Back to Journals » Cancer Management and Research » Volume 13
Predicting Lymph Node Involvement in Borderline Ovarian Tumors with a Quantitative Model and Nomogram: A Retrospective Cohort Study
Authors Zhang M, Zhou F, He Y, Tao X , Hua K , Ding J
Received 19 October 2020
Accepted for publication 9 January 2021
Published 16 February 2021 Volume 2021:13 Pages 1529—1539
DOI https://doi.org/10.2147/CMAR.S287509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Menglei Zhang,1,2,* Fangyue Zhou,1,* Yuan He,3 Xiang Tao,4 Keqin Hua,1,2 Jingxin Ding1,2
1Department of Gynecology, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, 200011, People’s Republic of China; 2Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Shanghai, 200011, People’s Republic of China; 3Public Health School of Fudan University, Shanghai, 200032, People’s Republic of China; 4Department of Pathology, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, 200011, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingxin Ding
Department of Gynecology, The Obstetrics and Gynecology Hospital of Fudan University, 128 Shen-Yang Road, Shanghai, 200011, People’s Republic of China
Tel +86-13918206746
Fax +86-21-63455090
Email [email protected]
Purpose: This study aimed to establish a predictive model for lymph node involvement (LNI) in patients with borderline ovarian tumor (BOT) using clinicopathological factors.
Patients and Methods: We collected clinical data from consecutive patients who underwent lymphadenectomy for BOT between 2001 and 2018 and analyzed their clinicopathological features. Multivariate logistic regression was used to identify all independent risk factors associated with LNI; these were then incorporated into the prediction model.
Results: In total, we included 248 patients with BOT who were undergoing lymphadenectomy. These were divided into a training cohort (n=174) and a validation cohort (n=74). When considering histopathological data, 16 and 5 patients were identified to have LNI in the training and validation cohorts, respectively. Overall, 13.5% (21/156) patients with serous BOT had LNI while 0% (0/92) patients with non-serous BOT had LNI. We identified several predictors of LNI: the largest tumor being ≥ 12.2cm in diameter, the presence of lesions on the ovarian surface, and the presence of pelvic or abdominal lesions. We created a prediction model and nomogram that incorporated these three risk factors for serous BOT. The model achieved good discriminatory abilities of 0.951 and 0.848 when predicting LNI in the training and validation cohorts, respectively. The LNI-predicting nomogram had an area under curve (AUC) of 0.951 and generated well-fitted calibration curves.
Conclusion: Non-serous BOT may not require lymphadenectomy as part of surgical staging. The individual risk of LNI in patients with serous BOT can be accurately estimated using our prediction model and nomogram. The use of LNI criteria provides a practical way to support the clinician in making an optimal decision relating to surgical scope for patients with BOT.
Keywords: lymph node involvement, borderline ovarian tumor, lymphadenectomy, prediction model, nomogram
Introduction
Borderline ovarian tumor (BOT) lies between benign and malignant ovarian epithelial tumors with regard to clinical manifestations and histomorphology; collectively, BOTs account for 10–20% of all ovarian epithelial tumors.1 The standard treatment for BOT is comprehensive surgical staging, although the role of lymphadenectomy remains controversial. According to the 2020 NCCN Guidelines, lymphadenectomy may upstage patients but does not affect overall survival.2 Some researchers3–5 have shown that lymphadenectomy is related to progression-free survival (PFS) but not to the overall survival of BOT patients. For example, Shazly et al6 did not recommend routine lymph node dissection for BOT because of procedure-associated trauma. However, surgery without lymphadenectomy may leave residual tumor tissue in patients with BOT who also have lymph node involvement (LNI). The long-term outcomes of these patients remain unknown.
