Back to Journals » Journal of Pain Research » Volume 12
Prediction of Hemodynamic Reactivity by Electroencephalographically Derived Pain Threshold Index in Children Undergoing General Anesthesia: A Prospective Observational Study
Authors Wu L, Wang S, Wang Y, Zhang K , Bai J, Zheng J
Received 19 September 2019
Accepted for publication 15 November 2019
Published 3 December 2019 Volume 2019:12 Pages 3245—3255
DOI https://doi.org/10.2147/JPR.S231596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Lei Wu,1,* Siyuan Wang,2,* Yanting Wang,1 Kan Zhang,1 Jie Bai,1 Jijian Zheng1,3
1Department of Anesthesiology, Shanghai Children’s Medical Center Affiliated to School of Medicine, Shanghai Jiao Tong University, Pudong, Shanghai, People’s Republic of China; 2Department of Anesthesiology, 3201 Hospital, Hanzhong City, Shaanxi, People’s Republic of China; 3Pediatric Clinical Pharmacology Laboratory, Shanghai Children’s Medical Center Affiliated to School of Medicine, Shanghai Jiao Tong University, Pudong, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jijian Zheng
Department of Anesthesiology, Shanghai Children’s Medical Center Affiliated to School of Medicine, Shanghai Jiao Tong University, 1678 Dongfang Road, Pudong, Shanghai 200127, People’s Republic of China
Tel +86 21 38626161
Fax +86 21 58393915
Email [email protected]
Purpose: The pain threshold index (PTI) is a novel measure of nociception based on integrated electroencephalogram parameters during general anesthesia. The wavelet index (WLI) reflects the depth of sedation. This study aims to evaluate the ability of the PTI and WLI to predict hemodynamic reactivity after tracheal intubation and skin incision in pediatric patients.
Patients and methods: Pediatric patients (n=134) undergoing elective general surgery or urinary surgery were analyzed. Measurements at predefined time-points during tracheal intubation and skin incision included the PTI, WLI, heart rate (HR), and mean blood pressure (MBP). Receiver-operating characteristic (ROC) curves were computed to evaluate the predictive performance of the PTI and WLI in measuring hemodynamic reactivity (an increase of more than 20% in either MBP or HR) during general anesthesia.
Results: Of the 134 patients evaluated, positive reactivity of HR and MBP was observed in 95 (70.9%) and 61 (45.5%) patients induced by intubation, respectively, and 19 (14.2%) and 24 (17.9%) patients induced by skin incision, respectively. Using either HR or MBP reactivity induced by intubation as a dichotomous variable, the areas under the curves (AUCs) [95% CI] of PTI and WLI were 0.81[0.73–0.87] and 0.58[0.49–0.67] with the best cutoff values of 62 and 49. The AUCs [95% CI] of PTI and WLI were 0.82[0.75–0.88] and 0.61[0.52–0.69] after skin incision. The best cutoff values of PTI and WLI were 60 and 46, respectively.
Conclusion: The PTI can predict hemodynamic reactivity with the best cutoff values of 62 and 60 after tracheal intubation and skin incision in pediatric patients during general anesthesia. The WLI failed in predicting hemodynamic changes.
Keywords: pain threshold index, wavelet index, hemodynamic reactivity, pediatric patients
Introduction
The response to noxious stimulation during general anesthesia primarily depends on a balance between nociceptive and anti-nociceptive forces.1–3 Nociception in anesthetized patients is typically demonstrated as a sympathetic response to surgery and other noxious stimuli.4,5 Excessive nociception not only affects the stability of hemodynamics but may also exacerbate postoperative pain, disturb the balance of inflammation, and produce adverse outcomes.6,7 Analgesic administration is the key component when enhancing anti-nociceptive forces. This is mainly determined by the anesthesiologist’s clinical experience.8 However, it is very challenging to reach an appreciable analgesic level with objective and effective assessment tool of the anti-nociceptive state of the patient. Errors in dosage may result in adverse side effects.9
Changes in hemodynamic parameters including heart rate and blood pressure are usually used to indicate whether the analgesic level is sufficient under general anesthesia. However, these parameters cannot be used to predict the response to noxious stimulation as they are the results of the noxious stimulation.10,11 Furthermore, many confounding factors such as hypovolemia, β-blockers, and anticholinergic drugs can interfere with hemodynamic parameters.12 In recent years, several noninvasive monitoring tools based on skin conductance (SC), pupil diameter, heart rate variability (HRV), amplitude from photoplethysmography (Surgical Pleth Index, SPI) or analysis of electroencephalogram (EEG) signals have been used to estimate the anti-nociceptive state of adult patients during general anesthesia. However, few tools are used in children. This may be due to the age-dependent characteristics of EEGs and electrocardiograms (ECG) or the lack of pediatric electrodes.11,13–17
