Back to Journals » Journal of Experimental Pharmacology » Volume 13
Preclinical Efficacy and Safety Studies of Formulation SSV-003, a Potent Anti-Viral Herbal Formulation
Received 21 March 2021
Accepted for publication 21 August 2021
Published 22 October 2021 Volume 2021:13 Pages 913—921
DOI https://doi.org/10.2147/JEP.S310452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Paola Rogliani
Yogesh Arun Dound,1 Rajesh Sehgal2
1Research and Development, Shreepad Shree Vallabh SSV Phytopharmaceuticals, Mumbai, Maharashtra, India; 2Research and Development, Pharma Instinct Pvt. Ltd, Noida, Uttar Pradesh, India
Correspondence: Yogesh Arun Dound
Research and Development, Shreepad Shree Vallabh SSV Phytopharmaceuticals, 201/2, Old Kashmiri Building, R R Thakur Marg, Majaswadi, Jogeshwari, Mumbai, Mahrashtra, 400060, India
Email [email protected]
Background: Recent viral pandemics have challenged the global scientific community to immediately develop new therapies. The fastest approach to develop these is to explore natural products for their efficacies and repurposing of already approved molecules. Keeping global emergency in view, researchers at Shreepad Shree Vallabh SSV Phytopharmaceuticals developed the CurvicTM (SSV-003) formulation, comprising of curcumin, vitamin C, vitamin K2-7, selenomethionine and Zinc.
Methods: Researchers have systematically studied the SSV-003 formulation for its in vitro efficacy against influenza A virus (H1N1) (ATCC® VR-219™) and human beta coronavirus (ATCC® VR1558™) using MDCK & HCT-8 cell lines, respectively, in vivo efficacy studies of SSV-003 on influenza A virus infected Balb/c mice, and acute toxicity studies as per OECD guidelines.
Results: Formulation SSV-003 showed potent antiviral activities against both the selected virus strains. Its IC50 was significantly lessthan ribavirin against influenza A-H1N1-VR219, with no cytopathic effect. SSV-003 showed IC50 of 2.26 μg/mL against human beta coronavirus, which was near to the IC50 of ribavirin (2.25 μg/mL) and was less than remedisivir (6.23 μg/mL) with no cytopathic effect. In-vivo studies in an influenza A virus infected mice model showed a significantly higher TCID50 value in the infected control group as compared to test groups. Animals treated with SSV-003 showed a dose dependent decrease in TCID50. Formulation SSV-003 at the dose of 500, 1,000, and 1,500 mg/kg body weight showed 85.9%, 94.6%, and 95.1% decreases in infection as compared to the infected control group. Dose-dependent significant increases in CD4+, CD8+ counts, IgG and IgM levels were observed in SSV-003 treated groups as compared to the infected control group and remedisivir treated group. In the acute oral toxicity study, no mortality or morbidity was observed.
Conclusion: The data from these preclinical studies provide strong evidence of potent and safe antiviral and immunomodulatory activity of SSV-003.
