using stepwise regression, a new compound model combined with six-and-twelve grade and four RLM radiomic features was established and validated as follows:
Meanwhile, a model using only NC-CT radiomic features is as follows:
Performance of Different Models
ROC curve was performed both in the training and validation groups to evaluate the efficacy of different models in predicting TACE response in our cohort. Six-and-twelve score was used to keep the type of data consistent with the new compound model we developed. As indicated in Supplementary Materials and Results (Table S1), the area under the ROC curve of the new compound model was 0.840 (95% CI, 0.716–0.964) in the training group, which was higher than the area of 0.754 found in the six-and-twelve model (95% CI, 0.609–0.898). Similar results were shown in the validation group and the whole cohort. Moreover, the ROC curve of the NC-CT radiomics model was equivalent to that of the six-and-twelve model. These data showed that NC-CT radiomics could be as effective a predictive method as the six-and-twelve model to directly estimate TACE response in HCC patients. After the combination of NC-CT radiomics and six-and-twelve model, the predictive effectiveness was significantly improved (Figure 2). Interestingly, we obtained similar predictive efficiency with the new radiomic features derived from Lasso and the details are shown in Supplementary Materials and Results (Figure S3). Moreover, we repeated this analysis with the whole cohort randomly dividing into a training group and a validation group at a ratio of 7:3 and similar results were obtained and presented in Supplementary Materials and Results (Figures S4 and S5).
 |
Figure 2 ROC curve of TACE short-term response in HCC patients. (A) Training group. (B) Validation group. |
Survival Analysis of Potential Risk Factors
The overall survival analysis was performed to evaluate the potential risk factors to survival based on the construction and verification of the aforementioned new compound model. The cutoff value of the new compound model was calculated as 2.7 by ROC curve using the maximal Youden’s index (equal to specificity plus sensitivity minus one): grade 1 ≤ 2.7 predicted TACE-response, whereas grade 2 > 2.7 predicted TACE-nonresponse. The estimated average OS in grade 1 was 25.30 months (95% CI: 21.02–29.57 months), but significantly decreased to 10.17 months (95% CI: 6.41–13.93 months) in grade 2 (Figure 3A, P = 0.006). Using six-and-twelve grade (Figure 3B), the estimated average OSs were 25.08 months (95% CI: 17.68–32.48 months), 24.00 months (95% CI: 18.85–29.14 months), and 9.79 months (95% CI: 5.85–13.73 months). Compared with grade 1, grade 3 showed a significant difference (P = 0.044), whereas grade 2 did not (P = 0.542), implying that our new compound model with two grades played a superior role in predicting OS performance. Similar results were shown in the validation group (Figure 3C and D). Nomogram of the compound model for TACE-nonresponse risk is shown in Figure 4 using R software.
 |
Figure 4 The radiomics nomogram of TACE-nonresponse risk in our compound model. The radiomics signature was calculated from four radiomic features. |
In terms of the findings of K-M survival analysis for OS prediction, Table S2 (Supplementary Materials and Results) shows that factors including general, clinical, sequential therapy, and radiomics compound features were involved in survival prediction. Univariate analysis showed that AFP, TACE response, BCLC stage, six-and-twelve grade, and compound grade were significant variables of survival. Multivariate analysis was further performed on these five features, and the compound grade was the only effective factor (P = 0.000). A Forest plot (Figure 5) showed the significantly increased odds ratio in compound grade compared with six-and-twelve grade and CT-radiomics grade, which indicates an improved effect of this model in prognosis.
 |
Figure 5 Forest plot of three models using Cox regression. |
Discussion
In this study, a compound model using radiomics and the six-and-twelve grade was constructed to predict the short-term ORR of TACE. TACE is a local treatment recommended for patients with different stages of HCC.3 One of the primary reasons for variations in TACE response is tumor heterogeneity,23–26 which increases the difficulty of determining distinct features between tumors for use in predicting TACE response and guiding treatment choice. Previous models, such as those using the six-and-twelve grade and HAP score, divided patients into three or four groups and indirectly predicted the efficiency of TACE using the probability of patient survival. These models could not draw direct conclusions about TACE response, limiting the application of the model to the prediction of short-term TACE efficacy.
The value of radiomics in HCC treatment has been steadily increasing.15–18,27 Several recent studies that combined radiomics with clinical features reported success in predicting the ORR for TACE in HCC patients. A model that combined T1WI and T2WI MRI-based radiomics with clinical features, including sex, tumor margin, peritumoral enhancement, BCLC stage, and AFP level, was generated to predict the recurrence-free survival (RFS) of HCC patients after TACE treatment, and the C-index was 0.681–0.861.28 Two other compound models that used 3D multiphase-CT radiomic features and clinical factors (Child–Pugh score, AFP lever, size of tumor, and number of tumors) were also constructed to predict the survival of HCC patients after TACE treatment.29,30 The information used in these two models was complex, which limited the clinical application of these models.