The pain threshold index (PTI), a novel measure reflecting the anti-nociceptive state under general anesthesia, is based on integrated EEG wavelet analysis. The pediatric model was developed by Beijing Easymonitor Technology Co., Ltd, China. According to the company’s specifications, the PTI measurements range from 0 to 100, which reflects the tolerance of the cerebral cortex and the subcortex to pain stimulation under general anesthesia. EEG wavelet analysis, acting like a mathematical microscope, can capture very minute details and sudden changes. It separates the small weak signals from high-frequency signals.18 Recent study clearly shows that scalp EEG contains both cortical and subcortical signals.19 Therefore, EEG wavelet analysis can be used to separate and analyze cortical and subcortical EEG signals, which is the theoretical base of the algorithm used by this new monitor. Theoretically, PTI can be used to predict the response to noxious stimulation instead of detecting the result of noxious stimulation during general anesthesia. Whether PTI can actually be used to predict the response to noxious stimulation during general anesthesia remains unknown. Wavelet index (WLI), a parameter for monitoring the depth of sedation, was developed by the same company and is based on wavelet analysis. It is similar to the bispectral index (BIS) in reflecting the patient’s sedation state and is enhanced when the patient is under muscle relaxation or facial paralysis.20,21 Our primary aim was to examine whether the PTI can be used to predict the hemodynamic reactivity induced by endotracheal intubation and skin incision in pediatric patients. Our secondary aim was to investigate the role of the WLI in predicting the hemodynamic reactivity induced in pediatric patients.
Materials and Methods
This prospective observational clinical study was approved by the Institutional Review Board of Shanghai Children’s Medical Center (reference number: SCMCRB-k2018050, May 20, 2018) and registered in the www.chictr.org.cn (ChiCTR1800015969, May 3, 2018). The study was performed between May 2018 and September 2018. Written informed consent was obtained from parents or legal guardians the day before data collection and surgery. All data acquisition took place in the operating room.
Patients
Patients in this study included the American Society of Anesthesiologists physical status (ASA) I or II and pediatric patients aged from 6 months to 12 years. These patients were scheduled for elective general surgery and urinary surgery under general anesthesia.
Exclusion criteria consisted of patients with brain diseases (history of epilepsy, autism, cognitive dysfunction), autonomic nervous system disorders, liver and renal diseases, endocrinological diseases, cardiac arrhythmias, preoperative chronic pain, symptoms of upper respiratory infection and those who received administration of anticholinergic drugs, vasopressors, vasodilators blockers, or ketamine before and during the study.
Anesthetic Technique
For pediatric patients younger than 4 years, 0.5 mg/kg midazolam was administered orally 20–30 mins before entering the operating room. Other patients did not receive premedication. On arrival in the operation room, intravenous access and basic monitoring with an electrocardiogram, and pulse oximetry were established. A crystalloid infusion was begun following the 4-2-1 principals.16 Brachial noninvasive blood pressure was measured every 1 min during the experiment.
For uncooperative patients older than 4 years, 0.1–0.15 mg/kg midazolam IV was administered initially to facilitate the measurement of baseline blood pressure and SpO2, as well as to place the EEG and the ECG electrodes. During the experiment, atropine or vasoactive drugs were contraindicated except when serious bradycardia and/or hypotension occurred. Anesthesia management was left to the discretion of the anesthesiologist in charge, with no restrictions imposed by the study except those mentioned above. Generally, children received an intravenous induction with fentanyl (1.5–3.0 µg/kg) or sufentanil (0.15–0.3 µg/kg), propofol (2–4 mg/kg), and rocuronium 0.6 mg/kg. All patients older than 4 years received 0.1–0.15 mg/kg midazolam IV during the entire induction process. Endotracheal intubation was performed by a skilled anesthesiologist who had used videolaryngoscopy at least 50 times. After successful intubation, anesthesia was maintained using propofol 4–8 mg/kg/h and remifentanil 0.25–0.4 μg/kg/min, with or without 1.0–2.0% sevoflurane according to the hemodynamic changes induced by skin incision. Mechanical ventilation was initiated after tracheal intubation with a mixture of 50% O2 and 50% air. The pressure-controlled ventilation mode was used, and tidal volume was 8–10 mL/kg. Inspiration pressure and ventilator frequency were adjusted to keep end-tidal CO2 pressure between 35 mmHg and 45 mmHg.