Therefore, it is crucial that we are able to accurately estimate the risk of LNI in BOT patients if we are to optimize the therapeutic effect. If a patient is considered to have a high risk of LNI, then lymph node resection should be needed for curative intent. On the other hand, for those at a low risk of LNI, lymph node dissection may increase the risk procedure-associated trauma and thus outweigh the potential benefit to such patients. Unfortunately, the diagnosis of LNI is not feasible for patients who undergo surgery without lymphadenectomy. This is due to the fact that the diagnosis of LNI is determined by histopathological examination of lymph node tissue obtained from surgical resection. Therefore, it is desirable to develop a predictive model that incorporates factors associated with LNI based on clinicopathological data. The aim of this study was to establish a prediction model that features clinicopathological factors and the risk of LNI to help doctors make surgery-related decisions for BOT patients.
Patients and Methods
Study Population
We retrospectively reviewed patients with BOT who underwent operative treatment in the Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China, between February 2001 and December 2018. Patients who met the following inclusion criteria were included in this study: (i) borderline ovarian tumor; (ii) primary surgical treatment including lymphadenectomy; and (iii) no adjuvant therapy prior to surgery. We excluded patients who met any one of the following conditions: (i) multiple primary cancers of different pathological types; (ii) incomplete systemic lymph node dissection during surgery; and (iii) incomplete clinical dataset and patients who refused surgery. Figure 1 depicts the process used to recruit participants: 248 patients met the inclusion criteria and entered the study. Of these, 174 patients were included into the training cohort to establish the model, and 74 patients were entered into the validation cohort.
 |
Figure 1 Diagram showing how participants were recruited. |
Surgical Procedures
We performed surgery for BOT patients as described in the 2020 NCCN Guidelines 2020.2 On entering the abdomen, we aspirated ascites or performed peritoneal lavage so that we could carry out peritoneal cytological examinations. All peritoneal surfaces were visualized and any peritoneal surface or adhesion that was suspicious for harboring involvement was selectively excised or biopsied. Some patients did not wish to preserve their fertility; for these, we performed bilateral salpingoophorectomyand hysterectomy, making sure that we kept the encapsulated mass intact during removal. For patients who wished to preserve their fertility, we performed unilateral or bilateral salpingoophorectomywith uterine preservation. Omentectomy was performed for every patient. The dissection of the pelvic lymph nodes included the bilateral removal of lymph nodes overlying and anterolateral to the common iliac vessel, overlying and medial to the external iliac vessel, overlying and medial to the hypogastric vessels, and from the obturator fossa at a minimum anterior to the obturator nerve. Para-aortic lymph node dissection was performed by stripping the nodal tissue from the vena cava and the aorta bilaterally to at least the level of the inferior mesenteric artery, and preferably to the level of the renal vessels.
Clinicopathological Variables
We collected a range of clinical characteristics, including age, the presence of comorbidities, previous histories of abdominal surgery, parity, menopause, and the serum levels of preoperative serum tumor markers, including CA-125, CA-199, and CEA; the criteria for a positive diagnosis when considering these three serum markers were are ≥35U/mL, ≥37U/mL, and ≥5ng/mL, respectively. We made detailed notes of any intraoperative evaluations, including tumor diameter, tumor location, tumor rupture, macroscopic lesions on the ovarian surface, macroscopic lesions (>1cm) in the pelvic or abdominal cavity, and frozen pathology. For histopathological assessment, the slides prepared from surgical specimens were assessed independently by two experienced pathologists. The final classification was based on the consensus diagnosis of these two pathologists.
Statistical Analysis
Comparisons between groups were performed using the Student’s t-test for continuous variables and the Chi-squared test for categorical variables (Fisher’s test was used when the number of variables was < 5). Data were randomly partitioned into a training cohort and a validation cohort. The risk factors for LNI in the training cohort were evaluated by both univariate analysis and multivariable analysis. A prediction model was established using the independent risk factors identified by multivariable analysis. Receiver operating characteristic (ROC) curve analysis was then used to calculate the optimal cut-off values by maximizing the Youden index. We also acquired the area under ROC curve (AUC) to calculate predicted values.
