Back to Journals » Journal of Pain Research » Volume 15
Predicting Response to Radiotherapy in Breast Cancer-Induced Bone Pain: Relationship Between Pain and Serum Cytokine Expression Levels After Radiotherapy
Authors Lou Y , Cao H, Wang R , Chen Y, Zhang H
Received 26 August 2022
Accepted for publication 3 November 2022
Published 11 November 2022 Volume 2022:15 Pages 3555—3562
DOI https://doi.org/10.2147/JPR.S387670
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Yaling Lou,1 Henbin Cao,1 Ronghua Wang,1 Yu Chen,2 Haibing Zhang3
1Department of Clinical Pharmacy, Huzhou Central Hospital, Huzhou, People’s Republic of China; 2Department of Clinical Laboratory, Huzhou Central Hospital, Huzhou, People’s Republic of China; 3Department of Radiotherapy Center, Huzhou Central Hospital, Huzhou, People’s Republic of China
Correspondence: Yaling Lou; Haibing Zhang, Email [email protected]; [email protected]
Introduction: Breast cancer is the second leading cause of cancer-associated death in women. Herein, we explored the associations of cytokines, pain, and bone metastasis between patients before and after radiotherapy in breast cancer with bone metastasis. The pain caused by metastasis was effectively relieved by external radiation therapy. However, the underlying mechanisms remain unknown.
Methods and Results: In this case-controlled study, we enrolled healthy individuals (n = 10) and bone metastatic cancer patients (n = 30). Peripheral venous blood samples were collected from healthy controls, and one week before and after radiotherapy, the peripheral venous blood and clinical characteristics of cancer patients were collected. We analyzed the blood cytokine profile, quality of life (QOL), and pain score of patients pre- and post-radiotherapy to explore the possible causes of pain relief. Both the pain score and QOL significantly improved after radiotherapy. The serum cytokine profiles of patients were significantly different before radiotherapy than after. Meanwhile, only three cytokines differed between post-radiotherapy and healthy controls. We believe radiotherapy stimulated local immune storms in bone tissue and promoted significant changes in cytokines pre- and post-radiotherapy. Therefore, the bone microenvironment of early breast cancer patients with bone metastasis pain can be restored after radiotherapy. Restoring a healthy bone environment can not only relieve the pain but also improve the patient’s QOL.
Conclusion: Tumor cells can effectively activate immune cells in the bone microenvironment through direct interaction, releasing many factors and promoting bone metastasis. Early local radiotherapy of bone metastases can restore the microenvironment and improve the QOL and prognosis of patients, thereby comprehending a novel target for prevention, treatment, and therapy of bone metastases.
Keywords: breast cancer, bone metastasis, radiotherapy, cytokines, pain, QOL, microenvironment, immune system
Introduction
Breast cancer is the second leading cause of cancer-associated death in women, with 2.3 million new cases reported in 2020 worldwide.1 In the United States, 279,100 new cases and 42,690 deaths were reported in 2020. Based on the statistics provided by the Cancer Statistics Bureau, breast cancer is the most common cancer in women (accounting for 30% of female cases) and the second cause of cancer-associated death (accounting for 15% of female cancer deaths).2 Bone metastasis is frequently observed in breast cancer patients, with the spine, ribs, pelvis, and long bones being the most common sites.3 Bone metastases often lead to bone-related problems, including spinal cord compression, bone marrow regeneration disorders, hypercalcemia, pain, and pathological fractures. The median time from the diagnosis to the first occurrence of bone-related events in breast cancer patients with bone metastases is only 1.8 months, and the 1-year incidence of SREs is as high as 40%.4 Therefore, bone adverse events negatively impact the survival, activity, and quality of life (QOL) of patients, posing a significant threat to women’s health.
Due to the constant and dynamic reshaping of bone tissue throughout life, the occurrence and progression of bone metastases is a complex process. Under physiological conditions, the crosstalks between osteoblasts and osteoclasts at the molecular level are significantly required for bone remodeling. For example, the differentiation of osteoclasts is significantly controlled by the signals provided by osteocytes and osteoblasts through the soluble or membrane-bound RANKL. By directly binding to the RANK receptor, RANKL can promote maturation and regulate the function of osteoclasts. Osteoclast differentiation can also be negatively mediated via another soluble decoy RANKL receptor secreted by osteoblasts, osteoprotegerin (OPG). In contrast, osteoclasts affect the differentiation and activity of osteoblasts by secreting semaphorin 4D (Sema 4D) and other factors.5 Furthermore, osteoclast and osteoblast functions can be significantly regulated by bone matrix-secreted cytokines, including insulin-like growth factor-1 (IGF-1) and TGF-β.6
Bone remodeling imbalance is dramatically associated with bone mass and significantly connected to pathological conditions. The bone metastasis of breast cancer has an organ-specific spreading pattern. Osteoblast factors expressed by breast cancer cells deregulate the function, recruitment, and differentiation of osteoblasts and osteoclasts, promoting the proliferation, survival, colonization, invasion, and homing of breast cancer cells in bones.7 Cytokine and chemokine signaling intervene and regulate different steps of metastatic processes, such as tumor cells detachment from the primary tumor mass, bone colonization, cell proliferation, dissemination, migration, and epithelial-to-mesenchymal transition (EMT).8 Therefore, bone formation and resorption balance disruption are closely associated with mixed, osteoblastic, or osteolytic bone lesions.