Keywords: curcumin, antiviral, coronavirus, vitamin C
Introduction
Coronaviruses are extensively researched Coronaviridae family viruses. They infect multiple hosts due to their ability to evolve in epidemiological situations that include mutations and crossing species barriers.1,2 The enveloped virions have a single-stranded, positive-sense ribonucleic acid (RNA) genome bordered by an extracellular membrane containing spike glycoproteins.3,4 Following an infection, severity varies from asymptomatic to severe acute respiratory distress syndrome. Matricardi et al5 hypothesized that the balance between the cumulative viral exposure and effectiveness of the local innate immune response is vital in the progression of COVID-19. Researchers have proposed that the first stage of COVID-19 is characterized by upper respiratory tract infection, fever, muscular pain, and fatigue. The second stage is marked by breathing difficulties and pneumonia. The third stage involves clinical deterioration dominated by a cytokine storm followed with hyper-inflammatory state causing arterial and venous vasculopathy in the lungs, leading to acute respiratory distress syndrome (ARDS). Sepsis, acute renal and cardiac damage add to the complications in this phase.6 The fourth stage is marked with death or recovery.5 Currently, no specific treatment is available for treating the COVID-19 infection, but antiviral, anti inflammatory drugs, low-molecular-weight heparins, herbs, and immunoglobulins are being used. In the early stages of infections, antiviral agents are being used, whilst immunomodulators along with antiviral agents are used in patients in the critical stage.7
Herbs and phytochemicals are used to treat various diseases. Several polyphenolic compounds have been studied for their varied antiviral mechanisms like inhibiting virus host specific interactions, its entry, replication, and assembly. In line with such observations, Curcumin is widely investigated for its antiviral activity.8 Curcumin, a powerful antioxidant, exhibits a range of medicinal properties such as anti-proliferative, anti-viral, anti-inflammatory, neuroprotective, and cardioprotective properties.9 It is also reported to exhibit antiviral activities against Influenza virus, Hepatitis virus, HSV-2, HPV viruses, Zikavirus, and Adenovirus.10 It modulates various cellular and molecular pathways, like COX-2, MMPs, glutathione, protein kinase C, ATPase, nuclear NF-kb, AP-1, P-gp, MRP-1, MRP-2, ErbB2, a1-acid-glycoprotein, Cyclin D1, and others.11 It directly interacts with many proteins such as protein kinase (PK), polymerases, thioredoxin reductase, tubulin, lipoxygenase, and focal adhesion kinase. It exerts post-transcriptional and post-translational changes impeding critical steps of viral replications and viral attachment. Various bio-activities of Curcumin are attributed to its metabolites, ie, dimethoxycurcumin and tetrahydrocurcumin. Recent molecular docking research suggested that COVID-19 Mpro was docked with dimethoxycurcumin and it can be its inhibitors.11 Vitamin K2-7 has strong anti-inflammatory activity and high bioavailability. Dound et al11 developed Vitamin K2-7 with more than 98.5% Trans-isomers and less than 0.2% cis-isomers and reported its antiviral activity against HIV and HBV (Patent number – IN202021007016). They also studied the in-silico activity of vitamin K2-7 against SARS-CoV’s main peptide and COVID-19 Mpro (Patent number– IN202021013430). Dound et al11 reported that Vitamin K2-7 has inhibitory activity against SARS CoV’s main peptide and COVID-19 Mpro with binding energy of −6.92 and −6.54, respectively, suggesting its beneficial role in management of COVID-19 infection. Vitamin C and Selenomethionine are also potent anti-oxidants, with immune boosting and anti inflammatory activities.12
Based on the outcomes of various experiments, scientific rationale, and medicinal potential, researchers at Shreepad Shree Vallabh SSV Pharmaceuticals developed the formulation CurvicTM (SSV-003), containing Curcumin, Vitamin C, Vitamin K2-7, Selenomethionine and Zinc, for effective management of COVID-19 patients.13 In the present research work, we evaluate the anti-viral activities of SSV-003 against Influenza A Virus (ATCC® VR-219™) and Human Beta Coronavirus (ATCC® VR1558™) and evaluate its acute toxicity profile in rats.
Materials and Methods
MDCK cells and HCT-8 cells were procured from NCCS, Pune, and ATCC, respectively. Influenza-A H1N1 virus (Strain: A/NWS/33) and Human Beta Coronavirus (Strain: OC43) were procured from ATCC. DMEM media, RPMI-1640 media, FBS, Penicillin Streptomycin solution, and Trypsin-EDTA were procured from HiMedia Laboratories Pvt. Ltd. Influenza-A H1N1 virus was propagated in the MDCK cell line and Human Beta Coronavirus was propagated in the HCT-8 cell line under Biosafety Level 2 category at TheraIndx Lifesciences Pvt Ltd (Karnataka, India). Test Item SSV-003 (batch Number CU.CO20-01) was provided by the GMP compliant production department of Shreepad Shree Vallabh SSV Phytopharmaceuticals (Maharashtra, India).