Study Protocol and the Analgesia Monitoring Device
The anesthesiologist in charge used the HXD-I multi-function combination monitor (for the WLI and PTI recording, Beijing Easymonitor Technology Co., Ltd., China) and was responsible for determining the anesthesia protocol and the timing of endotracheal intubation. The anesthesiologist was blind to the research protocol. The research team was responsible for recording the PTI, WLI, heart rate (HR), and mean blood pressure (MBP) just before intubation and skin incision, and 1 min after intubation and skin incision.22
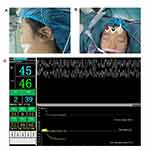
After the skin on the forehead and the mastoid was cleaned with water or alcohol cotton balls, the EEG collection electrodes were placed 1–1.5 cm above the center point between the eyebrows on the forehead (FZ), above the bilateral eyebrows (Left FP1, right FP2), and on the bilateral mastoid sites (left C1, right C2) (Figure 1A and B). Real-time EEG status and time trend of the PTI and the WLI data were displayed on the monitor screen in pediatric mode (Figure 1C).
Pain Threshold Index Calculation
The principle of the pain threshold index calculation was reported in China Medical Engineering in 2017.23 Briefly, two channels of EEG data were recorded and analyzed by the HXD-I monitor through an EEG analysis software package from Beijing Easymonitor Technology Co., Ltd, which is based on a wavelet algorithm. The specific EEG data vector is controlled by the continuous wavelet transforms, binary discrete wavelet transforms and the frequency domain reconstruction algorithm in wavelet analysis. The vector set of each waveform signal is produced by discrete processing: fi (x) = [x1 x2 x3 … xm‑2 xm‑1xm], i: the number of EEG leads, and m: the number of vector elements. Furthermore, the direct circuit components (Av) of a vector are removed by f (x) = f (x) – Av. The preprocessed EEG wave data are further analyzed by the waveform recognition algorithm, the spectral analysis algorithm, and the wavelet analysis algorithm. If f (x) function is the signal of space domain {−∞, +∞}, then, the continuous wavelet transform algorithm formula is as follows: 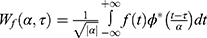 . The algorithm formula of the binary discrete wavelet transforms is as follows:
. The algorithm formula of the binary discrete wavelet transforms is as follows: 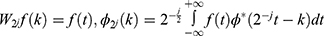 . The formula of the wavelet frequency domain calculation is as follows:
. The formula of the wavelet frequency domain calculation is as follows:
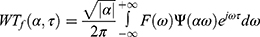
The spectral analysis algorithm follows the discrete Fourier formula: 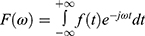 . The inverse transformation is completed by the following formula:
. The inverse transformation is completed by the following formula: 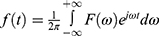 . For the brain wave data, the specific brain wave vector data are first processed by the binary conversion algorithm and the waveform reconstruction algorithm in wavelet analysis. It then selects the specific wavelet generating function and constructs an n scale. The following formula is used to conduct the binary conversion algorithm, from 20to 2n:
. For the brain wave data, the specific brain wave vector data are first processed by the binary conversion algorithm and the waveform reconstruction algorithm in wavelet analysis. It then selects the specific wavelet generating function and constructs an n scale. The following formula is used to conduct the binary conversion algorithm, from 20to 2n: 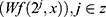 . Subsequently, a set of wavelets transforms the basis functions for the bandpass filter banks and is obtained by the following formula:
. Subsequently, a set of wavelets transforms the basis functions for the bandpass filter banks and is obtained by the following formula: 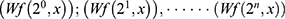 .
.
Reconstruction consists of the wavelet function reconstruction and each wavelet base reconstruction follows: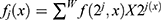 . The reconstructed wavelet is X2j(x); j is the order of the time domain function. A set of reconstruction functions is obtained by reconstruction of the various wavelet base reconstruction functions through the following formula:
. The reconstructed wavelet is X2j(x); j is the order of the time domain function. A set of reconstruction functions is obtained by reconstruction of the various wavelet base reconstruction functions through the following formula: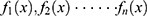 ;n=order. The power WLE(I) of the waveform potential of each wavelet base reconstruction function is calculated by
;n=order. The power WLE(I) of the waveform potential of each wavelet base reconstruction function is calculated by 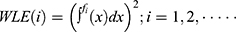 . The Fast Fourier transform is used to calculate the power spectrum function synchronously through the formula:
. The Fast Fourier transform is used to calculate the power spectrum function synchronously through the formula: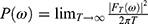 . The calculation window is n, where the alpha wave component of 8–13 Hz, the delta wave component of 0.5–4 Hz, the theta wave component of 4–8 Hz, the beta wave component of 13–30 Hz, the dominant frequency, edge frequency, central frequency, and the initial phase PH(Hz) of each frequency component can be obtained.