Furthermore, pain is significantly associated with bone metastases, but there are no strict dependencies between pain intensity and bone metastatic size and extent. The pain caused by bone metastasis is considered neuropathic pain, regulated and transmitted by the primary efferent nociceptor located around the nerve. Different types of receptors contribute to detecting harmful stimuli, such as inflammatory molecules, heat, lipid metabolites, and acids. The persistent inflammatory and acidic environment at metastatic lesions lead to persistent irritation, hyperalgesia (hypersensitivity to pain), and ectopic pain (central pain sensitization).9 Radiotherapy is the gold standard for treating bone metastases pain10 and can effectively relieve bone metastases pain and improve the QOL of metastatic patients.11 Nevertheless, the mechanisms underlying pain relief after radiotherapy are unclear. Candidate cytokines can predict the efficacy of radiotherapy for cancer-induced bone pain, and their expression levels pro- and post- radiotherapy are related to analgesia.12 However, there is no substantial evidence of an interaction between radiotherapy and cytokines.13 Therefore, in the present study, we analyzed the expression levels of cytokines in peripheral blood of breast cancer patients with bone metastasis pain pre- and post-radiotherapy. We also evaluated the pain relief degree and provided strong evidence for the association between cytokines and bone metastasis relief after radiotherapy.
Methods
Patients
Enrolled patients were divided into three groups: A: pre-radiotherapy, B: post-radiotherapy, and C: healthy individuals (controls). Except for the healthy controls, all patients who met the following criteria were included: 1) female; 2) breast cancer with bone metastasis; 3) no other internal organ metastases; 4) no radiotherapy or chemotherapy within four weeks; 5) bone metastases were diagnosed using positron emission or radiological computed tomography (CT). Magnetic resonance imaging (MRI) and CT were conducted to evaluate bone destruction. Additionally, patients with long-term nonsteroidal and glucocorticoid anti-inflammatory drugs, psychiatric disorders, immune system diseases, and infectious diseases were excluded. Ten healthy individuals between 18 and 50 years with no chronic disease or long-term medication were recruited from the physical examination center. All enrolled patients were followed up until the end of this study.
Measurement of Cytokines
After collecting peripheral venous blood, samples were centrifuged for 5 min at 3000 rpm and 4 °C to separate and collect blood serum. The BCA assay (KangChen KC-430) was conducted to determine protein concentrations. Serum samples were stored at −80 °C until further analysis.
The Human Cytokine Array G5 membrane (Raybiotech #AAH-CYH-G5) was designed and used to detect serum cytokines (n = 80). Briefly, after 30-min blocking with blocking buffer, samples were incubated overnight at 4 °C or for 1–2 h at room temperature. Then, membranes were washed and incubated for 1–2 h with diluted biotin-conjugated antibodies at room temperature. After washing, samples were incubated with streptavidin-coupled fluorescence at room temperature and rewashed. An Axon scanner was used to scan and record the signals. Densitometry was used to quantify the intensities of signals collected to evaluate the expression of cytokines. After eliminating background signals, cytokine expression values were normalized to median values, and protein expression fold changes (FCs) were calculated.
Statistical Analysis
SPSS 23.0 was used for data analysis. All data were normally distributed, and the Student’s t-test was carried out for pairwise comparisons. One-way ANOVA was performed to analyze the variance homogeneity and compare the results from multiple groups. Pearson’s correlation test was conducted to evaluate the correlation between indicated parameters. A p < 0.05 was considered statistically significant.