In vitro Antiviral Activity of SSV-003 against Influenza A Virus (H1N1) and Human Beta Coronavirus
In vitro efficacies of test compounds (SSV-003 and Vitamin C) were assessed by the MTT method to determine IC50 using Influenza-A H1N1 virus and Human Beta Coronavirus. The In vitro cytopathic effect (IC50) was determined by MTT method using MDCK cells (Influenza-A H1N1 virus Strain: A/NWS/33) and HCT-8 cells (Human Beta Corona Virus Strain: OC43) for 2–7 days.
Antiviral Activity Assay against Influenza-A H1N1 Virus (100TCID50)
MDCK cells were seeded into a 96-well tissue culture plate 1 day prior to the testing so as to get an approx. 80% monolayer on the next day. Test compounds were solubilized in DMSO to make master stock solutions. All subsequent working stock solutions were prepared in infectious PBS (iPBS). Test compounds and Influenza-A H1N1 virus (100TCID50) were mixed and added in 96-well plates and incubated at 37°C in 5% CO2 for 1 hour for drug–virus interaction as well as for virus adsorption. After incubation, the drug virus mixture was discarded and plates were washed with PBS followed by the addition of infectious DMEM (iDMEM) and the plates were further incubated for 3–7 days or till virus control showed a Cytopathic effect (CPE).
Antiviral Activity Assay against Human Beta Coronavirus (100TCID50)
HCT-8 cells were seeded into a 96-well tissue culture plate 1 day prior to the testing so as to get an approx. 80% monolayer on the next day. Test compounds were solubilized in DMSO to make master stock solutions. All subsequent working stock solutions were prepared in PBS. Test compounds and Human Beta Corona virus (100TCID50) were mixed and added in 96-well plates and incubated at 37°C in 5% CO2 for 1 hour for drug–virus interaction as well as for virus adsorption. After incubation, the drug–virus mixture was discarded and the platea washed with PBS followed by addition of RPMI-1640 medium and the plates were further incubated for 3–7 days or till virus control showed CPE. The viability of the infected and non-infected cells was evaluated by MTT method. The IC50 was determined by plotting percent cytopathy against the concentration. Propagated virus titre was determined by TCID50. The percent viability of cells in the untreated group was set to 100% and the percent viability of cells in the treated groups was estimated relative to the untreated control.
Efficacy of SSV-003 on Influenza A Virus Infection in Balb/c Mice
Male Balb/c mice having body weight of 25±1 g (aged 7 weeks, n=50) were anesthetized with intra-peritoneal (i.p.) injection of Ketamine 60 mg/kg IP + Xylazine 10 mg/kg. Anesthetized mice were infected intranasally by instillation of 30 μL of the virus inoculum (1x104) IAV (Plaque Forming Units (PFU)/mouse) into the nostrils (15 μL/nostril). The animals were divided into five groups containing 10 animals each: G1 (Infection Control), G2 (Positive ctrl, Remdisivir, 25 mg/kg, SC), G3 (SSV-003, 500 mg/kg) G4 (SSV-003, 1,000 mg/kg), and G5 (SSV-003, 1,500 mg/kg), which were treated every 24-hours post-infection for 9 days. SSV-003 was provided as a tablet, crushed, and resuspended in 0.25% CMC (Carboxy Methyl Cellulose) at the doses mentioned above. Animals were observed for general clinical signs, body temperature, and body weights morbidity and mortality twice daily throughout the study by the study veterinarian. All animals were sacrificed after 10 days’ post-infection to determine the viral load in the lungs. The blood samples were collected on Day −1 and Day 10 for assessment of cellular and humoral responses. IgG and IgM were estimated from serum, using commercially available kits. Flow cytometry was used to evaluate the CD4+ and CD8+ cells in blood as a measure of the cellular immune response. Lungs were aseptically excised and homogenized in a sterile homogenizer in 1 mL of cold infection media and stored at 4°C. The samples were centrifuged and the supernatant was collected in a new sterile tube and assayed for viral titer by TCID50 method as described above.