A nomogram was constructed to calculate a patient’s risk of LNI by considering points related to each risk factor and distinguishing patients who have a low probability of LNI. The predictive performance of the nomogram was measured by AUC and was calibrated with 1000 bootstrap samples to reduce the overfitting deviation and align agreement with the current state. Statistical analyses were performed using SPSS 22.0 software and R statistical software (version 3.3). Differences were considered to be statistically significant when P<0.05.
Results
Characteristics of the Study Population
During the study period, 1328 consecutive patients with BOT underwent surgery. Of these, 248 patients who met the inclusion criteria were included in this study; 174 and 74 patients were divided into the training and validation cohorts, respectively. The clinicopathological characteristics of the patients are listed in Table 1. The baseline clinicopathologic data were similar between the training and validation cohorts. The rate of LNI was 9.2% (16/174) and 6.8% (5/74) in the two cohorts, respectively.
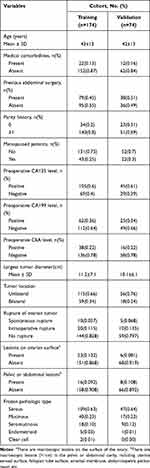 |
Table 1 Clinicopathologic Characteristics of the Study Population |
Risk Factors for LNI in the Training Cohort and the Creation of a Prediction Model
Next, we analyzed the association between clinicopathological factors and the presence of LNI in the patients in the training cohort (Table 2). Univariate analysis showed that preoperative CA125 level (P=0.009), largest tumor diameter (P=0.004), tumor location (P<0.001), the presence of lesions on the ovarian surface (P<0.001), the presence of pelvic or abdominal lesions (P<0.001), and frozen pathologic type (P<0.001), were significantly associated with LNI. The remaining variables had no significant association with LNI.
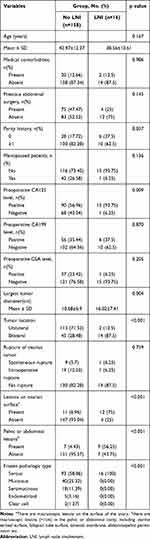 |
Table 2 Characteristics of Patients in Training Cohort |
We determined that the optimal largest tumor diameter cutoff value was 12.2cm by maximizing the Youden index (Table 3). Therefore, the tumors were stratified as ≥12.2cm or <12.2cm. Using largest tumor diameter alone, the area under the ROC curve was 0.723 (95% confidence interval [CI]: 0.603–0.843) (Figure 2). The frozen pathologic type of all of the LNI patients investigated was serous BOT; this factor was not included in the regression analysis.
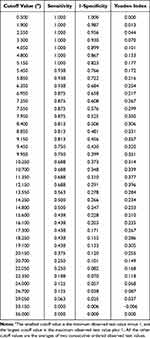 |
Table 3 Largest Tumor Diameter Cutoff Values with Their Sensitivity, Specificity, and Youden Index |
 |
Figure 2 Receiver operator curve (ROC) for the largest tumor diameter cutoff values in the training cohort. The area under the ROC was 0.723 (95% CI: 0.603–0.843). |
Univariate and multivariate logistic analysis demonstrated that a largest tumor diameter ≥12.2cm (odds ratio [OR]=5.66; P=0.029), the presence of lesions on the ovarian surface (OR=28.31; P<0.001), and the presence of pelvic or abdominal lesions (OR=5.98; P=0.034), were all significant predictors of LNI (Table 4).
 |
Table 4 Univariate and Multivariate Predictors of Lymph Node Involvement in Training Cohort |
Independent risk factors that had been identified and confirmed by multivariate analysis were then used to generate a predictive model. The estimated possibility of LNI was calculated for each patient. The ROC curve of the estimated values was generated and the area under the ROC curve that estimates the model’s discriminatory ability was 0.951 (95% CI: 0.911–0.991) (Figure 3A).
