Back to Journals » Journal of Experimental Pharmacology » Volume 13
Preclinical Evaluation of the Antihypertensive Effect of an Aqueous Extract of Anogeissus leiocarpa (DC) Guill et Perr. Bark of Trunk in L-NAME-Induced Hypertensive Rat
Authors Belemnaba L , Nitiéma M, Ilboudo S, Ouédraogo GG, Ouédraogo N, Belemlilga MB, Compaoré S, Ouédraogo S, Ouédraogo S
Received 14 May 2021
Accepted for publication 19 July 2021
Published 7 August 2021 Volume 2021:13 Pages 739—754
DOI https://doi.org/10.2147/JEP.S319787
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Paola Rogliani
Lazare Belemnaba,1 Mathieu Nitiéma,1 Sylvain Ilboudo,1 Gueswindé Geoffroy Ouédraogo,1 Noufou Ouédraogo,1 Mohamed Bonewendé Belemlilga,1 Souleymane Compaoré,1,2 Salfo Ouédraogo,1,2 Sylvin Ouédraogo1
1Institut de Recherche en Sciences de la Santé/Centre National de la Recherche Scientifique et Technologique (IRSS/CNRST), 03 BP 7047, Ouagadougou, 03, Burkina Faso; 2Université Joseph KI-ZERBO, 03 BP 7021, Ouagadougou, 03, Burkina Faso
Correspondence: Lazare Belemnaba
Research Institute for Health Sciences (IRSS), Department of Medicine and Traditional Pharmacopeia (MEPHATRA/Ph), National Centre for Scientific and Technological Research (CNRST), 03 BP 7047, Ouagadougou, 03, Burkina Faso
Tel +226 25 36 33 64/25 36 32 15
Fax +226 25 36 28 38/25 36 03 94
Email [email protected]
Background: The present study investigates the effect of an aqueous extract of Anogeissus leiocarpa (AEAL) on normotensive Wistar rats and its chronic antihypertensive effects in L-NAME-induced hypertensive rats by using a non-invasive tail-cuff model.
Methods: The effects of AEAL (50mg/kg) and NaCl 0.9% on blood pressure were investigated by daily oral administration in normotensive Wistar rats over four weeks. L-NAME-induced hypertensive rats were produced by L-NAME (40mg/kg) daily oral administration for two weeks. For chronic antihypertensive effects, induced hypertensive rats have received L-NAME in combination with AEAL (10 or 50mg/kg/day) for two following weeks.
Results: In normotensive rats, daily administration of AEAL (50mg/kg) has no significant effect on their blood pressure, which was similar to that of the control group. L-NAME’s daily oral administration induces a progressive increase in systolic blood pressure (SBP) from 115.8 ± 7.9mmHg to 153.5 ± 4.6mmHg after two weeks, which was maintained to the end of the treatment. In L-NAME-induced hypertensive rats, AEAL (50mg/kg/day) significantly decreases the SPB from 160.0 ± 5.8 mmHg to 108.8 ± 2.7mmHg after only four days of administration. However, the lower dose of AEAL (10mg/kg) also normalized the SBP of L-NAME-induced hypertensive rats but only evident after seven days of administration. Moreover, AEAL does not effect on the serum biochemical parameters (ALAT, ASAT, CREAT, etc.) and any macroscopic adverse effect was detected on the sensible organs involved during hypertension. In the aorta rings from treated rats, AEAL (50mg/kg/day) alone or in combination with L-NAME has enhanced the vasodilation effect of acetylcholine. However, the vasodilation effect of AEAL alone or in association with L-NAME has enhanced the sodium nitroprusside effect in treated rat aorta rings after autopsy.
Conclusion: These findings suggest that AEAL affords significant antihypertensive effects against L-NAME-induced hypertensive rats without modification of serum parameters and deleterious effects.
Keywords: Anogeissus leiocarpa, non invasive, L-NAME-induced hypertension, vasorelaxation
Introduction
Hypertension or high blood pressure is a multifactorial disease associated with modifiable and non-modifiable risk factors.1 Moreover, hypertension is a serious chronic disease that causes mortality and morbidity worldwide. It is characterized as a sustained elevated level of systolic and/or diastolic blood pressure (≥140/90 mmHg). It is one of the most common emerging chronic diseases of developed and developing countries. Called an insidious “silent killer”, hypertension is a major risk factor for cardiovascular disease and kidney disease which increased the risk of other diseases such as stroke, coronary artery disease, and heart failure.2–5 Taken the specific case of the vascular system, endothelial dysfunction and hyperactive adrenergic-mediated vasoconstriction were known to contribute to peripheral vascular resistance increases, which results in blood pressure elevation.6,7
Moreover, vascular oxidative stress has been associated with impaired endothelial-dependent vasorelaxation and enhanced agonist-induced vasoconstriction in oxidative stress-related hypertension.8 Therefore, improving vasorelaxation and inhibiting oxidative stress are valuable strategies to combat hypertension. Nowadays, natural substances that have antioxidant and vasorelaxant activities have been the focus of studies, and are increasingly recognized for their use to prevent and treat hypertension.9 Indeed, once declared, the care is done all the life, and about 30–60% of hypertensive patients do not achieve blood pressure targets, which are in part to the cost of treatment.10 Indeed, the treatment of hypertension in modern medicine is expensive for African populations in general.11,12 In addition, there are issues related to the accessibility, side effects and effectiveness of conventional medicines.5 This caused a renewed interest in medicine and traditional pharmacopoeia for primary health needs and is based on plants therapeutic properties among which there is Anogeissus leiocarpa (DC) Guill & Perr. (Combretaceae). This specie is widely used in Burkina Faso by traditional healers for the treatment of several diseases, including hypertension.13 In addition to its antihypertensive properties, studies already showed antibacterial, antifungal and pest-destroying properties of Anogeissus leiocarpa.14–17 Previous studies have shown that extracts from Anogeissus leiocarpa possess a potent antioxidant activity, an inhibitory effect of purified phosphodiesterases and an in vitro vasodilation effect.18,19 Specifically, the AEAL is known to have an antihypertensive potential impact in the anesthetized model.20 However, the long-term effects of Anogeissus leiocarpa on high blood pressure and arteriolar function from treated animals have not yet been reported. For that, experiment was undertaken to investigate the antihypertensive effects of AEAL in L-NAME-induced hypertensive rats with a focus on cardiac parameters and peripheral vascular responsiveness as the possible underlying mechanisms of its antihypertensive effect.
Methods and Methods
Chemicals and Reagents
Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), Sodium Chloride, Phenylephrine hydrochloride, Acetylcholine chloride and other unspecified chemicals were from Sigma-Aldrich (France), Captopril Denk 25 were from Denk Pharma GmbH&Co (Germany).
Plant Material and Preparation
The process to obtain the Anogeissus leiocarpa (DC) Guill et Perr (Combretaceae) plant material and the preparation method leading to AEAL has been previously described.20 Briefly, the plant material was constituted by the barks of the trunk of Anogeissus leiocarpa. It was collected in May 2006 in Loumbila area (savanna area, Burkina Faso) which was located at 20 km in the east of Ouagadougou. A voucher specimen (N°1544) was deposit at the herbarium of the Department of Forest Production, National Centre for Scientific and Technological Research, Ouagadougou after authenticated by a botanist of this section.
