Back to Journals » Clinical Ophthalmology » Volume 13
Precision pulse capsulotomy in challenging cataract surgery cases
Authors Park MJ , Bang CW , Han SY
Received 31 May 2019
Accepted for publication 2 July 2019
Published 25 July 2019 Volume 2019:13 Pages 1361—1368
DOI https://doi.org/10.2147/OPTH.S217919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Video 1 An example of using the precision pulse capsulotomy device in an eye with a poorly dilated pupil
Views: 1101
Min Ji Park, Chan Woo Bang, Sang Youp Han
Department of Cornea and Refractive Surgery, Busan Sungmo Eye Hospital, Busan, Republic of Korea
Purpose: To evaluate the use of the precision pulse capsulotomy (PPC) device for challenging cataract surgery cases.
Patients and methods: This single-center retrospective case series study comprised of 43 eyes (from 35 patients) that were challenging cataract surgery cases with poorly dilated pupils, anterior subcapsular opacity, white cataract, brunescent cataract, and corneal opacity. This was conducted at the Busan Sungmo Eye Hospital (Busan, Republic of Korea) to assess the performance of the PPC device through a 2.2-mm clear corneal incision width, followed by the phacoemulsification technique and intracapsular intraocular lens fixation. The main outcome measurement was the anterior capsulotomy performance of the PPC device and the development of intraoperative complications. At postoperative 2 months, visual acuity, endothelial cell count, and refractive error were measured.
Results: No cases of anterior capsule tears or tags occurred. All 43 eyes received circular, 360-degree, free-floating, and appropriately sized anterior capsulotomies. During 2 months of follow up, no postoperative complications occurred in association with the PPC device.
Conclusion: The PPC device facilitated the creation of a precise, round, appropriately sized anterior capsulotomy in challenging cataract surgery cases. Further investigations are required to understand the long-term safety and efficacy of the PPC device.
Keywords: Zepto, capsulorhexis, cataract, cataract extraction, phacoemulsification
Introduction
A continuous curvilinear capsulorhexis (CCC) is a very important step in phacoemulsification cataract surgery. The state of CCC such as size, shape, strength, and centration affects all subsequent steps of cataract surgery such as hydrodissection, nuclear removal, and insertion of the intraocular lens (IOL). Performing manual CCC is often difficult for clinicians—particularly in challenging cataract surgery cases such as patients with white cataract, anterior subcapsular opacity, and zonulopathy. To improve the precision, strength, safety, and ease of CCC creation, femtosecond laser-assisted cataract surgery (FLACS) was developed in recent years. With FLACS, surgeons have consistently achieved a round, well-centered, and reproducible CCC.1–5 However, the high cost of FLACS, the extra space required for the bulky equipment, and the increased operative time are prohibitive. The femtosecond laser also has limitations in patients who have corneal opacity, a poorly dilated pupil, dense cataracts, or other issues that make the suction and docking steps more challenging.4,6
Precision pulse capsulotomy (PPC) with the Zepto device (Mynosys Cellular Devices, Inc., Fremont, CA, USA). has recently been introduced and commercialized (Figure 1). Precision pulse capsulotomy uses a highly focused, fast, multipulse, low-energy discharge to produce a perfectly round anterior capsulotomy instantaneously and simultaneously along all 360 degrees.7 In cadaver eyes, a previous study8 demonstrated that the capsulotomy edge was significantly stronger with PPC than with femtosecond laser or manual CCC. In an actual human eye, the PPC device also creates a complete precise circular anterior capsulotomy. The PPC device works well independently of corneal clarity, pupil size, and grade of lens opacification.9–12 We report our experiences with using the PPC device in patients with challenging cases of cataract surgery who were treated with the phacoemulsification technique and intracapsular IOL implantation.
Materials and methods
Study design
This retrospective case series study assessed the use of the PPC device in challenging cataract surgery cases at a single center (Sungmo Eye Hospital) in Busan, Republic of Korea. All surgeries were performed between April 2018 and November 2018 by one experienced surgeon (SYH). All patients had a complicated cataract status characterised by poorly dilated pupils, corneal opacity, anterior subcapsular opacity, white cataract and/or brunescent cataract. The exclusion criteria were as follows: age under 18 years old; a history of glaucoma, retinal detachment, macular degeneration, retinopathy, neuro-ophthalmic disease, or ocular inflammation; and patients with diabetes. All tenets of the Declaration of Helsinki were followed. Ethics review board approval for the study was obtained from the Sungmo Eye Hospital Ethical Committee. Informed written consent was obtained preoperatively from all patients.