According to the maximized Youden’s index, the optimal clinically applicable cutoff value for estimated risks was 0.13 (Table 5). Therefore, we defined the predicted risk groups using the cutoff values at 13% of the estimated possibility of LNI (Table 6). As listed in Table 6, LNI was observed in 1.35% (2/148) and 53.85% (14/26) of the low-risk and high-risk groups in the training cohort, respectively.
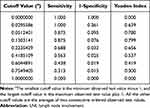 |
Table 5 Cutoff Values of Estimated LNI Risks with Their Sensitivity, Specificity, and Youden’s Index |
 |
Table 6 Predicted Risk Groups Based on Estimated LNI Possibility with Observed LNI Rate in Two Cohorts |
Development of a Nomogram to Predict LNI
Independently associated risk factors were used to form a nomogram to predict LNI and thus facilitate clinicians to use the prediction model described above (Figure 4A). The nomogram demonstrated good levels of accuracy for estimating the risk of LNI with an AUC of 0.951 (95% CI, 0.911–0.991). In addition, calibration plots showed good levels of agreement with regard to the presence of LNI when compared between risk estimation by the nomogram and the histopathological confirmation on surgical specimens in the training cohort (Figure 4B).
Validation of the Prediction Model
For model validation, the fitted model derived from the training cohort was applied to the validation cohort. This allowed us to produce an estimated value of the possibility of risk for LNI in each patient. All patients in the validation cohort were assigned to a risk group based on their resulting value and LNI was identified in 1 out of 65 cases (1.54%) in the low-risk group and 14 out of 26 cases (53.85%) in the high-risk group (Table 6). A ROC curve for estimated value in the validation cohort was generated and the area under the ROC was 0.848 (95% CI: 0.590–1.000) (Figure 3B), thus demonstrating that this model had good discriminatory power. Statistical analysis also demonstrated that the ROC curves showed high levels of uniformity when determining risk scores between training and validation cohorts (Table 7).
 |
Table 7 Statistical Analysis of ROC Curves of Risk Scores Between Training and Validation Cohorts |
The performance of the model was also examined in the nomogram using the validation cohort. Calibration plots showed good levels of agreement for the presence of LNI when estimating risk by the nomogram and by the histopathological confirmation of surgical specimens in the validation cohort (Figure 4C).
LNI Criteria for Lymphadenectomy
The “LNI Criteria” are a series of intermediate risk factors that are used to guide lymphadenectomy decisions, including: 1) frozen pathological type; 2) largest tumor diameter; 3) pelvic or abdominal lesions; or 4) lesions on the ovarian surface (Table 8). If BOT patients meet the LNI Criteria then lymphadenectomy might be recommended.
 |
Table 8 LNI Criteria for Lymphadenectomy in Borderline Ovarian Tumor |
Discussion
Complete staging is currently the standard surgery treatment for patients with BOT. However, lymphadenectomy as a component of surgical staging remains a subject of significant debate. The lymph nodes may represent the recurrent site of BOT and the first or only site of transformation to a carcinoma.7,8 Longacre et al previously reported that 10% of such carcinoma transformations occurred in lymph nodes.7 In another study, lymph nodes represented the only site of extraovarian disease in 22% of LNI cases; furthermore, disease recurred exclusively in the lymph nodes as low-grade serous carcinoma in 6% of these cases.9 It is theoretically possible that such transformed nodes will be present during the initial tumor resection and will be missed if surgical staging does not include lymphadenectomy. These observations underscore the importance of lymphadenectomy as a necessary part of the staging procedure for BOT surgeries.