Acute Oral Toxicity Study
For acute toxicity studies of SSV-003, a stepwise procedure using three female animals per step was used in the study as described in the OECD guidelines for testing of chemicals, number 423. A starting dose of 300 mg/kg body weight was selected for step 1. If no morbidity was observed the next set of animals were given an increased dose SSV-003 (2,000 mg/kg body weight). All the animals received a single dose of the SSV-003 (P.O.), after fasting for approximately 10 hours, but with free access to water. Feed was offered after 3 hours of test item administration. Clinical observations were performed to look for signs of ill health or overt toxicity during the first 30 minutes and at approximately 1, 2, 3, and 4 hours after dose administration on Day 0 and daily during days 1–14. Any abnormalities of appearance, behavior, or other signs of reaction were recorded for each animal. All the surviving animals were necropsied at the end of the 14-day observation period and subjected to gross pathology.
The animal room was monitored for temperature and relative humidity daily. The temperature range was 22°C±3°C and the humidity range was 30–70%. The animals were provided with 12 hours light and 12 hours darkness, and housed in IVC systems. Animal feed and autoclaved drinking water were provided ad libitum. The studies were performed as per ethical practices laid down in the CPCSEA guidelines for animal care.14
Ethics Approval
The research work was approved by the Institutional Animals Ethics Committee (IAEC) (Approval number: IAEC/14/2020/172) of the test facility, TheraIndx Lifesciences Pvt Ltd, Karnataka, India, under biosafety level 2 conditions.
Data Analysis
In the case of in-vitro anti-viral activity studies, the percent viability of cells in the untreated group was set to 100% and the percent viability of cells in the treated groups was estimated relative to the untreated control. Based on the dose–response relationships, an appropriate model was fit to estimate the Imax and IC50 by plotting the percent viability against the concentration.
All values of the parameters CD4+, CD8+, IgG, & IgM are expressed as the mean±SD. Significant differences between the treated group and the Virus control were analyzed by One-way ANOVA, followed by a Dunnett’s multiple comparison test, using GraphPad Prism at 95% confidence levels. *P<0.05 vs Virus control and $P<0.05 vs Remdesivir, nsno significant difference and n=3, pooled samples of three animals in triplicates.
Results
In vitro Antiviral Activity against Influenza A-H1N1-VR219
SSV-003 had IC50 values lessthan the concentration tested, ie, lessthan 0.39 µg/mL against Influenza A-H1N1-VR219, where as IC50 was more than 50 (µg/mL) for vitamin C and, for Ribavirin, the observed IC50 was 1.38 (µg/mL). Significantly high cytopathic effects (91%) were observed in the case of vitamin C, while no cytopathic effect was observed in the cases of SSV-003 and Ribavirin. None of the tested compounds had cytotoxic effects against MDCK cells (Tables 1 and 2).
 |
Table 1 IC50, Percent Cytotoxicity, and Percent CPE of Test Compounds (Influenza A-H1N1-VR219) |
 |
Table 2 Percent CPE of Test Compounds at Different Tested Concentrations (Influenza A-H1N1-VR219) |
In vitro Antiviral Activity against Human Beta Coronavirus
SSV-003 had an IC50 of 2.26 µg/mL against human beta coronavirus. Ribavirin and Remedisivir showed IC50 values of 2.25 µg/mL and 6.23 µg/mL, respectively. Vitamin C showed a 93% cytopathic effect, whereas no cytopathic effect was observed in the cases of SSV-003, Ribavirin, and Remedisivir. None of the tested compound had cytotoxic effects against HCT-8 cells (Tables 3 and 4).