In our cohort, the predicting ROC curve of the six-and-twelve grade was consistent with the previous report.10 The six-and-twelve grade was developed from a multicenter randomized controlled study and proven to be the only significant clinical feature for the evaluation of TACE response in our training group. Only one NC-CT image with maximum diameter was used to extract radiomics information. The model could therefore be repeatably used, was easy to implement, and was not affected by scanning time and parameters. Four features that were extracted through radiomics in our model represented the heterogeneity of the tumor, something that is difficult to quantify with traditional imaging due to subjective image assessment.31 Moreover, all the features selected in this compound model were extracted by NC-CT, making the model easy to implement. Compared with the six-and-twelve grade, our model improved the TACE-response predictive ability from 0.757 to 0.829. A cutoff value of 2.7 may therefore be used as a direct evaluation index for TACE-response before surgery in HCC patients. Furthermore, our model also significantly predicted survival. However, our study had the limitation that validation in other centers was deficient. Thus, our preliminary results should be validated in a future multicenter study with large sample sizes.
In conclusion, the compound model using general and radiomic features from NC-CT imaging created in this study was reliable and efficient for predicting both short-term TACE response and long-term survival in HCC patients. The model can be further applied for clinical decision-making to reduce unnecessary TACE surgery and financial burden. Other recommended treatments should be selected earlier in TACE non-response patients after use of our combination model.
Ethics Approval and Informed Consent
The retrospective study was conducted in accordance with the Declaration of Helsinki of 1975, revised in 2013. The institutional committee of the First Affiliated Hospital of Gannan Medical University approved the proposal of our study and the informed consent of patients was waived. The reason for exemption of informed consent is that this non-invasive radiomics study is based on clinical history and imaging data, which does not involve personal privacy and commercial interests. All the data was anonymized and maintained with confidentiality. Specifically, all the original images were desensitized to delete the subject’s personal information (name, hospitalization number, telephone number, address, etc.). During the radiomic analysis, all imaging data were stored anonymously in our hospital database, with the anonymous code saved by our team leaders (Yingwei Qiu and Fuping Tu).
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author Contributions
Zheng Guo and Yingwei Qiu: designed the study, wrote the manuscript and edited the manuscript for important intellectual content; Nanying Zhong, Xueming Xu and Fuping Tu: collected the information of patients, and contributed to writing the manuscript and edited the manuscript for important intellectual content; Yu Zhang, Xiaoning Luo, Huabin Zhu and Xiufang Zhang: performed statistic analysis and contributed to writing the manuscript; Di Wu and Yingwei Qiu: reviewed the CT images and drew the ROI.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Nature Science Foundation of China (# 81860440), the Jiangxi Provincial Natural Science Foundation (# 20202BAB206047), and the Jiangxi Science and Technology Plan of Health Commission (# 20204501).
Disclosure
The authors have no financial or non-financial competing interests.
References
1. Zhu XD, Sun HC. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol. 2019;12(1):110. doi:10.1186/s13045-019-0794-6
2. Sacco R, Tapete G, Simonetti N, et al. Transarterial chemoembolization for the treatment of hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2017;4:105–110. doi:10.2147/JHC.S103661
3. Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31(3):334–351. doi:10.1016/j.annonc.2019.12.001
4. Li XH, Wei L. [The comparison among the guidelines for the diagnosis and treatment of hepatocellular carcinoma in China, AASLD and EASL]. Zhonghua Gan Zang Bing Za Zhi. 2019;27(3):236–240. (Chinese). doi:10.3760/cma.j.issn.1007-3418.2019.03.015
5. Rimola J. Heterogeneity of hepatocellular carcinoma on imaging. Semin Liver Dis. 2020;40(1):61–69. doi:10.1055/s-0039-1693512
6. Xu LX, He MH, Dai ZH, et al. Genomic and transcriptional heterogeneity of multifocal hepatocellular carcinoma. Ann Oncol. 2019;30(6):990–997. doi:10.1093/annonc/mdz103
7. Department of Medical Administration NH, Health Commission of the People’s Republic of C. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):112–128. (Chinese). doi:10.3760/cma.j.issn.1007-3418.2020.02.004