Several investigators have suggested that LNI does not exert impact on overall survival.7,10,11 However, LNI has been reported to reduce PFS in other studies.4,12,13 For example, McKenney et al11 analyzed the precise morphology of LNI in 31 cases of serous BOT and identified a significant reduction in disease-free survival among patients with LNI exceeding 1 mm in size. In another study, a total of 99 BOT patients underwent lymphadenectomy; this procedure was significantly associated with improved PFS.3 It was also noted that 8.4% of these patients had pelvic LNI and that 16.7% of patients had para-aortic LNI. It is plausible that lymphadenectomy may improve PFS by removing LNI.
However, there are also some drawbacks associated with lymphadenectomy, including prolonged operation time and increased surgical risk. The sequelae associated with lymphadenectomy can be life-long and reduce the quality of life.14 Therefore, performing lymphadenectomy only in a selected group of patients with a high risk of LNI would be ideal. No specific guidelines have yet been developed for selecting lymphadenectomy in BOT cases. The oncological safety of surgery without lymphadenectomy for BOT patients with potential LNI remains controversial. This is mostly due to the fact that the evidence of LNI is not available for patients who undergo surgery without lymphadenectomy; this is because LNI is determined by the histological examination of lymph nodes obtained during surgical resection. In the present study, we developed an accurate model to predict LNI in BOT using clinicopathological data that can be obtained before and during surgery.
We found that all BOT patients with LNI had serous tumors; this finding was consistent with previous literature.10,12,15 In a database analysis of 4943 cases of stage T1 BOT,5 cases of serous BOT had a greater prevalence of LNI compared to other mucinous candidates. In a previous study, Cho et al evaluated 264 cases involving early stage mucinous ovarian tumors, including both borderline tumors and invasive carcinomas; the authors identified no cases with LNI.16 In another study, Moroney et al failed to identify any LNIs in any case involving a malignant or borderline primary mucinous ovary.17 Taken together, these results could suggest that mucinous BOT may not require lymphadenectomy as a component of surgical staging. However for serous BOT, the morbidity risks associated with lymphadenectomy and the benefits of staging information need to be considered carefully.
Our study retrospectively collected clinicopathological data from the training cohort as constructive information, and from the validation cohort as confirmatory information. Having analyzed these data using univariate and multivariate regression models, we then established a prediction model and nomogram to estimate the potential rates of LNI. This prediction model and nomogram incorporates three easily available risk factors: a largest tumor diameter ≥12.2cm, the presence of lesions on the ovarian surface, and the presence of pelvic or abdominal lesions; all of these are independently associated with LNI. As all the patients with LNI had serous BOT in this study, the predictive indications of the model are readily applicable to patients who have serous BOT.
In a previous study, Matsuo et al reported that a tumor diameter >5cm was significantly associated with LNI.5 Our present study also demonstrated that the optimal tumor diameter cutoff value was 12.2cm. In a previous study, Lesieur et al reported that the presence of macroscopic implants on the ovary surface was not associated with LNI in BOT patients;18 however, this previous study only featured 49 cases. Another study, reported by Fadare, suggested that tumors which were confined to the ovary and without ovarian surface involvement were rarely associated with nodal involvement;19 these findings were similar to our present findings. Previous studies reported that the probability of LNI in serous BOT increased with invasive and non-invasive peritoneal implants.9,10,20 For example, Lesieur et al reported that the presence of macroscopic implants on the omentum was significantly associated with LNI.18 In a study reported by Fadare, microinvasive borderline tumors with lymphatic vessel involvement showed a remarkably low frequency of LNI; furthermore, there were no significant differences between LN-positive and LN-negative tumors in terms of lymphatic vessel density.19 All of these findings suggested that the possible route of LNI, at least in most cases, was via the peritoneum rather than lymphatic vasculature. This may explain why we found that the presence of pelvic or abdominal lesions was a significant predictor of LNI.
Using our prediction model and nomogram, we calculated a score that represented the risk of LNI for each patient in the training cohort. ROC analysis of these risk scores was then applied to acquire optimal cut-off values that were determined by maximizing the Youden index and area under the ROC. This strategy clearly demonstrated the model’s ability to discriminate between the estimated and observed LNI results.