Fresh samples were collected, dried safe from solar light and dust, and then grinded into a fine powder. An aqueous decoction was carried out with 120 g of bark powder in 2100 mL of water and boiled for 30 min with a reflux system in accordance with the traditional use. After cooling, filtration and centrifugation, the supernatant was collected and freeze-dried. The resulting extract named AEAL was then lyophilize and used for the pharmacological investigations.
In vivo Model of Hypertension and Experimental Protocol
Male normotensive Wistar rats, 191.70±24.25 g, from the Research Institute for Health Sciences (Burkina Faso) pet stores were used. Animals were placed in plastic cages in a room with controlled humidity (70%) at a temperature of 23 ± 2°C and on a 12:12 h light-dark cycle and had free access to rat chow and drinking water. They were fed on standard Rat chow and tap water ad libitum. During the experimental study, precautions were taken to ensure that pain and distress were minimized or relieved. Moreover, drinking fluid consumption and food quantity were controlled and adjusted, when necessary. All experimental animal procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health and the EU Directive 2010/63/EU for animal experiments.21 Ethical approval of the present study was obtained from the ethic committee of the University Joseph KI-ZERBO (Protocol number: CE-UOI/2019-04).
After one-week environmental acclimatization, Rats body weights were assessed and randomly divided into six (06) groups of five (05) animals each.
The first group was served as the control and has received a daily oral administration via gavage of Normal Saline (NaCl 0.9% group) for 4 weeks;
The second group was given a daily oral administration of AEAL at a dose of 50 mg/kg/day (AEAL 50 group) for 4 weeks;
The third group were given a daily oral administration of L-NAME at a dose of 40 mg/kg/day (L-NAME group) for 4 weeks.
From the fourth to the sixth group, all animals were given a daily oral administration of L-NAME at a dose of 40 mg/kg/day for 4 weeks. Particularly, after the L-NAME administration for the first two weeks, the fourth, fifth and sixth groups were simultaneously treated orally respectively with captopril at a dose of 5 mg/kg/day (L-NAME+Captopril group), AEAL at the doses of 50 mg/kg/day (L-NAME+AEAL 50 group) and 10 mg/kg/day (L-NAME+AEAL 10 group) for the two following weeks.
Measurement of Systolic Blood Pressure and Heart Rate
One week prior to the administration of drugs and chemicals substances, all animals were trained to the systolic blood pressure and heart rate measurement by non-invasive tail-cuff plethysmography (58,500 Blood Pressure Recorder System, Ugo Basile). For that, a group of 5 animals were placed every day in a Heating Box for Rats, each in its individual holder. After 30 min in the compact temperature-controlled “cupboard” (37°C), the tail-cuff plethysmography method was applied, and their blood pressure was measured. The value was taken as the average of at least four measurements after removing the outliers and any associated with excess noise or animal movement on each occasion. During the time of the experiment, a daily rat weight, systolic blood pressure and heart rate measurement were applied. The mean value of water and aliment consumption was estimated every week.
Autopsy and Vital Organs Weight Analysis
At the end of the fifth week, the animals were sacrificed by intraperitoneal administration of ketamine (150 mg/kg bw) and blood sample were collected by cardiac puncture. The heart, liver, lungs, kidneys, spleen and testes were excised, autopsied by macroscopic observation to detect any damage. All organs were then weighed on a Sartorius sensitive balance (Precision 0.1 mg) after they have been dried with a hygienic paper.
Biochemical Analysis
The Rat blood samples collected by cardiac puncture after the experiment end were transferred into dry tubes vacutainers. Samples were centrifuged at 3000 rpm for 10 min by using a table centrifuge (ROTOFIX 32A, Hettich Zentrifugen, Germany) and the sera were collected in Eppendorf tubes for the biochemical analysis. The automatic biochemistry analyzer (Mindray BS-300, China) has been used for the sera biochemical parameters determination which was transaminases (aspartate aminotransferase or ASAT and alanine aminotransferase or ALAT), Total proteins, Urea, Uric Acid, chloride, phosphorus, Calcium, Cholesterol, Creatinine, Glucose and triglyceride.
Vascular Reactivity Experiment
Beyond the organs removed for the autopsy, the thoracic aorta of each rat was gently removed for the vascular reactivity studies, which were performed as previously described.20 Briefly, the descending thoracic aorta was excised and immersed in a physiological Krebs–Henseleit solution (composition in mM: NaCl 119, KCl 4.7, CaCl2 1.5, NaHCO3 25, KH2PO4 1.2, MgSO4 1.1, and D-glucose 10) and bubbled with a pneumatic pump. The aorta was carefully cleaned of adhering fat and connective tissue and then cut into rings about 3–4 mm in length. Rings were then mounted vertically in a 20 mL organ bath chamber containing Krebs–Henseleit solution maintained at 37°C and equilibrated at a basal tension of 2 g for 1 h with washes every 15 min.
After the equilibration period, the vascular reactivity of rings was conduct by determining the contractile reference response to an isotonic KCl solution (80 mM). At the maximum of constriction, the rings were wash out of the responses to high K+. The presence of functional endothelium was assessed in all preparations by the ability of the endothelium-dependent vasodilator acetylcholine (10 µM) to induce more than 60% relaxation of rings precontracted with phenylephrine (1 µM).
The rings were then rewashed. Next, the relaxation responses to the cumulatively increasing concentration of acetylcholine (Ach, 10−9 M to 10–5 M) and of the endothelium-independent vasodilator sodium nitroprusside (SNP, 10−9 M to 10–5 M) were evaluated in precontracted aortic rings with phenylephrine (1 µM).
Statistical Analysis
Statistical analyses were carried out using either a two-way analysis of variance or a one-way analysis of variance (ANOVA) followed by ‘Bonferroni’s post hoc test to determine significant differences between treatments groups (GraphPad Prism 5.00.288 for Windows, GraphPad Software Inc., CA, USA). A P value less than 0.05 was considered statistically significant. All results were expressed as the mean ± standard error of the mean (SEM).
Results
Effect of AEAL on the SBP of Normotensive Wistar Rat
Results presented in Figure 1 showed no significant difference in SBP for all groups during the seven days before treatment. Daily oral administration of L-NAME (40 mg/kg) has progressively and significantly increased the SBP from 116.25±24.94 mmHg (day 7) in the L-NAME 40 group to 149.25±2.25 mmHg after two weeks of treatment (day 21), which was maintained to the end of the treatment (four weeks).
The daily oral administration of NaCl 0.9% in control NaCl 0.9% group did not modify the SBP of the group animals, which was of 108.83±1.45 mmHg at the beginning of the experiment and remained stable to the end of the administration.
The different results also indicate that in AEAL 50 group, daily per os administration of AEAL (50 mg/kg) has no significant effect on their SBP compared to the Control NaCl 0.9% group (105.88±9.90 mmHg to 102.94±7.05 mmHg respectively at the start and the end of the experiment).