. The calculation window is n, where the alpha wave component of 8–13 Hz, the delta wave component of 0.5–4 Hz, the theta wave component of 4–8 Hz, the beta wave component of 13–30 Hz, the dominant frequency, edge frequency, central frequency, and the initial phase PH(Hz) of each frequency component can be obtained.
The generating function is referred to as the first derivative of the smoothing function (spline function), and 64 points are constructed by the dyadic wavelet transform, scaling from 20 to 26. The weighted items of each sub‑index extracted from the EEG (i‑series metadata) are obtained by decomposing the various EEG data vectors on transformation characteristic weighting sequences by using a multilayer calculation and a multiple regression iteration method. PTI is calculated by combining the weighted items of each sub‑index (a1, a2 … … an as the multiple regression weighting coefficients). The PTI= {a1, a2 … … an} & {i_22, i_24, i_35, i_52, i_60, i_70}.
Statistical Analysis
Continuous data are presented as the mean ± SD, or median with the interquartile range [IQR] according to the normality of the distribution. Categorical variables are presented as category counts and percentages. The continuous outcome variables are the PTI, WLI, HR, and MBP. The occurrence of positive pain response after applying endotracheal intubation and surgical incision was reflected by hemodynamic reactivity, which was defined as more than a 20% increase of either MBP or HR, 1 min after nociception stimulus.22,24 Receiver-operating characteristics (ROC) curves and the associated areas under the curves (AUC) were computed to characterize the sensitivity, specificity, and the ability of the PTI and WLI (pre-stimulation values) to predict hemodynamic reactivity. The asymptotic 95% CI of each AUC was calculated, as well as the asymptotic P value under the null hypothesis that the true AUC = 0.5. The reaction of HR and MBP after tracheal intubation and skin incision was compared using the Wilcoxon signed-rank test.
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Inc., USA) and MedCalc® version 12.1.4.0 (MedCalc Software, Mariakerke, Belgium). The P values of less than 0.05 were considered significant.
Results
Patient Characteristics
We initially recruited 163 pediatric patients during the study period. Thirteen patients were excluded due to fever and/or cough or they refused to sign the informed consent document prior to surgery. Pediatric patients who met the inclusion criteria and obtained signed informed consent by their parents or legal guardians (n=150) were included in this study. During the study, ten cases were eliminated due to poor electrode-skin contact or noise interference, and another six cases were excluded due to the use of atropine or vasoactive drugs. In the final analysis, 134 patients were included (Figure 2). Patient characteristics are shown in Table 1. The study patients were comprised of 114 males and 20 females. The median age of patients was 40 [IQR 17–64] months and the median weight was 15 [IQR 12–20] kg.
 |
Table 1 Patient Characteristics (N=134) |
 |
Figure 2 The CONSORT flow diagram. |
Hemodynamic Reactivity
During the induction of general anesthesia, the WLI values decreased much more quickly than the PTI values (Figure 1C). The changes of HR and MBP induced by endotracheal intubation and skin incision are, respectively, shown in Table 2. According to the criteria of hemodynamic reactivity, positive reactivity of HR and MBP induced by endotracheal intubation was observed in 95 (70.9%) and 61 (45.5%) patients, respectively. The patients’ HR and MBP were significantly increased (P < 0.001) after intubation. The median values of HR and MBP changes were 30.4% (IQR, 15.7–44.2%) and 17.3% (IQR, 5.5–35.8%), respectively. The positive reactivity of HR and MBP induced by skin incision was found in 19 (14.2%) and 24 (17.9%) patients, respectively. Skin incision significantly increased the patient’s HR and MBP (P < 0.001). The median values of HR and MBP changes were 1.8% (IQR, −2.7 to 9.9%) and 7.1% (IQR, 2.4–17.7%).