Results
Patient Clinical Characteristics
To meet the minimum standard for statistical analysis,14 we enrolled 30 bone metastasis-associated pain cancer patients from June 2017 to May 2021. Cytological, radiological, and clinical examinations were carried out to diagnose patients, and a visual analog scale (VAS) was used to evaluate pain levels. The mean age of patients was 48.06 ± 11.94 years (minimum 25 and maximum 70). Ten healthy women without chronic disease or long-term medication history were enrolled as controls (average age of 40.5 ± 11.1 years). Since the normal expression levels of cytokines were not obtained after the inclusion of tumor patients, we used the expression of cytokines in healthy controls as the baseline to measure increases or decreases in tumor patients before and after radiotherapy. The mean VAS pre- and post-radiotherapy were 5.56 ± 1.34 and 3.90 ± 2.31, respectively. The mean QOL scores were 27.00 ± 5.70 and 47.87 ± 9.17. Both the pain score [odds ration (OR): 0.004, 95% confidence interval (CI): 0.000–0.081, p < 0.001] and QOL score (OR: 0.278, 95% CI: 0.094–0.823, p < 0.001) significantly improved after radiotherapy. The baseline characteristics of patients are presented in Table 1.
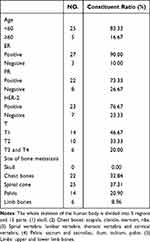 |
Table 1 Baseline Characteristics |
Identification of Pain-Associated Cytokines
We used the Human Cytokine Array G5 antibody chip to measure the production of 80 pain-related cytokines in serum. Volcano plots (FC ≥ 1.5 or ≤ −1.5, p < 0.05) were used to determine differentially expressed cytokines between group pairs (Figure 1). Twenty-one cytokines were differentially expressed between groups A and C. Three cytokines were differentially expressed between groups B and C. Additionally, 24 cytokines were differentially expressed between groups B and A. Differentially expressed cytokines in the three groups are shown in Figure 2. Compared to healthy controls, LEPTIN, MIP-1δ, MIF, and IL-3 were downregulated in group A, while MDC, LIGHT, THPO, IGPBP1, CKβ8-1, BDNF, OPG, IGFBP3, PDGF-BB, TGF-β3, CCL26, VEGF-A, CCL24, TGF-β2, FGF4, MCP-2, and Flt-3LG were upregulated. Compared to group B, IL-3, MIP-3α, MCP-4, FGF7, SCF, and BLC were downregulated in group A, and HGF, IGFBP4, MIG, MDC, Flt-3LG, IL-5, THPO, CX3CL1, OPG, IL-6, IFN-γ, VEGF-A, I-309, GRO, OSM, LIF, IGFBP3, and NAP-2 were upregulated. Only MIP, GRO, and LEPTIN were upregulated in group B compared to healthy controls.
Clustering Analysis of Differentially Expressed Cytokines
Further, we performed cluster analysis and built heatmaps for the 24 differentially expressed cytokines pre- and post-radiotherapy and the 21 differentially expressed cytokines pre- and post-radiotherapy compared to healthy controls (Figure 3).
The corresponding MIP-3α, IL-3, FGF7, SCF, MCP4, and BLC regions are mainly red in Figure 3A, indicating that the samples represented by these cytokines were highly expressed in this region. HGF, IGFBP4, MIG, MDC, Flt-3LG, IL-5, THPO, CX3CL1, OPG, IL-6, IFN-γ, VEGF-A, I-309, GRO, OSM, LIF, IGFBP3, and NAP-2 were higher post-radiotherapy than pre-radiotherapy, and the post-radiotherapy area is dominated by red in the heatmap. The expression levels of LEPTIN, MIP-1δ, MIF, and IL-3 were higher in healthy controls than in pre-radiotherapy. The corresponding area of healthy controls in the heatmap is redder than in pre-radiotherapy. The expression levels of MDC, LIGHT, THPO, IGPBP1, CKβ8-1, OPG, IGFBP3, TGF-β3, CCL26, VEGF-A, CCL24, TGF-β2, FGF4, MCP-2, and Flt-3LG were higher in healthy controls than in tumor patients pre-radiotherapy. In the heatmap, the pre-radiotherapy corresponding area is dominated by green, and in healthy controls is dominated by red. However, PDGF-BB and BDNF are not evident in the heatmap.
Discussion
Although significant progress has been made in the early diagnosis and treatment of breast cancer, 20 to 30% of early breast cancer patients might develop tumor recurrence or distant metastasis, with the most common site being the bone.15 Bone adverse events significantly negatively impact patients’ survival, activity, and QOL, thereby posing a significant threat to women’s health. We believe that local radiotherapy for breast cancer patients with bone metastases can help restore the bone microenvironment, reduce the occurrence of bone adverse events, and improve patient prognosis.