Effect of AEAL on the SBP in L-NAME-Induced Hypertensive Rats
Before L-NAME administration, the SBP of L-NAME+Captopril group, L-NAME+AEAL 50 group and L-NAME+AEAL 10 group were of 121.92±15.11 mmHg, 103.27±2.67 mmHg and 111.13±10.98 mmHg respectively. After two weeks of L-NAME 40 mg/kg daily administration to each group, their SBP values reached to 147.46±4.60 mmHg, 160.00±8.79 mmHg and 150.83±8.45 mmHg respectively for L-NAME+Captopril group, L-NAME+AEAL 50 group and L-NAME+AEAL 10 group. These SBP values were not significantly different from those of the L-NAME group, which was added in Figure 2 for comparison.
The concomitant treatment with L-NAME 40 mg/kg/day plus Captopril 5 mg/kg/day for the following two weeks has shown that captopril significantly decreased the SBP values of L-NAME+Captopril group from 147.46±4.60 mmHg to 116.88±8.20 mmHg after only one day of treatment that was not significantly different to those of the Control NaCl 0.9% group. The return to normal blood pressure values was maintained until the end of the experiment.
Moreover, in L-NAME-induced hypertensive rats, the daily per os administration with L-NAME 40 mg/kg/day plus AEAL 50 mg/kg/day has significantly decreased the SBP of the L-NAME+AEAL 50 group in a dose-dependent manner from 160.00±8.79 mmHg to 108.78±4.31 mmHg. However, this return to the normal SBP values has been reached after four days of treatment and has been maintained until the end of the experiment.
In addition, administration of L-NAME 40 mg/kg plus AEAL 10 mg/kg also normalized the SBP of L-NAME-induced hypertensive rats; and this was obvious from the seventh days of AEAL 10 mg/kg/day administration (Day 28, SBP = 105.91±3.08 mmHg). A significant difference was observed between the effect of AEAL 50 mg/kg and AEAL 10 mg/kg during the three first days compared to L-NAME+Captopril group and L-NAME+AEAL 50 group respectively (Figure 2). A significant difference was also noted in the five first days between L-NAME+Captopril group and L-NAME+AEAL 10 group after treatment (see Figure 1).
Effect of AEAL on the Heart Rate of the Treated Rats
The results in Figure 3 showed that the heart rate in the different animal groups averaged 426.15±3.31 bpm at one week before the start of treatment. After 2 weeks of daily oral administration with L-NAME 40 mg/kg, the heart rate values were significantly reduced compared to those of the NaCl 0.9% group (405.82±18.00 bpm, beat per min). They have decreased from 404.82±7.20 bpm; 399.88±14.07 bpm; 398.80±11.040 bpm and 373.97±7.99 bpm to 353.53±7.23 bpm; 302.85±11 bpm; 314.75±10.55 and 328.25±11.32 bpm for L-NAME group; L-NAME+Captopril group; L-NAME+AEAL 50 group and L-NAME+AEAL 10 group respectively. Concomitant administration with L-NAME plus Captopril or L-NAME plus AEAL (50 or 10) returned the heart rate values not significantly different from that of the NaCl 0.9% group after one week of treatment. In contrast, the heart rate of the L-NAME 40 group (324.52±7.00 bpm) remained low and significantly different from the control NaCl 0.9% group (378.13±11.15 bpm) at the 3rd week of administration. In addition, at the end of the 4 weeks of experimentation, only the L-NAME group had a significantly different heart rate than the L-NAME plus AEAL 50 group.
Effect of AEAL on the Body Weight of Treated Animals
Results showed that the mass body gain in all groups was significantly increased compared to the baseline weight (week 0) from week 1 until the end of the experiment (week 5).
They also showed that there was no difference in mass gain among the control NaCl 0.9% group and the AEAL 50 group at any point of time with a maximal mass gain of 38.78±7.63 g and 36.85±6.30 g for the control NaCl 0.9% group and AEAL 50 group respectively at the end of the treatment.
Since the second week of L-NAME administration, there was a progressive and significant decrease in the mass gain of all groups compared to the control NaCl 0.9% group (Figure 4). The maximal mass gain was of 26.61±2.43 g, 25.44±5.70 g, 14.93±8.88 g and 19.89±4.67 g for the L-NAME group, L-NAME+Captopril group, L-NAME+AEAL 10 group and L-NAME+AEAL 50 group respectively. Specifically, the mass gain of L-NAME+AEAL 50 group was significantly decreased during the fifth days before the end of the experiment compared to the L-NAME+Captopril group.
Effect of AEAL on the Food Intake of Treated Rat
At the beginning of the experiment (week 0 or S0), the average food consumption was of 45.88±2.92 g; 43.81±5.34 g; 37.23±2.98 g; 37.48±3.97 g and of 40.66±7.65 g/100 g bw for the group’s Control NaCl 0.9%; AEAL 50; L-NAME; L-NAME+Captopril; L-NAME+AEAL 50 and the group L-NAME+AEAL 10 respectively (Data not show).
After two (02) weeks (S1-S2) of experimentation, this average food consumption in all groups was reduced to 10.37%; 12.58%; 4.17%; 9.59%; 9.10% and 8.80% respectively for the Control NaCl 0.9% groups; AEAL 50; L-NAME; L-NAME+Captopril; L-NAME+AEAL 50 and L-NAME+AEAL 10 groups compared to the initial amounts of S0 (Figure 5A).
During the two following weeks (S3-S4) of treatment with the combination of L-NAME plus AEAL 50 and L-NAME plus AEAL 10, the average dietary intake of animals from these groups further decreased of 24.92% and 20.13% compared to the initial amount consumed at inclusion week (S0). However, the AEAL 50 group showed a decrease but not significant during these last 2 weeks of experimentation (S3-S4) to reach an average consumption decrease of 22.68% compared to that in S0. In addition, the results show a small but significant reduction for food consumed in the L-NAME 40 group (8.17%) compared to the AEAL 50 and L-NAME plus AEAL 50 groups (Figure 5A).
A difference was also observed when the analysis takes into account all the experimental time (S1-S4). The decrease in average food consumption was of 12.80%; 17.63%; 6.17% 10.88%; 17.01% and of 14.46% respectively for the Control NaCl 0.9% groups; AEAL 50; L-NAME; L-NAME+Captopril; L-NAME+AEAL 50 and L-NAME+AEAL 10 after 4 weeks of inclusion (Figure 5B).
Effect of AEAL on the Water Consumption of Treated Rat
The histogram in Figure 6B showed that the average water consumption was varied during the experiment in all groups.
At the inclusion step (S0), the average water consumption was of 84.22±9.68; 90.03±11.59; 81.29±8.61; 75.58±3.86; 71.55±5.20 and 67.59±1.86 mL/100 g bw per animal from the NaCl Control 0.9% groups; AEAL 50; L-NAME; L-NAME + Captopril; L-NAME + AEAL 50 and L-NAME + AEAL 10 respectively (Data not show).