 |
Table 3 IC50, Percent Cytotoxicity, and Percent CPE of Test Compounds (Beta Corona-OC43) |
 |
Table 4 Percent CPE of Test Compounds at Different Tested Concentrations (Beta Corona-OC43) |
Efficacy of SSV-003 on Influenza A Virus Infection in Balb/c Mice
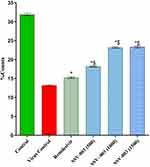
The TCID50 (Median Tissue Culture Infectious Dose) assay is indicative of viral titer and viral infection. A significantly higher TCID50 was observed in the infected control group as compared to test groups. Groups treated with SSV-003 showed a dose dependent decrease in TCID50. The percent decrease in infection also happened in a dose-dependent manner. SSV-003 at the doses of 500, 1,000, and 1,500 mg/kg body weight showed 85.9%, 94.6%, and 95.1% decreases in infection as compared to the infected control group. The positive control group showed a 99.6% drop in infection as compared to the infected control group (Table 5). No significant change in animal body weight was observed in all the groups over the treatment period of 10 days. No mortality was observed.
 |
Table 5 Median Tissue Culture Infectious Dose in Mice Lungs following Treatment with Test Compounds |
Effect on CD4+ and CD8+ Counts
CD4+ and CD8+ counts were significantly reduced in viral control as compared with the Control group before Infection, whereas all the treatment groups showed improvement in CD4+ and CD8+ counts. SSV-003 showed a dose-dependent increase in CD4+ and CD8+ counts. SSV-003 at 500, 1,000, and 1,500 mg/Kg exhibited increased CD4+ counts by 37.6%, 75.75%, and 76.74%, respectively. Remdisivir at a concentration of 25 mg/kg showed an increase in CD4+ counts by 15%. SSV-003 at the dose of 500, 1,000, and 1,500 mg/Kg exhibited increased CD8+ counts by 57.77%, 64.44%, and 66.66%, respectively. Remdisivir showed an increase in CD8+ counts by 22.22% (Figures 1 and 2).
Effect on IgG and IgM Levels
IgG and IgM levels were decreased in virus control by 5.6% and 31.5%, respectively, as compared with the Normal control (before infection). SSV-003 at the dose of 500, 1,000, and 1,500 mg/kg showed an increase in IgG and IgM concentration by 8.6%, 17%, 16%, and 7.6%, 23%, 23%, respectively. Positive control Remdisivir exhibited a mild increase in IgG and IgM concentration by 3.3% and 7.6%, respectively (Table 6).
 |
Table 6 Percent Increase in Immune Parameters as Compared to the Virus Control Group |
Acute Oral Toxicity of SSV-003
No mortality or morbidity was observed during the study. Animals showed an increase in body weight. All animals were apparently normal condition following test item administration. Based on the results obtained, SSV-003 (test item) falls under “Category 5 (>2,000–5,000)” according to the Globally Harmonized System (GHS) for the classification of chemicals. The cut-off LD50 value is greater than 2,000 mg/kg.
Discussion
The development of novel antiviral drugs is a challenging task and persistent threat of viral pandemics and epidemics such as COVID-19, Zika virus, and Chikungunya virus has prompted fast track drug development to check the spread of viruses. To fasten anti-viral drug development, repurposing of existing molecules and exploring herbs and phytochemicals for their antiviral activities can be an efficient strategy. Keeping current global health emergency in-view, researchers at SSV phytopharmaceuticals developed a SSV-003 formulation for better management of COVID-19 patients. The SSV-003 formulation was developed after thorough research. SSV-003 comprises of Curcumin, Vitamin C, Vitamin K2-7, Selenomethionine and Zinc. In this research paper, we discuss outcomes of in-vitro and in-vivo studies and acute toxicity studies.