8. Pesapane F, Nezami N, Patella F, Geschwind JF. New concepts in embolotherapy of HCC. Med Oncol. 2017;34(4):58. doi:10.1007/s12032-017-0917-2
9. Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24(10):2565–2570. doi:10.1093/annonc/mdt247
10. Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70(5):893–903. doi:10.1016/j.jhep.2019.01.013
11. Han G, Berhane S, Toyoda H, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology. 2020;72(1):198–212. doi:10.1002/hep.31022
12. Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762. doi:10.1038/nrclinonc.2017.141
13. Fave X, Zhang L, Yang J, et al. Delta-radiomics features for the prediction of patient outcomes in non-small cell lung cancer. Sci Rep. 2017;7(1):588. doi:10.1038/s41598-017-00665-z
14. Liu Z, Zhang XY, Shi YJ, et al. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin Cancer Res. 2017;23(23):7253–7262. doi:10.1158/1078-0432.CCR-17-1038
15. Liu D, Liu F, Xie X, et al. Accurate prediction of responses to transarterial chemoembolization for patients with hepatocellular carcinoma by using artificial intelligence in contrast-enhanced ultrasound. Eur Radiol. 2020;30(4):2365–2376. doi:10.1007/s00330-019-06553-6
16. Sun Y, Bai H, Xia W, et al. Predicting the outcome of transcatheter arterial embolization therapy for unresectable hepatocellular carcinoma based on radiomics of preoperative multiparameter MRI. J Magn Reson Imaging. 2020;52(4):1083–1090. doi:10.1002/jmri.27143
17. Weng W, Lu XL, Zhang QQ, et al. [Prediction of short-term prognosis of hepatocellular carcinoma after TACE surgery based on MRI texture analysis technology]. Zhonghua Yi Xue Za Zhi. 2020;100(11):828–832. (Chinese). doi:10.3760/cma.j.cn112137-20190705-01502
18. Peng J, Kang S, Ning Z, et al. Residual convolutional neural network for predicting response of transarterial chemoembolization in hepatocellular carcinoma from CT imaging. Eur Radiol. 2020;30(1):413–424. doi:10.1007/s00330-019-06318-1
19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
20. Szczypinski PM, Strzelecki M, Materka A, Klepaczko A. MaZda--a software package for image texture analysis. Comput Methods Programs Biomed. 2009;94(1):66–76. doi:10.1016/j.cmpb.2008.08.005
21. Wang R, Su Y, Mao C, Li S, You M, Xiang S. Laser lithotripsy for proximal ureteral calculi in adults: can 3D CT texture analysis help predict treatment success? Eur Radiol. 2020.
22. Cavallo AU, Troisi J, Forcina M, et al. Texture analysis in the evaluation of Covid-19 pneumonia in chest X-Ray images: a Proof of Concept Study. Curr Med Imaging. 2021;17. doi:10.2174/1573405617999210112195450
23. de Baere T, Arai Y, Lencioni R, et al. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovasc Intervent Radiol. 2016;39(3):334–343. doi:10.1007/s00270-015-1208-y
24. Golfieri R, Bargellini I, Spreafico C, Trevisani F. Patients with Barcelona clinic liver cancer stages B and C hepatocellular carcinoma: time for a subclassification. Liver Cancer. 2019;8(2):78–91. doi:10.1159/000489791
25. Jeong SO, Kim EB, Jeong SW, et al. Predictive factors for complete response and recurrence after transarterial chemoembolization in hepatocellular carcinoma. Gut Liver. 2017;11(3):409–416. doi:10.5009/gnl16001
26. Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. 2018;16(1):69. doi:10.1186/s12957-018-1368-8
27. Zhang W, Xu AH, Wang W, Wu YH, Sun QL, Shu C. Radiological appearance of hepatocellular carcinoma predicts the response to trans-arterial chemoembolization in patients undergoing liver transplantation. BMC Cancer. 2019;19(1):1041. doi:10.1186/s12885-019-6265-1
28. Song W, Yu X, Guo D, et al. MRI-based radiomics: associations with the recurrence-free survival of patients with hepatocellular carcinoma treated with conventional transcatheter arterial chemoembolization. J Magn Reson Imaging. 2020;52(2):461–473. doi:10.1002/jmri.26977
29. Kim J, Choi SJ, Lee SH, Lee HY, Park H. Predicting survival using pretreatment CT for patients with hepatocellular carcinoma treated with transarterial chemoembolization: comparison of models using radiomics. AJR Am J Roentgenol. 2018;211(5):1026–1034. doi:10.2214/AJR.18.19507
30. Meng XP, Wang YC, Ju S, et al. Radiomics analysis on multiphase contrast-enhanced CT: a survival prediction tool in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Front Oncol. 2020;10:1196. doi:10.3389/fonc.2020.01196
31. Casadei-Gardini A, Giulia O, Francesco C, Giorgio E. Developments in predictive biomarkers for hepatocellular carcinoma therapy. Expert Rev Anticancer Ther. 2020;20(1):63–74. doi:10.1080/14737140.2020.1712198
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.