The predictive model and nomogram also demonstrated good levels of accuracy when estimating the risk of LNI in the validation cohort. Based on the cut-off values, patients were divided into two categories: low-risk and high-risk. According to this classification, the rates of LNI for the two groups were 1.35% and 53.85% in the training cohort and 1.54% and 44.44% in the validation cohort. Statistical differences were evident between the low-risk and high-risk groups both in the training and validation cohorts; there was no significant difference when comparing the same risk groups between the two cohorts. Furthermore, there were no statistical differences between the two cohorts in terms of basic characteristics. These results further illustrated that the predictive model and nomogram established from the training cohort could be applied to the validation cohort.
When using our nomogram clinically, we defined 81 as the cutoff value for estimating the risk of LNI. Patients with a score > 81 represent a high-risk subgroup of LNI with an estimated risk of LNI that is higher than 13%. Patients with a score of 81 or less are a low-risk subgroup of LNI with an estimated risk of 13% or lower. More conveniently, we prepared a list of “LNI Criteria” for lymphadenectomy in BOT patients. Based on these criteria, doctors could make further surgical choices for BOT patients so that they could achieve better long-term outcomes. For patients meeting these criteria, lymph node resection might be recommended for curative reasons. For patients not meeting the criteria, a surgical approach without lymphadenectomy might be safe and sufficient.
This study had some limitations that need to be considered. First, there may be statistical bias associated with the small sample size of LNI cases. The low number of LNI cases featured in this study may affect the validity of the predictive model. Second, our analysis was based on data from a single institution; it is possible that the results would be more generalizable if external validation was performed. Third, we did not evaluate the clinical outcomes of patients with BOT who did not undergo lymphadenectomy but potentially had LNI, as predicted by our model. Therefore, perspective clinical trials with long-term outcomes might be needed in order to further evaluate the accuracy and efficacy of this prediction model in BOT patients.
Conclusions
Non-serous BOT may not require lymphadenectomy as a component of surgical staging. The individual risk of LNI in patients with serous BOT can be accurately estimated using our predictive model and nomogram. Our newly developed “LNI Criteria” provides a practical way to support the clinician in making an optimal decision relating to surgical scope for patients with BOT.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was performed in accordance with the Declaration of Helsinki and with approval by the Obstetrics and Gynecology Hospital of Fudan University Institutional Review Board.
Informed Consent
Patients provided informed consent for the use of their clinical data for research purposes.
Acknowledgments
This study was supported by Fudan University’s “Tomorrow Star” Famous Physicians Cultivation Project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ludovisi M, Foo X, Mainenti S, Testa AC, Arora R, Jurkovic D. Ultrasound diagnosis of serous surface papillary borderline ovarian tumor: a case series with a review of the literature. J Clin Ultrasound. 2015;43(9):573–577. doi:10.1002/jcu.22266
2. Armstrong DK, Alvarez RD. NCCN guidelines version 1.2019 ovarian cancer. Natl Compr Canc Netw. 2019;17(8):896–909. doi:10.6004/jnccn.2019.0039
3. Chen X, Fang C, Zhu T, Zhang P, Yu A, Wang S. Identification of factors that impact recurrence in patients with borderline ovarian tumors. J Ovarian Res. 2017;10:1–8. doi:10.1186/s13048-017-0316-5