During the first 02 weeks of treatment, there was a significant decrease in pet consumption of NaCl 0.9% (at 16.06%) compared to L-NAME 40 (4.46%); L-NAME+AEAL 50 (−0.96%) and L-NAME+AEAL 10 (1.85%). Specifically, the average consumption of animals in the AEAL 50 (11.00%) and L-NAME plus Captopril (9.10%) groups were significantly reduce compared to the L-NAME+AEAL 50 group.
In addition, during the last 2 weeks of treatment, all groups included in the study reduced their average water consumption to 25.67%; 26.17%; 19.41%; 18.87%; 23.62% and 19.65% respectively for the animals groups Control NaCl 0.9%; AEAL 50; L-NAME; L-NAME + Captopril; L-NAME + AEAL 50 and L-NAME + AEAL 10 (Figure 6A).
During the four weeks of inclusion, the results show a substantial decrease in the water consumption of all treated groups compared to the week of inclusion (Figure 6B). However, no significant difference was found between the different groups in the study and the maximum reduction (20.86%) in average water consumption was obtained with the control group NaCl 0.9%.
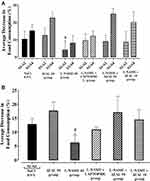
Effect of AEAL on Organs Weight from Treated Rat
After the autopsy, macroscopic examination of vital organs (heart, liver, lung, spleen, kidneys and testicles) of all treated animals groups did not show any damage of vital organs compared to the control NaCl 0.9% group. There was no change in aspect and colour of the different organs compared to the control NaCl 0.9% group (Table 1).
Effect of AEAL on the Biochemical Parameters of Serum from Treated Rat
Table 2 summarizes the results of serum biochemistry tests for the treated groups after the experiment time and autopsy. No significant difference was observed for any serum biochemical between the Control NaCl 0.9% group and treated group. Exceptionally, results showed that the urea parameters from all treated groups were increased but not significantly different from those of the control NaCl 0.%9 group.
Vascular Reactivity
Verification of the Integrity of Aortas
Before investigating the effects of the treatments on the vascular system of the vessels from the study animals, the vascular integrity of the various thoracic aorta rings was verified. To do this, the maximum effect induced by acetylcholine (10 µM) on each ring precontracted with phenylephrine (1 µM) was determined. The results were shown in Figure 7.
Results showed that the Emax of the rings from the control NaCl 0.9% and AEAL 50 groups were greater than 60% with values of 65.35±8.26% and 62.74±2.96% for the control NaCl 0.9% group and AEAL 50 group respectively. In addition, a significant difference was obtained with rings from the L-NAME and L-NAME+AEAL 10 groups compared to the control NaCl 0.9% group with respective Emax of 30.72±9.34% and 18.57±8.94%. In addition, the Emax of the rings from the L-NAME+Captopril and L-NAME+AEAL 50 groups were of 34.98±17.38% and 32.35±12.86% respectively but not significantly different to those of the control NaCl 0.9 group.
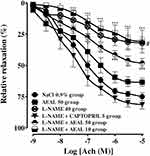
Effect of the Concentration-Response to Acetylcholine in Rings from Treated Rats Precontracted with Phenylephrine
In isolated thoracic rat aortas from all groups, the cumulative response to acetylcholine from 10−9 M to 10–5 M, induced a concentration-dependent vasodilation effect with a better relaxation in rings from L-NAME+Captopril group. The Emax values were of 81.50±6.77%, 75.05±6.11%, 63.41±5.74% and 48.13±7.38% in L-NAME+Captopril, control NaCl 0.9% group, AEAL 50 group and L-NAME+AEAL 50 group respectively. Chronic treatment with AEAL 50 mg/kg/day alone did not affect the acetylcholine-induced concentration-dependent relaxation of the thoracic aorta precontracted by phenylephrine (1 µM) compared to the control NaCl 0.9% group. In comparison to control NaCl 0.9% group, administration of L-NAME alone (Emax = 31.97±8.17%) and L-NAME together with AEAL 10 mg/kg/day (Emax = 30.83±15.82%) or with AEAL 50 mg/kg/day significantly attenuated the acetylcholine-induced relaxation of the thoracic aorta. Moreover, chronic treatment with L-NAME plus Captopril has enhanced the vasodilation effect of acetylcholine on rat aorta, but this effect was not significantly different from that of the control NaCl 0.9% group. The EC50 values of acetylcholine in rings from control NaCl 0.9%, L-NAME, AEAL 50, L-NAME+Captopril, L-NAME+AEAL 50 and L-NAME+AEAL 10 groups were of (5.47±2.99)x10−08 M, (10.31±0.85)x10−08 M, (9.26±7.78)x10−08 M, (3.47±4.16)x10−08 M, (8.23±6.66)x10−08 M, and (13.73±1.64)x10−08 M respectively. In addition, a significant difference to the acetylcholine-induced relaxation has been shown in aortas from L-NAME and L-NAME+AEAL 10 groups compared to L-NAME+Captopril group (Figure 8).
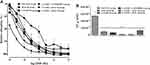
Effect of the Concentration-Response to SNP in Rings from Treated Rats Precontracted with Phenylephrine
Cumulative concentration-response and EC50 values of isolate aortic rings from all groups were presented in Figure 9A and B. Results showed that SNP had induced a concentration-dependent vasodilation effect, which was significantly shifted to the left in all treated groups compared to the control NaCl 0.9% group. While no significant difference was obtained in their Emax values that were of 102.57±3.39% and 89.96±6.96%, respectively, in rings from AEAL 50 group and L-NAME+AEAL 50 group compared to the control NaCl 0.9% group (92.673±13.32%) (Figure 9A). Moreover, the EC50 values from treated groups were all significantly enhance than those from the control NaCl 0.9% group (Figure 9B).
Discussion
Numerous studies have demonstrated the effectiveness of medicinal plant extracts or their fractions for the treatment of devastating metabolic diseases such as hypertension.22–26 The organs often affected by the hypertensive pathology include the heart, vessels, kidneys, eyes and brain.27–29
Specifically, the endothelium is recognized as a monolayer of cells playing a major role in vascular physiology and pathophysiology. Studies suggest that arterial hypertension causes a decrease in endothelial function correlated with a reduction in the efficacy of NO-dependent vasodilatation and an increase in the production of reactive oxygen species (ROS). However, other research papers suggest that endothelial dysfunction may also predispose to the development of HTA.30 In addition, endothelial dysfunction is known to be responsible for a reduction in the expression and/or activity of eNOS, the “decoupling” of eNOS, the increase in NO uptake or degradation and the alteration of the transmission of NO-initiated signal events.31–34 Thus, efficacy studies of plant extracts for their potential antihypertensive properties rely primarily on in vivo, in vitro or ex vivo evaluations with various scientifically valid methodologies. It is for this interest that studies have already demonstrated the vasodilation effect of AEAL from Anogeissus leiocarpa.18 AEAL were also known to be a potent inhibitor of purified cyclic nucleotide phosphodiesterase.35 A recent study has also shown that a dichloromethane fraction from AEAL has an endothelium-independent vasodilator effect involving inhibition of PDE1, 2 and 5 and involve a reduction in intracellular calcium.36 Also, a preliminary study showed that this extract has an antihypertensive effect in an anesthetized rat model.20 The present study was undertaken to investigate the antihypertensive effect of AEAL in L-NAME-induced hypertensive rats in comparison to captopril that was an existing sulfur-containing angiotensin-converting enzyme inhibitor.