 |
Table 2 Prestimulus, Poststimulus (Median [25–75th Percentile]) of MBP, HR After Intubation and Skin Incision |
Prognostic Power of the PTI and the WLI
To further analyze the ability of the PTI and WLI (pre-stimulation values) to predict hemodynamic reactivity induced by endotracheal intubation and skin incision, receiver-operating characteristics (ROCs) curves and the associated areas under the curves (AUCs) were computed (Table 3). Using either HR or MBP reactivity induced by tracheal intubation as a dichotomous variable, the AUCs [95% CI] of the PTI and WLI were 0.81 [0.73–0.87] and 0.58 [0.49–0.67], respectively. The best cutoff values (the optimal threshold) of the PTI and WLI were 62 and 49. Using either HR or MBP reactivity induced by skin incision as a dichotomous variable, the AUCs [95% CI] of the PTI and WLI were 0.82 [0.75–0.88] and 0.61 [0.52–0.69], respectively. The best cutoff values of the PTI and WLI were 60 and 46, respectively. The sensitivity and specificity of the PTI and WLI with corresponding cutoff values in predicting hemodynamic reactivity are also included in Table 3. The ROC curves of the PTI and WLI to predict hemodynamic reactivity, HR change, and MBP change are displayed in Figure 3.
 |
Table 3 Prognostic Power of PTI and WLI (Pre-Stimulation Values) in Predicting Hemodynamic Response |
Discussion
Using more than a 20% increase of either MBP or HR 1 min after tracheal intubation and skin incision as a criteria of analgesic insufficiency, we found that the PTI, a newly derived EEG parameter designed to reflect the anti-nociceptive state for surgical patients under general anesthesia, could be used to predict the hemodynamic reactivity induced by tracheal intubation and skin incision. The areas under ROC curves were 0.81 and 0.82, respectively. The best PTI cutoff values for predicting hemodynamic reactivity induced by tracheal intubation and skin incision were 62 and 60, respectively. The WLI, a similar parameter to the BIS for monitoring the depth of sedation, cannot be used to predict the hemodynamic reactivity induced by tracheal intubation and skin incision as the areas under ROC curves were only 0.58 and 0.61, respectively. In addition, our study also demonstrated that the WLI decreased significantly more rapidly than the PTI during the induction of anesthesia. This indicated that the WLI and the PTI may reflect different characteristics of the EEGs, the EEG signals from other brain regions or the effects of various anesthetics. Further study is required in order to confirm these results.
Self-reporting is considered the gold standard in determining the presence and degree of pain perception in clinical practice. However, it is not feasible for patients who are under general anesthesia, who suffer from consciousness disorders or who are in coma. Therefore, more and more recent studies focus on methods of objective pain assessment. Thus far, there are two major strategies that are used to detect and assess the response to nociceptive stimuli.10 One strategy is based on the autonomic nervous system response to nociceptive stimuli. This includes the analgesia nociception index (ANI), the surgical plethysmography index (SPI), skin conductance, the nociception level index, and pupillometry. Another strategy is based on electroencephalogram (EEG) readings, which includes the bispectral index (BIS), the composite variability index, Spectral Entropy, the qNOX index, and the qCON index.25 The nociceptive flexion reflex measured by electromyography (RIII reflex) is an additional strategy, which determines the depth of analgesia by measuring the inhibition of motor response to nociceptive stimuli.26 The advantages and disadvantages of these parameters used for detecting and assessing the response to nociceptive stimuli have been systematically reviewed. None of these parameters can be used to detect and assess the nociceptive response during anesthesia in pediatric patients.10,27 The NIPE (newborn infant parasympathetic evaluation) is the neonatal version of the ANI used in adults. Our previous study demonstrated that the NIPE may not serve as a sensitive and specific predictor of changes in hemodynamics induced by endotracheal intubation and skin incision during general anesthesia.28
General anesthesia significantly inhibits the activity of cerebral cortex. This illustrates that the BIS is reduced; however, it leaves the activity of subcortical autonomous nervous system relatively intact.7 Noxious stimulations can continue to alter the activity of the subcortical autonomous nervous system under general anesthesia. Thus, demonstrating the changes of heart rate, blood pressure, pupillary diameter, pulse wave amplitude, sweating, and other clinical signs due to the inhibition of the sympathetic and/or parasympathetic activity or a shift in the sympathetic/parasympathetic balance.10,25 Therefore, the monitoring tools based on the autonomic nervous system’s response to nociceptive stimuli can detect the nociceptive response more sensitively than hemodynamic parameters during general anesthesia. However, they cannot predict the response of nociceptive stimuli.10,25 These parameters are affected by the depth of sedation, arrhythmia, emotional stress, and drugs affecting hemodynamic stability and autonomic nervous system activity.7