4. Ureyen I, Karalok A, Tasci T, et al. The factors predicting recurrence in patients with serous borderline ovarian tumor. Int J Gynecol Cancer. 2016;26(1):66–72. doi:10.1097/igc.0000000000000568
5. Matsuo K, Machida H, Takiuchi T, et al. Role of hysterectomy and lymphadenectomy in the management of early-stage borderline ovarian tumors. Gynecol Oncol. 2017;144(3):496–502. doi:10.1016/j.ygyno.2017.01.019
6. Shazly SA, Laughlin-Tommaso SK, Dowdy SC, Famuyide AO. Staging for low malignant potential ovarian tumors: a global perspective. Am J Obstet Gynecol. 2016;215(2):153–168.e2. doi:10.1016/j.ajog.2016.04.035
7. Longacre TA, McKenney JK, Tazelaar HD, Kempson RL, Hendrickson MR. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29(6):707–723. doi:10.1097/01.pas.0000164030.82810.db
8. Malpica A, Deavers MT, Gershenson D, Tortolero-Luna G, Silva EG. Serous tumors involving extra-abdominal/extra-pelvic sites after the diagnosis of an ovarian serous neoplasm of low malignant potential. Am J Surg Pathol. 2001;25(8):988–996. doi:10.1097/00000478-200108000-00002
9. Bojana Djordjevic M, Anais Malpica M. Lymph node involvement in ovarian serous tumors of low malignant potential: a clinicopathologic study of thirty-six cases. Am J Surg Pathol. 2010;34(1):1–9. doi:10.1097/PAS.0b013e3181c0a5ab
10. Camatte S, Morice P, Atallah D, et al. Lymph node disorders and prognostic value of nodal involvement in patients treated for a borderline ovarian tumor: an analysis of a series of 42 lymphadenectomies. J Am Coll Surg. 2002;195(3):332–338. doi:10.1016/s1072-7515(02)01250-4
11. McKenney JK, Balzer BL, Longacre TA. Lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors): pathology, prognosis, and proposed classification. Am J Surg Pathol. 2006;30(5):614–624. doi:10.1097/01.pas.0000194743.33540.e6
12. Leake JF, Rader JS, Woodruff JD, Rosenshein NB. Retroperitoneal lymphatic involvement with epithelial ovarian tumors of low malignant potential. Gynecol Oncol. 1991;42(2):124–130. doi:10.1016/0090-8258(91)90331-x
13. Leake JF, Currie JL, Rosenshein NB, Woodruff JD. Long-term follow-up of serous ovarian tumors of low malignant potential. Gynecol Oncol. 1992;47(2):150–158. doi:10.1016/0090-8258(92)90099-5
14. Leung EY, Tirlapur SA, Meads C. The management of secondary lower limb lymphoedema in cancer patients: a systematic review. Palliat Med. 2015;29(2):112–119. doi:10.1177/0269216314545803
15. Tamakoshi K, Kikkawa F, Nakashima N, et al. Clinical behavior of borderline ovarian tumors: a study of 150 cases. J Surg Oncol. 1997;64(2):147–152. doi:10.1002/(SICI)1096-9098(199702)64:2<147::AID-JSO11>3.0.CO;2-3
16. Cho YH, Kim DY, Kim JH, et al. Is complete surgical staging necessary in patients with stage I mucinous epithelial ovarian tumors? Gynecol Oncol. 2006;103(3):878–882. doi:10.1016/j.ygyno.2006.05.022
17. Moroney MR, Post MD, Berning AA, Sheeder J, Corr BR. An evaluation of frozen section and lymph node dissection results for mucinous ovarian tumors. Int J Gynecol Cancer. 2018;28(1):92–98. doi:10.1097/igc.0000000000001150
18. Lesieur B, Kane A, Duvillard P, et al. Prognostic value of lymph node involvement in ovarian serous borderline tumors. Am J Obstet Gynecol. 2011;204(5):
19. Oluwole Fadare M. Recent developments on the significance and pathogenesis of lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors). Int J Gynecol Cancer. 2009;19(1):103–108. doi:10.1111/IGC.0b013e3181991a49
20. Qian XQ, Hua XP, Wu JH, Shen YM, Cheng XD, Wan XY. Clinical predictors of recurrence and prognostic value of lymph node involvement in the serous borderline ovarian tumor. Int J Gynecol Cancer. 2018;28(2):279–284. doi:10.1097/igc.0000000000001154
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