Moreover, the AEAL effect on the vascular system of chronically treated animals as well as its possible damages on the sensible organs of hypertension was investigated. To our knowledge, it constitutes the first study on this plant extract in a chronic administration and which used L-NAME-induced hypertension model. This model is very interesting in that it is close to the treatment of hypertensive patients that is for all the life.
The L-NAME has known to be a potent inhibitor of the endothelial nitric oxide synthase (eNOS) activity, which lead to endothelial dysfunction and used as L-NAME-induced hypertension model in the experiment. In addition, authors demonstrated that a prominent endothelial dysfunction occurred during L-NAME-induced hypertension with a subsequent increase in peripheral resistance leading to the animal blood pressure increase.37,38
As expected, the present findings show that chronic treatment of animals with L-NAME for 4 weeks was associated with a very significant increase in systolic blood pressure, while the treatment with AEAL (50 mg/kg/day) alone during the same time did not present any significant difference in systolic blood pressure (SBP) compared to those of the Control NaCl 0.9% group. Moreover, AEAL at 50 mg/kg/day during the experiment time has no impact on the heart rate (HR) of normotensive rats compared to the Control NaCl 0.9% group.
Interestingly, concomitant intake of Captopril with L-NAME used as positive control completely reversed the SBP of L-NAME-induced hypertensive rat back to normal values after two times administration indicating a rapid action and efficacy of this antihypertensive drug. Likewise, repeated AEAL administration to L-NAME-induced hypertensive rat, resulting in a gradual and significant dose-dependent reduction in the experimental animal SBP with efficacy after 04 and 07 times administration with AEAL 50 mg/kg/day and AEAL 10 mg/kg/day respectively. These results suggested that AEAL has an antihypertensive effect in L-NAME-induced hypertensive rat and confirms the previous results obtained with this extract and tested in anesthetized rats model.20 In addition, the present results indicate a significant decrease in the HR of the hypertensive animals after 02 weeks of L-NAME-induced hypertensive rat followed by a return to normal values with or without treatment by plant extract or captopril. These results corroborate those of other authors, which showed that continuous administration of L-NAME had substantially decreased the HR during the first week with a return to the normal values when treatment was maintained.39
In one part, the antihypertensive effect of AEAL in the present study may probably due to its ability to mediate vasodilation action in vessel since L-NAME-induced hypertension involve sympathetic drive effects.25,40,41 Indeed, AEAL has been known to have vasodilation properties via Src/PI3-Akt kinase relaxation pathway responsible for the activation of eNOS followed by the release of NO.18 Moreover, AEAL was known to inhibit in vitro, the purified cyclic nucleotides PDEs found in vascular smooth muscle cell (VSMC), whose might induce vessels relaxation.35 Previous studies raised the presence of tannins, sterols and triterpenoids in AEAL, which were known to have vasodilator effect.20 In other parts, AEAL antihypertensive effect could be explained by its possible cardiac action since this extract has known to possess an antihypertensive effect in hypertensive rat induced by adrenaline in the invasive model.20 This antihypertensive effect of AEAL may also be attributable to its contains in saponins. Indeed, numerous studies indicated that saponins from plants extract were responsible for the management of hypertension in the experimental model were associated with vascular relaxation.42–44 The presence of tannin may consolidate the antihypertensive effect of AEAL since study demonstrated that a phenolic compound-like tannic acid was able to reduced blood pressure in L-NAME-induced hypertensive rats.45,46
Beyond the confirmed antihypertensive effect of AEAL, this study looked at the effect of the chronic administration of this extract on the animals, including its effects on the weight gain, as well as the water and food consumption. Indeed, several authors showed that animals in L-NAME chronic administration inexorably present a decrease in their weight, water and food consumption, in addition to the installation of hypertension.47–49 These findings were in the line of those of the present study in where the gain weight, as well as the water and food consumption of normotensive rats treated with AEAL 50 mg/kg/day, has not affected; while, the weight of L-NAME-induced hypertensive rat was significantly affected even in concomitant treatment with AEAL or captopril. Nevertheless, it should be notified that the decrease in food and water intake during the first two weeks of chronic L-NAME administration was improved after treatment with AEAL and Captopril, although the L-NAME 40 group recorded a slight but significant decrease in food consumption.
More interesting, chronic administration of L-NAME alone, NaCl 0.9% or AEAL (50 mg/g/day) during the 4 weeks were resulted in no mortality and without any evidence of apparent toxicity. Similarly, concomitant administration of L-NAME with AEAL (10 and 50 mg/kg/day) or captopril for two weeks resulted in no deaths or signs of toxicity.
Numerous studies indicated that organ weight constitutes one of the most sensitive drug toxicities indicators, and its changes often precede morphological changes. Indeed, several studies revealed that a significant difference in organ weight between control and the treated animal might exist without any morphological changes.50–53 The current study showed that the relative weight of the target organs of hypertension, which are the heart, lung, kidneys, spleen, liver and testes of the different groups treated were comparable to those of the control NaCl 0.9% group indicating that AEAL did not have a manifest impact on these organs (Table 1). However, a study has shown that L-NAME caused deleterious effects on these organs when chronically administrate in chickens and may be justify by the difference of the experimental used animals.54
To corroborate the results on organs normal function, biochemical analysis was undertaken with the serum of all experiment animals. Indeed, it has been showed that hepatic and renal function analysis is indispensable for any plant extract used, as they are important for the functioning of an organism.55–57 For that, impact of AEAL, L-NAME and Captopril treatment in liver and kidney functions for all experiment rats has been assessed. The results showed that the changes in the biochemical parameters were not significant in both tested groups and control NaCl 0.9% group. Then, AEAL seems to be safe of use and confirm no damage observable on sensible organs during this experiment.
Furthermore, L-NAME is known to interact with the vascular system leading to its dysfunction when chronically administrate. Indeed, in L-NAME-induced hypertensive model, authors have confirmed the involvement of both renin-angiotensin system and a reduction in the thick of the vascular walls, which were significantly reverse by captopril.58–60 Then, vascular reactivity study were undertaken on tissues from treated animals for to characterize the effects of AEAL or Captopril and or in combination with L-NAME at the end of the experiment. Before started, a verification of the integrity of vessels from the different groups has been made. Results showed that there was a significant reduction of the maximum response to acetylcholine of vessels from L-NAME group and L-NAME+AEAL 10 group compare to the control NaCl 0.9% group indicating a possible destruction of these aortas. While, rings from L-NAME+Captopril group and L-NAME+AEAL 50 group were slightly reduce but not significantly different to those of control NaCl 0.9% group.