Recently, electroencephalogram monitoring has been widely used during the perioperative period. It is a cheap, easy-to-use, and noninvasive technique.29 Of those EEG-derived parameters, the BIS, the qCON index and state Entropy have been shown to be associated with the depth of sedation rather than nociception.10 Our study confirmed that the WLI, a similar EEG-derived parameter to the BIS, was used for monitoring the depth of sedation and could not predict the hemodynamic reactivity induced by noxious stimuli. Both Composite Variability Index and response Entropy incorporated frontal electromyography (EMG) signals in the EEG analysis and have been reported to be more likely to provide early detection of somatic events induced by stimulation than hemodynamic monitoring. However, they could not predict an inadequate level of analgesia under general anesthesia.12,30 Furthermore, the depth of neuromuscular blockade and the type of hypnotic agent used to influence the EMG variability.10 The qNOX index is obtained from a single channel from the frontal EEG signals spectral analysis. It uses an advanced digital processing algorithm (the Adaptive Neuro-Fuzzy Interference System, ANFIS) with four frequency bands. A previous study illustrates that the increasing likelihood of a response to a noxious stimulus is associated with increasing qNOX score values.4 The qNOX represents an interesting alternative to most other monitors that rely on autonomic activity changes for the assessment of nociception. It is promising for predicting nociception under general anesthesia; however, additional solid and in-depth studies are required.25
In our study, the pain threshold index (PTI) is a newly developed EEG-derived parameter for nociception assessment. The PTI is obtained from two channels from frontal EEG signals wavelet analysis using an advanced digital processing algorithm (Beijing Easymonitor Technology Co., Ltd., China) with an alpha, delta, theta and beta wave component as well as the dominant frequency, edge frequency, central frequency, and the initial phase PH (Hz) of each frequency component. The PTI score (0–100) is an integration of the cortical and the subcortical EEG activity-based dimensionless proprietary score for nociceptive assessment under unconsciousness, indicating various cerebral pain tolerant states. The optional PTI score is 40–60 as recommended by the developer. This is in contrast to the rapid reduction of the WLI value, which represents the rapid sedation initiation by propofol during anesthesia induction. The reduction of the PTI value is much slower, which may reflect the combined effects of the activity of various brain regions and opioids. This requires further study. Furthermore, our study illustrates that the PTI values before noxious stimulation can better predict hemodynamic reactivity following noxious stimulations in pediatric patients. The AUCs of the PTI for intubation and skin incision were 0.81 and 0.82, respectively. Therefore, PTI might be clinically helpful to predetermine whether an upcoming noxious stimulation would cause nociceptive response and titrate the doses of opioids in advance in pediatric patients. This may be different from our routine practice model which requires the doses of opioids to be titrated based on hemodynamic changes induced by noxious stimulation during general anesthesia. The PTI may be used as a target to guide the administration of opioids during surgery.
The Pain index (PI), another EEG-derived parameter used for pain assessment in conscious patients from the same monitor (HXD-I multi-function combination monitor Beijing Easymonitor Technology Co., Ltd., China), was found to be significantly correlated with the pain visual analogue scale/numerical rating scales.29 However, it cannot be used to assess the nociception of patients under general anesthesia, which may be due to the influence of general anesthetics on the EEG.
In this study, we defined hemodynamic reactivity as an increase of more than 20% of either MBP or HR after 1 min of painful stimulus. In adult patients, hemodynamic reactivity is often defined as an increase of more than 20% in HR and/or blood pressure within 5 mins or following 2 mins after noxious stimulation.6,24,31 Through preliminary experiments, we found that in pediatric patients the hemodynamic response caused by nociception fluctuates quickly. Hemodynamic changes may return to normal 1 min after intubation or skin incision. In a study of children, MBP and HR values were recorded within 1 min of the laryngeal mask airway insertion.22 Therefore, we chose the values of HR and MBP 1 min after stimulation to detect hemodynamic reactivity caused by nociception more accurately.
Our study presents some limitations. First, some patients were excluded such as children with a history of epilepsy, autism and cognitive dysfunction. Patients receiving vasopressors or ketamine before and during the experiment were also excluded. Therefore, our results may not be generalized to all patients. Second, our study was observational and anesthesia management including propofol and remifentanil dosing were left to the discretion of the anesthesiologist in charge. This may have induced bias in the analysis of the predictive ability of our method. Third, our sample size was relatively small, and the preliminary validation study was conducted in a single center. Larger sample and multicenter studies are required in the future. Currently, due to the confidential issues surrounding the algorithm used by this new monitor, we cannot provide additional information regarding the mechanisms of the monitor. We can only speculate that the PTI may reflect the cortical pain threshold state before noxious stimulations through the analysis of the correlated changes from cortical and subcortical neuronal activities using EEG wavelet analysis. Additional studies are required in order to validate this approach.