For In vitro studies, MDCK cell lines for Influenza A-H1N1-VR219 and HCT-8 cell lines for Beta Corona-OC43 were used. Influenza vaccine derived from MDCK culture has been approved by the European Medicines Agency (EMEA).15,16 HCT-8 are colon adenocarcinoma cells that are used for culture HCoV-OC43, a beta corona virus 1 that most commonly infects humans.17 In the current study, SSV-003 had an IC50 lessthan Ribavirin and vitamin against Influenza A-H1N1-VR219 with no cytopathic effect. SSV-003 had an IC50 of 2.26 µg/mL against Human Beta Coronavirus, similar to IC50 of Ribavirin (2.25 µg/mL), but much lessthan that of Remedisivir (6.23 µg/mL). Richart et al18 reported that curcumin and mono-acetylcurcumin inhibited influenza virus infection. In vitro studies on MDCK cells showed significant antiviral activity of curcuma longa against H5N1 virus (avian influenza virus) by interfering with viral hemagglutination (HA) activity. It up-regulates the mRNA expression of tumor necrosis factor‐α and interferon‐β in MDCK cells. TNFα and interferon‐β are potent antiviral agents. Also curcumin inhibited the (PI3K)/AKT cellular pathway in tumor cells, whereas influenza virus infection activates PI3K/AKT.19
SSV-003 was found to be effective against Influenza A Virus in Balb/c mice. SSV-003 treated groups showed a dose-dependent decrease in TCID50. All the treatment groups showed improvement in CD4+ and CD8+ counts. Dose-dependent increases in IgG and IgM levels were observed in SSV-003 treated groups. Fani et al19 reported the anti-viral activity of curcumin against herpes simplex virus. The average number of HSV plaque was significantly more in the control group and least in the Curcumin extract group (2 mg/mL). Curcumin inhibited viral plaque formation. Ichsyani et al20 studied the anti-viral activity of curcumin against dengue virus (DENV), reporting the acquired value of IC50 as 17.91 µg/mL, while the CC50 value was 85.4 µg/mL. They reported a significant reduction in viral load on treatment with C. longa within 24 hours.
T-cells are responsible for immune response and provide strength to fight against multiple infections. If the count of CD4+ and CD8+ cells is high, T-cell activation occurs and it represents a strong immune system. The study data showed that SSV-003 significantly improved the CD4+ and CD8+ counts, thus improving cellular immunity, which is beneficial to fight against infections. Chai et al21 studied the effect of curcumin on acute lung injury/acute respiratory distress syndrome (ALI/ARDS) in cecal ligation and a puncture (CLP)-induced acute lung injury mouse model. They reported that Curcumin suppressed inflammatory mediators, alleviated lung injury, and increased CD4+CD25+Foxp3+ Tregs. It up-regulated the differentiation from CD4+ naïve T-cells to regulatory T-cells (Tregs) in vitro. Avasarala et al22 studied the anti-inflammatory effect of curcumin on reovirus 1/L-induced acute viral pneumonia, which resembles characteristics of the human ALI/ARDS. They concluded the role for curcumin in modulating the pathogenesis of viral-induced ALI/ARDS through its anti-inflammatory and immune modulating activities. Curcumin can inhibit the infiltration of Polymorphonuclear neutrophils (PMNs), including GR1+, CD4+, CD19+ B-cells, NK cells, and CD8+ T-cells, and promotes the apoptosis of PMN by increasing the level of P-p38. Xiao et al23 reported that curcumin analog C66 protects lipopolysaccharide (LPS)-induced ALI through suppression of the JNK pathway and subsequent inhibition of inflammatory cytokine expression. IgG and IgM are major immunoglobulins, which play significant roles in fights against infections. SSV-003 showed improved concentration of IgG and IgM, suggesting its immuno-modulatory activity.