Multivariate analysis of risk factors for early bone metastasis of breast cancer found that age, primary tumor size, and the number of axillary metastatic lymph nodes may be the influencing factors for early bone metastasis of breast cancer. For example, age > 59 years old, tumor stage T2 and above, and the number of axillary lymph node metastasis > 10 were independent risk factors for early bone metastasis of breast cancer. At the same time, the above two factors are also related to the OS of breast cancer patients. The larger the primary tumor, the more the number of axillary lymph node metastases, which often indicates a worse prognosis.16–18 The local pain score and quality of life of the patients with early bone metastasis of breast cancer who were included in this study were significantly improved after radiotherapy. We were surprised to find that the expression level of cytokines after radiotherapy was not significantly different from that of healthy people, except for three cytokines. We speculate that the improvement of OS in patients with early bone metastasis of breast cancer by radiotherapy may be related to the expression level of cytokines. However, the human immune system, tumor microenvironment, and local microenvironment of bone tissue are closely related to cytokines.
The underlying mechanisms of the crosstalk between breast cancer cells, immune system, and bone have been intensively investigated, and many studies have determined the specific roles of different cells in bone metastasis. The bone microenvironment significantly participates in bone metastasis and the growth of some tumors. Moreover, various immunomodulatory cytokines influence the fate of osteocytes. Many studies have shown the relationship between cytokine expression and prognosis during radiotherapy. Recent reports have also shown the effects of TGF-β in the adaptation of cancer cells to radiotherapy. For example, TGF-β family members can regulate the proliferation, differentiation, apoptosis, EMT, and metastasis of cancer cells. TGF-β is a well-known and multitasking cytokine that regulates various reactions and interactions within the tumor and normal tissues, protecting normal tissue and inhibiting tumor sensitization.19,20 Platelet-derived growth factor (PDGF) is also associated with a poor prognosis of various malignancies. Er et al21 found that the decrease of PDGF-BB expression in peripheral blood after radiotherapy can improve the prognosis of patients. It is speculated that radiotherapy would regulate the expression of cytokines through TGF-β/PDGF pathways. Besides, breast cancer risk is significantly connected to IGF-I and its binding proteins (BPs). Radiotherapy can dramatically decrease IGF-I production in patients, suggesting its independent prognostic value and influence on breast cancer recurrence.22 Additionally, low baseline PDGF-AA, MCP-2, TGF-β1, TNF-α, and high IFN-γ are significantly associated with prolonged progression-free survival of patients.23
Herein, we found that 21 cytokines were differentially expressed between pre-radiotherapy samples and healthy controls, 24 between pre- and post-radiotherapy, and three between post-radiotherapy and healthy controls. No significant difference was detected in cytokine expression between breast cancer patients with bone metastases and healthy controls after radiotherapy. Moreover, breast cancer patients experienced significantly improved pain and QOL after radiotherapy, correlated with cytokine expression levels in peripheral blood. Thus, we believe that the pain of breast cancer patients with bone metastases treated with radiotherapy is closely related to cytokines. The abscopal effect induced by radiotherapy might depend on the immune system’s activation. The molecular and cellular effects of radiotherapy on the tumor microenvironment can also help prime and propagate antitumor immunity.24 Bone metastases represent the local tumor microenvironment of breast cancer in bone tissue, and radiotherapy for bone metastases activates the production of proinflammatory cytokines. Therefore, our current results provided evidence for a close link between radiotherapy and immunotherapy.
Pain is common in breast cancer patients with bone metastases. If the bone is the only metastatic site of breast cancer patients, early local radiotherapy can restore the microenvironment, reduce the occurrence of bone adverse events, and improve the QOL and prognosis. However, our current study also has limitations. For example, due to the economic constraints and strict inclusion criteria, this study had a small sample size. Therefore, a larger cohort is needed to validate our conclusions in the future.
Conclusion
Tumor cells can effectively activate immune cells in the bone microenvironment through direct interaction, releasing many factors and promoting bone metastasis. Early local radiotherapy of bone metastases can restore the microenvironment and improve the QOL and prognosis of patients, comprehending a novel target for prevention, treatment, and therapy.
Ethics Approval
This study was approved by the ethics committee of Huzhou Hospital, School of Medicine, Zhejiang University (Huzhou Central Hospital) (LS20191114-01), China. Patients from Huzhou Central Hospital were obtained in our preclinical research with informed consent (Version 2.0, 2019-11-18).
Patient Consent for Publication
Obtained.