Conclusion
In conclusion, the PTI can predict hemodynamic reactivity after tracheal intubation and skin incision in pediatric patients during general anesthesia. The WLI failed to predict hemodynamic changes. This study suggests that the PTI may be used to predict the nociceptive response induced by noxious stimuli during general anesthesia in children.
Abbreviations
ANI, analgesia/nociception index; ASA, anesthesiologists physical status; AUCs, areas under the curves; BIS, bispectral index; EEG, electroencephalogram; PTI, pain threshold index; HR, heart rate, HRV, heart rate variability; IQR, interquartile range; MBP, meanblood pressure; NoL, nociception level; ROC, receiver-operating characteristic; SC, skin conductance; SPI, surgical pleth index; WLI, wavelet index.
Ethics Approval and Consent to Participate
This trial was conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of Shanghai Children’s Medical Center Affiliated to School of Medicine (reference number: SCMCRB-k2018050, May 20, 2018) and registered in the www.chictr.org.cn (ChiCTR1800015969, May 3, 2018).
Consent for Publication
Written informed consent was obtained from the parent(s) and/or guardian(s) of all of the participants
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Medical Guide Project of the Shanghai Science and Technology Commission (16411967500) and the Shanghai Municipal Commission of Health and Family Planning, the Key Developing Disciplines (2015ZB0106). The funding bodies were not involved in the design of the study or the collection, analysis, and interpretation of data.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Edry R, Recea V, Dikust Y, et al. Preliminary intraoperative validation of the nociception level index: a noninvasive nociception monitor. Anesthesiology. 2016;125:193–203. doi:10.1097/ALN.0000000000001130
2. Brown EN, Pavone KJ, Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg. 2018;127:1246–1258. doi:10.1213/ANE.0000000000003668
3. Mashour GA. Neurophysiology and intraoperative nociception: new potentials? Anesthesiology. 2013;118:239–240. doi:10.1097/ALN.0b013e318279fb40
4. Jensen EW, Valencia JF, Lopez A, et al. Monitoring hypnotic effect and nociception with two EEG-derived indices, qCON and qNOX, during general anaesthesia. Acta Anaesthesiol Scand. 2014;58:933–941. doi:10.1111/aas.12359
5. Stockle PA, Julien M, Issa R, et al. Validation of the PMD100 and its NOL Index to detect nociception at different infusion regimen of remifentanil in patients under general anesthesia. Minerva Anestesiol. 2018;84:1160–1168. doi:10.23736/S0375-9393.18.12720-9
6. Funcke S, Sauerlaender S, Pinnschmidt HO, et al. Validation of innovative techniques for monitoring nociception during general anesthesia: a clinical study using tetanic and intracutaneous electrical stimulation. Anesthesiology. 2017;127:272–283. doi:10.1097/ALN.0000000000001670
7. von Dincklage F. Monitoring of pain, nociception, and analgesia under general anesthesia: relevance, current scientific status, and clinical practice. Anaesthesist. 2015;64:758–764. doi:10.1007/s00101-015-0080-0
8. Malver LP, Brokjaer A, Staahl C, et al. Electroencephalography and analgesics. Br J Clin Pharmacol. 2014;77:72–95. doi:10.1111/bcp.12137
9. Guignard B. Monitoring analgesia. Best Pract Res Clin Anaesthesiol. 2006;20:161–180. doi:10.1016/j.bpa.2005.09.002
10. Abad-Gurumeta A, Ripolles-Melchor J, Casans-Frances R, et al. Monitoring of nociception, reality or fiction? Rev Esp Anestesiol Reanim. 2017;64:406–414. doi:10.1016/j.redar.2017.01.009
11. De Jonckheere J, Bonhomme V, Jeanne M, et al. Physiological signal processing for individualized anti-nociception management during general anesthesia: a review. Yearb Med Inform. 2015;10:95–101. doi:10.15265/IY-2015-004
12. Mathews DM, Clark L, Johansen J, et al. Increases in electroencephalogram and electromyogram variability are associated with an increased incidence of intraoperative somatic response. Anesth Analg. 2012;114:759–770.