Each constituent of SSV-003 has its own biological activity and plays a crucial role in boosting immunity and reducing disease related damage. Bio-activities of Curcumin against dengue virus, human influenza virus, herpes simplex virus, and human immune deficiency virus are widely reported. Its anti-viral activity is ascribed to different mechanisms and pathways. Chen et al24 reported that Curcumin inhibits dengue virus entry and Padilla-S et al25 reported it inhibited particle production. Several researchers have reported curcumin inhibit Influenza A virus uptake, replication, and particle production. It induces post-transcriptional and post-translational modifications and impedes key steps of viral replication as well as its attachment.26 Dimethoxycurcumin is the bioactive metabolite of curcumin and molecular docking studies suggested that it docked Mpro. It is reported to alter viral surface proteins, thus blocking its entry to the host cell.11 Our published molecular docking studies clearly indicated that curcumin can bind to the receptors and inhibit ACE2, thus blocking the entry of COVID-19 virus.27 Vitamin K2-7 is effective in reducing pro inflammatory cytokines. Selenomethionine is a natural form of Selenium (Se) which plays a critical role in prevention of infection. Selenium (Se) is reported to be directly involved in combating against various viruses like influenza virus, HSV-1, HCV, and HIV. Sanna et al28 reported anti-viral activity essential oils isolated from Hornstedtia bella Škorničk. Moderate supplementation of Se could enhance tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) levels leading to an improved immune response.29 Wang et al30 reported Se inhibited the cytopathic effect of HSV and promoted cell apoptosis. Vitamin C is very effective in reducing cell mediators such as interleukin-6 and endothelin-1 levels which are regarded as pro-inflammatory factors.31 Our recent clinical studies on SSV-003 on mild-to-moderate COVID-19 infected patients (n=200) clearly established that it reduced inflammatory markers, improved patients disease condition to asymptomatic with relief from symptoms within 48 to 72 hours.13
The limitation of the current study is that biological markers establishing a mechanism of action of the SSV-003 should have been studied. Keeping these results and their good correlation with reported bioactivities of individual constituent of SSV-003 in-view, we can conclude that SSV-003 possessws potent anti-viral activities and is non-toxic to animals.
Acknowledgment
The authors greatly appreciate and thank Dr Ramesh Jayaraman and Mr Mahesh Nanjundappa, for providing infrastructural and facility support for cell culture and in-vivo studies at Theraindx Lifesciences Pvt. Ltd, Karnataka, India.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Complete research work was performed independent of production department of M/s Shreepad Shree Vallabh SSV Phytopharmaceuticals. Dr Yogesh Arun Dound reports grants from Shreepad Shree Vallabh SSV Phytopharmaceuticals and is the proprietor of the Company, during the conduct of the study. In addition, Dr Yogesh Arun Dound has a patent of the studies formulations pending. The authors do not have any other conflicts of interest.
References
1. Decaro N, Mari V, Elia G, et al. Recombinant canine coronaviruses in dogs, Europe. Emerg Infect Dis. 2010;16(1):41–47. doi:10.3201/eid1601.090726
2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi:10.1038/s41586-020-2008-3
3. Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–664. doi:10.1128/MMBR.69.4.635-664.2005
4. Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11):S223–S227. doi:10.1097/01.inf.0000188166.17324.60
5. Matricardi P, Dal Negro R, Nisini R. The first, holistic immunological model of COVID‐19: implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol. 2020;31(5):454–470. doi:10.1111/pai.13271
6. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
7. Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID-19: an overview. Eur J Pharmacol. 2020;889:173644. doi:10.1016/j.ejphar.2020.173644
8. Praditya D, Kirchhoff L, Brüning J, Rachmawati H, Steinmann J, Steinmann E. Anti-infective properties of the golden spice curcumin. Front Microbiol. 2019;10:912. doi:10.3389/fmicb.2019.00912
9. Pang XF, Zhang LH, Bai F, et al. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des Devel Ther. 2015;9:6043–6054. doi:10.2147/DDDT.S95333
10. Abdollahi E, Momtazi AA, Johnston TP, Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J Cell Physiol. 2018;233(2):830–848. doi:10.1002/jcp.25778
11. Dound YA, Chaudhary S, Sehgal R, Chaudhary SS, Dound BA. Plant based molecules for the management of Covid-19. J Infect Dis Ther. 2020;S2:008.