The cumulative response to acetylcholine (10−9 M to 10–5 M) in phenylephrine precontracted rat aortas from treated animals have shown a concentration-dependent vasodilation effect with a better relaxation in rings from L-NAME+Captopril group indicating that captopril improved the vasodilation effect of acetylcholine even in the presence of L-NAME. The impairment of endothelium intact vasodilation in L-NAME-induced hypertension was associated with oxidative stress but also due to a decrease of eNOS expression and cyclic GMP or NO metabolite levels.61,62 Moreover, several studies already showed that captopril had a beneficial effect of angiotensin-converting enzyme either in reversing or preventing endothelium dysfunction.63–66 While, a relative relaxation was obtained with rings from L-NAME+AEAL 50 group but not those from L-NAME group or in L-NAME+AEAL 10 group in acetylcholine concentration-response curves indicating that in high dose, AEAL may have the main way of action like captopril. Then, AEAL antihypertensive effect might be due to its antioxidant properties since it is well document that the principal mechanisms in L-NAME-induced hypertension is oxidative stress but also to AEAL capacity to inhibit PDEs localized in the vascular smooth muscle cells.9,36,60,67,68 Indeed, previous studies indicate that extract from Anogeissus leiocarpa has confirmed this potential antioxidant activity.69–71
In contrast, the vascular response of rings from all treated rats to SNP (NO donor) did not differ, indicating normal smooth muscle cells function. Nevertheless, a significant difference was obtained between the vasodilation effect to SNP of all treated group compared to the control NaCl 0.9% group. Thus, this impaired relaxation to Acetylcholine in the present study might be due to a decreased production and or an inactivation eNOS rather than any damage in smooth muscle function which lead to the maintained of response to the NO donor. Indeed, the present finding confirms others studies that demonstrated the potency of SNP to directly acted on vascular smooth muscle cells leading to cGMP activation and vasodilation via intracellular calcium decrease.72,73 These results were in line with a previous study, which demonstrates that a dichlorometanolic fraction from A. leiocarpa induced endothelium-independent activity via this pathway.36
Conclusion
In conclusion, AEAL affords significant antihypertensive effect in L-NAME-induced hypertensive rats without aversive incidence in the biochemical parameters and in the ‘sensitive’s organs. This antihypertensive is probably due to the vasodilator property of AEAL, which was known to involve the Src/PI3-Kinase Akt/eNOS pathway in the vascular system but also by a possible involvement of cardiac system regulation. Thus, AEAL has the potential to be developed as a medicinal substance in the management of hypertension.
Abbreviations
AEAL, aqueous extract of Anogeissus leiocarpa; HTA, hypertension; ASAT, aspartate aminotransferase); ALAT, alanine aminotransferase; acetylcholine, Ach; SNP, sodium nitroprusside; NO, nitric oxide; eNOS, endothelial nitric oxide synthase; SBP, systolic blood pressure; PDE, phosphodiesterase.
Data Sharing Statement
All data described in this manuscript are available from the corresponding author on reasonable request.
Ethics Approval
All animal experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health and the EU Directive 2010/63/EU for animal experiments. Ethical approval was obtained from the ethic committee of the University Joseph KI-ZERBO (Protocol number: CE-UOI/2019-04).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for the present work and declare that there is no conflict of interest for the publication of this manuscript.
References
1. Zhang Q, Mahapatra T, Huang F, et al. Association between anthropometric measures and indicators for hypertension control among Kazakh-Chinese hypertension patients in Xinjiang, China: results from a cross-sectional study. PLoS One. 2017;12(1):e0170959. doi:10.1371/journal.pone.0170959
2. JNC7. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda (MD); 2004.
3. Rust P, Ekmekcioglu C. Impact of salt intake on the pathogenesis and treatment of hypertension. Adv Exp Med Biol. 2017;956:61–84.
4. Touyz RM, Alves-Lopes R, Rios FJ, et al. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018;114(4):529–539. doi:10.1093/cvr/cvy023
5. WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: WHO; 2013:104.
6. Aekthammarat D, Pannangpetch P, Tangsucharit P. Moringa oleifera leaf extract lowers high blood pressure by alleviating vascular dysfunction and decreasing oxidative stress in L-NAME hypertensive rats. Phytomedicine. 2019;54:9–16. doi:10.1016/j.phymed.2018.10.023
7. Yilmaz E, Kaya-Sezginer E, Yilmaz-Oral D, Cengiz T, Bayatli N, Gur S. Effects of hydrogen sulphide donor, sodium hydrosulphide treatment on the erectile dysfunction in L-NAME-induced hypertensive rats. Andrologia. 2019;51(5):e13240. doi:10.1111/and.13240
8. Sorriento D, De Luca N, Trimarco B, Iaccarino G. The antioxidant therapy: new insights in the treatment of hypertension. Front Physiol. 2018;9:258. doi:10.3389/fphys.2018.00258
9. Tome-Carneiro J, Visioli F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: review of human evidence. Phytomedicine. 2016;23(11):1145–1174.
10. Braam B, Taler SJ, Rahman M, et al. Recognition and management of resistant hypertension. Clin J Am Soc Nephrol. 2017;12(3):524–535. doi:10.2215/CJN.06180616
11. Rachana P, Anuradha H, Shivamurthy M. Anti hypertensive prescribing patterns and cost analysis for primary hypertension: a retrospective study. J Clin Diagn Res. 2014;8(9):HC19–22.
12. Seeley A, Prynn J, Perera R, Street R, Davis D, Etyang AO. Pharmacotherapy for hypertension in Sub-Saharan Africa: a systematic review and network meta-analysis. BMC Med. 2020;18(1):75. doi:10.1186/s12916-020-01530-z
13. Belemnaba L, Nitiema M, Traoré S, et al. Research for plants with antihypertensive potentialities in the biodiversity of Burkina Faso. Revue CAMES–Série Pharm Méd Trad Afr. 2014;17(1):
14. Okpekon T, Yolou S, Gleye C, et al. Antiparasitic activities of medicinal plants used in Ivory Coast. J Ethnopharmacol. 2004;90(1):91–97. doi:10.1016/j.jep.2003.09.029
15. Mann A, Amupitan J, Oyewale A, Okogun J, Ibrahim K. Antibacterial activity of terpenoidal fractions from Anogeissus leiocarpus and Terminalia avicennioides against community acquired infections. Afr J Pharm Pharmaco. 2009;3(1):022–025.
16. Mann A, Banso A, Clifford L. An antifungal property of crude plant extracts from Anogeissus leiocarpus and Terminalia avicennioides. Tanzan J Health Res. 2008;10(1):34–38.
17. Fyhrquist P, Mwasumbi L, Haeggstrom CA, Vuorela H, Hiltunen R, Vuorela P. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J Ethnopharmacol. 2002;79(2):169–177. doi:10.1016/S0378-8741(01)00375-0
18. Belemnaba L, Ouedraogo S, Nitiema M, et al. An aqueous extract of the Anogeissus leiocarpus bark (AEAL) induces the endothelium-dependent relaxation of porcine coronary artery rings involving predominantly nitric oxide. J Basic Clin Physiol Pharmacol. 2018;29(6):599–608. doi:10.1515/jbcpp-2017-0084
19. Konaté K, Kiendrébéogo M, Ouattara MB, et al. Antibacterial potential of aqueous acetone extracts from five medicinal plants used traditionally to treat infectious diseases in Burkina Faso. Current Res J Biol Sci. 2011;3:435–442.