13. Gjerstad AC, Storm H, Hagen R, et al. Comparison of skin conductance with entropy during intubation, tetanic stimulation and emergence from general anaesthesia. Acta Anaesthesiol Scand. 2007;51:8–15. doi:10.1111/aas.2007.51.issue-1
14. Sabourdin N, Barrois J, Louvet N, et al. Pupillometry-guided intraoperative remifentanil administration versus standard practice influences opioid use: a randomized study. Anesthesiology. 2017;127:284–292. doi:10.1097/ALN.0000000000001705
15. Okkesim S, Celik G, Yildirim MS, et al. Comparison of pulse rate variability and heart rate variability for hypoglycemia syndrome. Methods Inf Med. 2016;55:250–257. doi:10.3414/ME15-01-0088
16. Won YJ, Lim BG, Lee SH, et al. Comparison of relative oxycodone consumption in surgical pleth index-guided analgesia versus conventional analgesia during sevoflurane anesthesia: a randomized controlled trial. Medicine (Baltimore). 2016;95:e4743. doi:10.1097/MD.0000000000004743
17. Shoushtarian M, Sahinovic MM, Absalom AR, et al. Comparisons of electroencephalographically derived measures of hypnosis and antinociception in response to standardized stimuli during target-controlled propofol-remifentanil anesthesia. Anesth Analg. 2016;122:382–392. doi:10.1213/ANE.0000000000001029
18. Faust O, Acharya UR, Adeli H, et al. Wavelet-based EEG processing for computer-aided seizure detection and epilepsy diagnosis. Seizure. 2015;26:56–64. doi:10.1016/j.seizure.2015.01.012
19. Seeber M, Cantonas LM, Hoevels M, et al. Subcortical electrophysiological activity is detectable with high-density EEG source imaging. Nat Commun. 2019;10:753. doi:10.1038/s41467-019-08725-w
20. Yang N, Ge MF, Wang TL, et al. Feasibility analysis of wavelet index for monitoring the depth of anesthesia in patients undergoing general anesthesia. Zhonghua Yi Xue Za Zhi. 2011;91:2849–2852.
21. Zhang XT, Cheng H, Xiong W, et al. Comparison of the ability of wavelet index and bispectral index for reflecting regain of consciousness in patients undergone surgery. Chin Med J (Engl). 2010;123:1520–1523.
22. Kim HS, Park HJ, Kim CS, et al. Combination of propofol and remifentanil target-controlled infusion for laryngeal mask airway insertion in children. Minerva Anestesiol. 2011;77:687–692.
23. Wu YB. Extraction of objective and quantitative indexes of pain, anxiety, depression and other brain function states from electroencephalogram. China Med Eng. 2017;25:4–10. Chinese. doi:10.19338/j.issn.1672-2019.2017.04.001
24. Boselli E, Bouvet L, Begou G, et al. Prediction of hemodynamic reactivity during total intravenous anesthesia for suspension laryngoscopy using Analgesia/Nociception Index (ANI): a prospective observational study. Minerva Anestesiol. 2015;81:288–297.
25. Ledowski T. Objective monitoring of nociception: a review of current commercial solutions. Br J Anaesth. 2019;123:e312–e321. doi:10.1016/j.bja.2019.03.024
26. von Dincklage F, Hackbarth M, Mager R, et al. Monitoring of the responsiveness to noxious stimuli during anaesthesia with propofol and remifentanil by using RIII reflex threshold and bispectral index. Br J Anaesth. 2010;104:201–208. doi:10.1093/bja/aep357
27. Cowen R, Stasiowska MK, Laycock H, et al. Assessing pain objectively: the use of physiological markers. Anaesthesia. 2015;70:828–847. doi:10.1111/anae.2015.70.issue-7
28. Zhang K, Wang S, Wu L, et al. Newborn infant parasympathetic evaluation (NIPE) as a predictor of hemodynamic response in children younger than 2 years under general anesthesia: an observational pilot study. BMC Anesthesiol. 2019;19:98. doi:10.1186/s12871-019-0774-y
29. An JX, Wang Y, Cope DK, et al. Quantitative evaluation of pain with pain index extracted from electroencephalogram. Chin Med J (Engl). 2017;130:1926–1931.
30. Ellerkmann RK, Grass A, Hoeft A, et al. response of the composite variability index to a standardized noxious stimulus during propofol-remifentanil anesthesia. Anesth Analg. 2013;116:580–588. doi:10.1213/ANE.0b013e31827ced18
31. Boselli E, Logier R, Bouvet L, et al. Prediction of hemodynamic reactivity using dynamic variations of Analgesia/Nociception Index (ANI). J Clin Monit Comput. 2016;30:977–984. doi:10.1007/s10877-015-9802-8
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.