Acknowledgment
This work was supported by the Funds of Huzhou Municipal Science and Technology Bureau (Grant No. 2019GY35).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Yardley DA. Pharmacologic management of bone-related complications and bone metastases in postmenopausal women with hormone receptor-positive breast cancer. Breast Cancer. 2016;8:73–82. doi:10.2147/BCTT.S97963
4. Jensen A, Jacobsen JB, Nørgaard M, et al. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer. 2011;11(1):29. doi:10.1186/1471-2407-11-29
5. Negishi-Koga T, Shinohara M, Komatsu N, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17(11):1473–1480. doi:10.1038/nm.2489
6. Paiva KBS, Granjeiro JM. Matrix metalloproteinases in bone resorption, remodeling, and repair. Prog Mol Biol Transl Sci. 2017;148:203–303. doi:10.1016/bs.pmbts.2017.05.001
7. Brook N, Brook E, Dharmarajan A, et al. Breast cancer bone metastases: pathogenesis and therapeutic targets. Int J Biochem Cell Biol. 2018;96:63–78. doi:10.1016/j.biocel.2018.01.003
8. Yao M, Brummer G, Acevedo D, et al. Cytokine regulation of metastasis and tumorigenicity. Adv Cancer Res. 2016;132:265–367. doi:10.1016/bs.acr.2016.05.005
9. Irshad I, Varamini P. Different targeting strategies for treating breast cancer bone metastases. Curr Pharm Des. 2018;24(28):3320–3331. doi:10.2174/1381612824666180619165728
10. Rich SE, Chow R, Raman S, et al. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol. 2018;126(3):547–557. doi:10.1016/j.radonc.2018.01.003
11. Faris A, Expósito J, Martínez-única A, et al. The efficacy of three-dimensional conformal radiation therapy on pain and quality of life in patients with painful bone metastases: a prospective study. Croat Med J. 2020;61(3):215–222. doi:10.3325/cmj.2020.61.215
12. MacLeod K, Laird BJA, Carragher NO, et al. Predicting response to radiotherapy in cancer-induced bone pain: cytokines as a potential biomarker? Clin Oncol. 2020;32(10):e203–e208. doi:10.1016/j.clon.2020.03.010
13. Koukourakis IM, Koukourakis MI. Combining the past and present to advance immuno-radiotherapy of cancer. Int Rev Immunol. 2021;1–17. doi:10.1080/08830185.2021.1974020
14. Box JF. Guinness, gosset, fisher, and small samples. Statist Sci. 1987;2(1). doi:10.1214/ss/1177013437
15. Cook GJ, Azad GK, Goh V. Imaging bone metastases in breast cancer: staging and response assessment. J Nucl Med. 2016;57(Suppl 1):27s–33s. doi:10.2967/jnumed.115.157867
16. Pulido C, Vendrell I, Ferreira AR, et al. Bone metastasis risk factors in breast cancer. Ecancermedicalscience. 2017;11:715. doi:10.3332/ecancer.2017.715
17. Purushotham A, Shamil E, Cariati M, et al. Age at diagnosis and distant metastasis in breast cancer--a surprising inverse relationship. Eur J Cancer. 2014;50(10):1697–1705. doi:10.1016/j.ejca.2014.04.002
18. Diessner J, Wischnewsky M, Stüber T, et al. Evaluation of clinical parameters influencing the development of bone metastasis in breast cancer. BMC Cancer. 2016;16:307. doi:10.1186/s12885-016-2345-7
19. Farhood B, Khodamoradi E, Hoseini-Ghahfarokhi M, et al. TGF-β in radiotherapy: mechanisms of tumor resistance and normal tissues injury. Pharmacol Res. 2020;155:104745. doi:10.1016/j.phrs.2020.104745
20. Wang J, Xu Z, Wang Z, et al. TGF-beta signaling in cancer radiotherapy. Cytokine. 2021;148:155709. doi:10.1016/j.cyto.2021.155709
21. Er P, Qian D, Zhang W, et al. The expression of PDGF-BB predicts curative effect in locally advanced esophageal squamous cell carcinoma treated by radiotherapy. Aging. 2020;12(8):6586–6599. doi:10.18632/aging.102993
22. Rosendahl AH, Björner S, Ygland Rödström M, et al. Pre- and postoperative circulating IGF-I, IGFBP-3, and IGFBP-7 levels in relation to endocrine treatment and breast cancer recurrence: a nested case-control study. Front Oncol. 2021;11:626058. doi:10.3389/fonc.2021.626058
23. Lyu X, Luo B. Prognostic factors and survival prediction in HER2-positive breast cancer with bone metastases: a retrospective cohort study. Cancer Med. 2021;10(22):8114–8126. doi:10.1002/cam4.4326
24. Patel RB, Hernandez R, Carlson P, et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci Transl Med. 2021;13:602. doi:10.1126/scitranslmed.abb3631
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