20. Ouédraogo S, Belemnaba L, Traoré A, Lompo M, Bucher B, Guissou IP. Etude de la toxicité et des propriétés pharmacologiques de l’extrait aqueux de Anogeissus leiocarpus (DC) Guill. et Perr. (Combretaceae). Pharmacopée Et Médecine Traditionnelle Africaine. 2008;15:18–22.
21. The National Academies Collection. In Guide for the Care and Use of Laboratory Animals. 8th, Ed. Reports funded by National Institutes of Health, Washington (DC) ;2011.
22. Lagunas-Herrera H, Tortoriello J, Herrera-Ruiz M, et al. Acute and chronic antihypertensive effect of fractions, tiliroside and scopoletin from Malva parviflora. Biol Pharm Bull. 2019;42(1):18–25. doi:10.1248/bpb.b18-00355
23. Khan S, Khan T, Shah AJ. Total phenolic and flavonoid contents and antihypertensive effect of the crude extract and fractions of Calamintha vulgaris. Phytomedicine. 2018;47:174–183. doi:10.1016/j.phymed.2018.04.046
24. Ikarashi N, Toda T, Hatakeyama Y, et al. Anti-hypertensive effects of acacia polyphenol in spontaneously hypertensive rats. Int J Mol Sci. 2018;19:3. doi:10.3390/ijms19030700
25. Abdel-Rahman RF, Hessin AF, Abdelbaset M, Ogaly HA, Abd-Elsalam RM, Hassan SM. Antihypertensive effects of roselle-olive combination in l-NAME-induced hypertensive rats. Oxid Med Cell Longev. 2017;2017:9460653. doi:10.1155/2017/9460653
26. Bello I, Usman NS, Dewa A, et al. Blood pressure lowering effect and vascular activity of Phyllanthus niruri extract: the role of NO/cGMP signaling pathway and beta-adrenoceptor mediated relaxation of isolated aortic rings. J Ethnopharmacol. 2020;250:112461. doi:10.1016/j.jep.2019.112461
27. Munoz-Durango N, Fuentes CA, Castillo AE, et al. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int J Mol Sci. 2016;17:7. doi:10.3390/ijms17070797
28. Sun N, Mu J, Li Y. Working Committee of Salt evaluation BPMCMAHPCHGCSoC. An expert recommendation on salt intake and blood pressure management in Chinese patients with hypertension: a statement of the Chinese Medical Association Hypertension Professional Committee. J Clin Hypertens (Greenwich). 2019;21(4):446–450. doi:10.1111/jch.13501
29. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041.
30. Favero G, Paganelli C, Buffoli B, Rodella LF, Rezzani R. Endothelium and its alterations in cardiovascular diseases: life style intervention. Biomed Res Int. 2014;2014:801896. doi:10.1155/2014/801896
31. Lataro RM, Silva MAB, Mestriner FL, Cau SBA, Tostes RCA, Salgado HC. Chronic treatment with acetylcholinesterase inhibitors attenuates vascular dysfunction in spontaneously hypertensive rats. Am J Hypertens. 2019;32(6):579–587. doi:10.1093/ajh/hpz036
32. Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–540.
33. Kostov K, Halacheva L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int J Mol Sci. 2018;19:6. doi:10.3390/ijms19061724
34. Cobos-Segarra L, Lopez-Jaramillo P, Ponte-Negretti CI C, et al. Pharmacological treatment of hypertension: effects in endothelial function. Curr Hypertens Rev. 2018;14(2):123–127. doi:10.2174/1573402114666180508104828
35. Belemnaba L, Ouedraogo S, Auger C, et al. Endothelium-independent and endothelium-dependent vasorelaxation by a dichloromethane fraction from Anogeissus Leiocarpus (DC) Guill. Et Perr. (Combretaceae): possible involvement of cyclic nucleotide phosphodiesterase inhibition. Afr J Tradit Complement Altern Med. 2013;10(2):173–179.
36. Belemnaba L, Nitiéma M, Ouédraogo S, Auger C, Schini-Kerth VB, Bernard B. Endothelium-independent vasorelaxation by dichloromethanolic fraction from Anogeissus leiocarpa (DC) Guill. Et Perr. (Combretaceae) bark of trunk on porcine coronary artery rings: involvement of [Ca2+]i decreased and phosphodiesterases inhibition.African Journal of Pharmacy and Pharmacology. 2019;13(4):25–35. doi:10.5897/AJPP2018.4992
37. Gardiner SM, Dunn WR, Bennett T. Chronic nitric oxide inhibition model six years on. Hypertension. 1999;34(5):e4. doi:10.1161/01.HYP.34.5.e4
38. Basrali F, Kocer G, Ulker Karadamar P, et al. Effect of magnesium supplementation on blood pressure and vascular reactivity in nitric oxide synthase inhibition-induced hypertension model. Clin Exp Hypertens. 2015;37(8):633–642. doi:10.3109/10641963.2015.1036063
39. Simko F, Baka T, Poglitsch M, et al. Effect of ivabradine on a hypertensive heart and the renin-angiotensin-aldosterone system in L-NAME-induced hypertension. Int J Mol Sci. 2018;19:10. doi:10.3390/ijms19103017
40. Biancardi VC, Bergamaschi CT, Lopes OU, Campos RR. Sympathetic activation in rats with L-NAME-induced hypertension. Braz J Med Biol Res. 2007;40(3):401–408. doi:10.1590/S0100-879X2006005000077
41. Amssayef A, Ajebli M, Eddouks M. Aqueous extract of oakmoss produces antihypertensive activity in L-NAME-induced hypertensive rats through sGC-cGMP pathway. Clin Exp Hypertens. 2020;1–7.
42. Chen M, Long Z, Wang Y, et al. Protective effects of saponin on a hypertension target organ in spontaneously hypertensive rats. Exp Ther Med. 2013;5(2):429–432. doi:10.3892/etm.2012.856
43. Lee KH, Bae IY, Park SI, Park JD, Lee HG. Antihypertensive effect of Korean Red Ginseng by enrichment of ginsenoside Rg3 and arginine-fructose. J Ginseng Res. 2016;40(3):237–244. doi:10.1016/j.jgr.2015.08.002
44. Liu JC, Hsu FL, Tsai JC, et al. Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003;73(12):1543–1555. doi:10.1016/S0024-3205(03)00481-8
45. Saravanakumar M, Raja B. Veratric acid, a phenolic acid attenuates blood pressure and oxidative stress in L-NAME induced hypertensive rats. Eur J Pharmacol. 2011;671(1–3):87–94. doi:10.1016/j.ejphar.2011.08.052
46. Turgut Cosan D, Saydam F, Ozbayer C, et al. Impact of tannic acid on blood pressure, oxidative stress and urinary parameters in L-NNA-induced hypertensive rats. Cytotechnology. 2015;67(1):97–105. doi:10.1007/s10616-013-9661-4
47. Simko F, Baka T, Krajcirovicova K, et al. Effect of melatonin on the renin-angiotensin-aldosterone system in l-NAME-induced hypertension. Molecules. 2018;23:2. doi:10.3390/molecules23020265
48. Moslemi F, Nematbakhsh M, Eshraghi-Jazi F, et al. Inhibition of nitric oxide synthase by L-NAME promotes cisplatin-induced nephrotoxicity in male rats. ISRN Toxicol. 2013;2013:242345. doi:10.1155/2013/242345
49. Joost HG, Tschop MH. NO to obesity: does nitric oxide regulate fat oxidation and insulin sensitivity?. Endocrinology. 2007;148(10):4545–4547.