12. Johnson LJ, Meacham SL, Kruskall LJ. The antioxidants–vitamin C, vitamin E, selenium, and carotenoids. J Agromedicine. 2003;9(1):65–82. PMID: 14563626. doi:10.1300/J096v09n01_07
13. Dound YA, Mandlik S, Suryavanshi S, Sehgal R, Naik A. A randomized, comparative clinical study to evaluate the activity of SSV-003 (SSV-003) formulation for management of SARS-COV-2 infection (COVID-19). J Clin Trials. 2020;S3:1000004.
14. CPCSEA. Guidelines for laboratory animal facility. Indian J Pharmacol. 2003;35:257–274.
15. Luo H, Zhao M, Tan D, et al. Anti-COVID-19 drug screening: frontier concepts and core technologies. Chin Med. 2020;15(1):115. doi:10.1186/s13020-020-00393-z
16. Tsai HC, Lehman CW, Lin CC, et al. Functional evaluation for adequacy of MDCK-lineage cells in influenza research. BMC Res Notes. 2019;12(1):101. doi:10.1186/s13104-019-4134-2
17. Owczarek K, Szczepanski A, Milewska A, et al. Early events during human coronavirus OC43 entry to the cell. Sci Rep. 2018;8(1):7124. doi:10.1038/s41598-018-25640-0
18. Richart SM, Li YL, Mizushina Y, et al. Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti‐influenza virus infection. J Food Drug Anal. 2018;26(3):1015–1023. doi:10.1016/j.jfda.2017.12.006
19. Fani MM, Motamedifar M, Kordshouli MZ. In vitro assessment of the anti-viral effect of Curcumin longa on Herpes Simplex virus type 1. J Biol Todays World. 2015;4(5):115–119. doi:10.15412/J.JBTW.01040502
20. Ichsyani M, Ridhanya A, Risanti M, et al. Antiviral effects of Curcuma longa L. against dengue virus in vitro and in-vivo. IOP Conf Ser Earth Environ Sci. 2017;101:012005. doi:10.1088/1755-1315/101/1/012005
21. Chai YS, Chen YG, Lin SH, et al. Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed Pharmacother. 2020;125:109946. doi:10.1016/j.biopha.2020.109946
22. Avasarala S, Zhang F, Liu G, et al. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS One. 2013;8(2):e57285. doi:10.1371/journal.pone.0057285
23. Xiao Z, Xu F, Zhu X, et al. Inhibition of JNK phosphorylation by curcumin analog C66 protects LPS-induced acute lung injury. Drug Des Dev Ther. 2019;13:4161–4171. doi:10.2147/dddt.S215712
24. Chen TY, Chen DY, Wen HW, et al. Inhibition of enveloped viruses infectivity by curcumin. PLoS One. 2013;8(5):e62482. doi:10.1371/journal.pone.0062482
25. Padilla-S L, Rodríguez A, Gonzales MM, Gallego-G JC, Castaño-O JC. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch Virol. 2014;159(3):573–579. doi:10.1007/s00705-013-1849-6
26. Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12(3):332–347. doi:10.2174/138945011794815356
27. Dound YA, Chaudhary S, Chaudhary SS, Ahmad MM, Geesi MH. Preventive strategies for management of COVID-19 using natural molecules. Res Sq. 2020. doi:10.21203/rs.3.rs-24587/v1
28. Sanna G, Madeddu S, Serreli G, et al. Antiviral effect of Hornstedtia bella Škorničk essential oil from the whole plant against vaccinia virus (VV). Nat Prod Res. 2020;(25):1–7. doi:10.1080/14786419.2020.1824228
29. Yu L, Sun L, Nan Y, Zhu LY. Protection from H1N1 influenza virus infections in mice by supplementation with selenium: a comparison with selenium-deficient mice. Biol Trace Elem Res. 2011;141(1–3):254–261. doi:10.1007/s12011-010-8726-x
30. Wang A, Yu K, Zou W, Song K. Anti-herpes simplex virus type 1 activity of trace element selenium In vitro. J Nanchang Univ. 2012;52(9):1–4.
31. Chen Y, Luo G, Yuan J, et al. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014:426740.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.