50. Piao Y, Liu Y, Xie X. Change trends of organ weight background data in sprague dawley rats at different ages. J Toxicol Pathol. 2013;26(1):29–34. doi:10.1293/tox.26.29
51. Long GG, Symanowski JT, Roback K. Precision in data acquisition and reporting of organ weights in rats and mice. Toxicol Pathol. 1998;26(3):316–318. doi:10.1177/019262339802600304
52. Gur E, Waner T. The variability of organ weight background data in rats. Lab Anim. 1993;27(1):65–72. doi:10.1258/002367793781082368
53. Michael B, Yano B, Sellers RS, et al. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007;35(5):742–750. doi:10.1080/01926230701595292
54. Tisljar M, Grabarevic Z, Artukovic B, et al. The impact of L-NAME and L-arginine chronic toxicity induced lesions on ascites–pulmonary hypertension syndrome development in broiler chickens. Coll Antropol. 2011;35(2):547–556.
55. Oyagbemi AA, Omobowale TO, Azeez IO, Abiola JO, Adedokun RA, Nottidge HO. Toxicological evaluations of methanolic extract of Moringa oleifera leaves in liver and kidney of male Wistar rats. J Basic Clin Physiol Pharmacol. 2013;24(4):307–312. doi:10.1515/jbcpp-2012-0061
56. Nigatu TA, Afework M, Urga K, Ergete W, Makonnen E. Toxicological investigation of acute and chronic treatment with Gnidia stenophylla Gilg root extract on some blood parameters and histopathology of spleen, liver and kidney in mice. BMC Res Notes. 2017;10(1):625. doi:10.1186/s13104-017-2964-3
57. Ibrahim MB, Sowemimo AA, Sofidiya MO, et al. Sub-acute and chronic toxicity profiles of Markhamia tomentosa ethanolic leaf extract in rats. J Ethnopharmacol. 2016;193:68–75. doi:10.1016/j.jep.2016.07.036
58. Zicha J, Dobesova Z, Kunes J. Antihypertensive mechanisms of chronic captopril or N-acetylcysteine treatment in L-NAME hypertensive rats. Hypertens Res. 2006;29(12):1021–1027. doi:10.1291/hypres.29.1021
59. Nguelefack-Mbuyo PE, Nguelefack TB, Dongmo AB, et al. Anti-hypertensive effects of the methanol/methylene chloride stem bark extract of Mammea africana in l-NAME-induced hypertensive rats. J Ethnopharmacol. 2008;117(3):446–450. doi:10.1016/j.jep.2008.02.028
60. Tata CM, Sewani-Rusike CR, Oyedeji OO, Gwebu ET, Mahlakata F, Nkeh-Chungag BN. Antihypertensive effects of the hydro-ethanol extract of Senecio serratuloides DC in rats. BMC Complement Altern Med. 2019;19(1):52. doi:10.1186/s12906-019-2463-2
61. Alp Yildirim FI, Eker Kizilay D, Ergin B, et al. Barnidipine ameliorates the vascular and renal injury in L-NAME-induced hypertensive rats. Eur J Pharmacol. 2015;764:433–442. doi:10.1016/j.ejphar.2015.07.033
62. Pechanova O, Bernatova I, Babal P, et al. Red wine polyphenols prevent cardiovascular alterations in L-NAME-induced hypertension. J Hypertens. 2004;22(8):1551–1559. doi:10.1097/01.hjh.0000133734.32125.c7
63. Tschudi MR, Criscione L, Novosel D, Pfeiffer K, Luscher TF. Antihypertensive therapy augments endothelium-dependent relaxations in coronary arteries of spontaneously hypertensive rats. Circulation. 1994;89(5):2212–2218. doi:10.1161/01.CIR.89.5.2212
64. Duarte J, Martinez A, Bermejo A, et al. Cardiovascular effects of captopril and enalapril in obese Zucker rats. Eur J Pharmacol. 1999;365(2–3):225–232. doi:10.1016/S0014-2999(98)00879-6
65. Paredes MD, Romecin P, Atucha NM, et al. Moderate effect of flavonoids on vascular and renal function in spontaneously hypertensive rats. Nutrients. 2018;10:8.
66. Gauthier KM, Cepura CJ, Campbell WB. ACE inhibition enhances bradykinin relaxations through nitric oxide and B1 receptor activation in bovine coronary arteries. Biol Chem. 2013;394(9):1205–1212. doi:10.1515/hsz-2012-0348
67. Marquez-Ramirez CA, Hernandez de la Paz JL, Ortiz-Avila Oet al. Comparative effects of avocado oil and losartan on blood pressure, renal vascular function, and mitochondrial oxidative stress in hypertensive rats. Nutrition. 2018;54:60–67. doi:10.1016/j.nut.2018.02.024
68. Raja B, Saranya D, Prabhu R. Role of flavonoid troxerutin on blood pressure, oxidative stress and regulation of lipid metabolism. Front Biosci (Elite Ed). 2019;11:121–129. doi:10.2741/e851
69. Ademosun AO, Adebayo AA, Oboh G. Anogeissus leiocarpus attenuates paroxetine-induced erectile dysfunction in male rats via enhanced sexual behavior, nitric oxide level and antioxidant status. Biomed Pharmacother. 2019;111:1029–1035. doi:10.1016/j.biopha.2019.01.022
70. Ouedraogo V, Kiendrebeogo M. Methanol extract from Anogeissus leiocarpus (DC) Guill. et Perr. (Combretaceae) stem bark quenches the quorum sensing of Pseudomonas aeruginosa PAO1. Medicines (Basel). 2016;3:4.
71. Akanbi OM, Omonkhua AA, Cyril-Olutayo CM, Fasimoye RY. The antiplasmodial activity of Anogeissus leiocarpus and its effect on oxidative stress and lipid profile in mice infected with Plasmodium bergheii. Parasitol Res. 2012;110(1):219–226. doi:10.1007/s00436-011-2472-7
72. Ali SF, Woodman OL. Tocomin restores endothelium-dependent relaxation in the diabetic rat aorta by increasing NO bioavailability and improving the expression of eNOS. Front Physiol. 2019;10:186. doi:10.3389/fphys.2019.00186
73. Caniffi C, Cerniello FM, Gobetto MN, Sueiro ML, Costa MA, Arranz C. Vascular tone regulation induced by C-type natriuretic peptide: differences in endothelium-dependent and -independent mechanisms involved in normotensive and spontaneously hypertensive rats. PLoS One. 2016;11(12):e0167817. doi:10.1371/journal.pone.0167817
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.