Preoperative assessment
All patients had a standard full preoperative assessment, which included biometry; keratometry and anterior segment and fundus examination with slit lamp microscopy; optical biometry (OA2000 optical biometer; Tomey Corporation, Nagoya, Japan); ultrasound biometry (Axis Nano; Quantel Medical, Rockwall, TX, USA); B-scan (UD-800 system; Tomey Corporation); visual quality analyzer (KR1W; Topcon, Tokyo, Japan); optical coherence tomography (OCT) (Cirrus HD OCT model 5000; Carl Zeiss Meditec; Inc., Jana, Germany); anterior segment OCT (CASIA SS-1000; Tomey Corporation); specular microscopy (ROBO-CA; Konan Medical Inc., Hyogo, Japan); non-contact tonometry (NT-530P; Nidek Co., Ltd., Gamagori, Japan); autorefraction (KR-8100PA; Topcon, Tokyo, Japan); and manual refraction. Cataracts were subdivided into nuclear opalescence grades I–V, based on the Lens Opacity Classification System III (LOCS III) criteria.13
Surgical technique
All patients preoperatively received topical 1.5% levofloxacin (Cravit Ophthalmic Solution 1.5%; Santen Pharmaceutical Co., Ltd., Osaka, Japan), 0.1% bromfenac sodium hydrate (Bronuck Ophthalmic Solution; Taejoon, Seoul, Korea) and 3% diquafosol (Diquas; Santen, Inc., Osaka, Japan) four times daily for 3 days. On the day of the surgery, pupil-dilating eye drops were administered with a solution containing 0.5% tropicamide and 0.5% phenylephrine (Mydrin-P; Santen, Inc., Osaka, Japan). After establishing standard topical anesthesia, sterile preparation and placement of the lid speculum, paracentesis was performed and 1.5% sodium hyaluronate (Hyalu; Hanmi Pharm.Co.,Ltd., Seoul, Korea) was injected to fill the anterior chamber. A 2.2-mm clear corneal incision was formed on the temporal side.
The PPC device was primed with a balanced salt solution. The surgeon manually extended the slider on the PPC handpiece. The PPC silicone cup was elongated. While stabilizing the eye with corneal forceps and providing counter traction, the elongated PPC tip was inserted through the incision into the anterior chamber. The slider was moved backward by retracting the push rod. The device regained its original circular shape within the anterior chamber. The tip was positioned on the desired anterior capsulotomy site. Suction was applied via the PPC control console and allowed to reach its desired maximum vacuum. The maximum desired vacuum was achieved when the bubble within the ophthalmic viscosurgical device stopped moving within the barrel of the handpiece. Electrical energy was discharged through the ring via the PPC control console. After suction was released, the collapsible PPC tip was manually removed from the anterior chamber. The surgeon removed the free-floating anterior capsule using forceps.
The remaining surgery was conducted with the standard phacoemulsification technique by using the Centurion Vision System (Alcon Laboratories, Inc., Fort Worth, TX, USA) or the Whitestar Signature Pro Phacoemulsification System (Advanced Medical Optics, Inc., Santa Ana, CA, USA). A single piece IOL was placed in the capsular bag for bag fixation. The desired anterior capsulotomy site was measured against the expected visual axis by using the VERION Image Guided System (Alcon Laboratories, Inc., Fort Worth, TX, USA).
Intraoperative and postoperative assessment
During surgery, the shape and size of the capsulotomy were observed, and the capsule and capsulotomy edge were checked for any tags or tears. Patients were followed up postoperatively at 1 day, 1 week, 3 weeks, and 2 months. Patients had a slit lamp microscopy examination and intraocular pressure measurement at each follow up. The best corrected visual acuity (BCVA) and the refractive error were postoperatively evaluated at 1 day and at 2 months. The endothelial cell count was examined postoperatively at 2 months. After surgery, patients instilled at home topical 1.5% levofloxacin (Cravit Ophthalmic Solution 1.5%; Santen Pharmaceutical Co., Ltd.), 0.1% bromfenac sodium hydrate (Bronuck Ophthalmic Solution; Taejoon), 3% diquafosol (Diquas; Santen, Inc.), and 0.1% fluorometholone (Flumetholon; Santen, Inc.). All medications were administered four times daily for 2 months.
Outcome measures
The main outcome measures were capsulotomy performance of the PPC device and intraoperative complications associated with using the PPC device. The secondary outcome measures were postoperative BCVA, loss in the endothelial cell count, early refractive outcome, intraocular pressure, and other postoperative complications.
Statistical analysis
Data were collected and tabulated using Excel (Office 365; Microsoft, Redmond, WA, USA) and analysed using SPSS version 18 (IBM corporation, Armonk, NY, USA). Data obtained before and after surgery were compared using the Wilcoxon signed-rank test. p-values less than 0.05 were considered statistically significant.
Results
This study comprised 43 eyes from 35 patients who were 35–92 years old. Twenty-two patients were male and 13 patients were female. Table 1 shows the patients’ demographics and preoperative data. Fifteen eyes had an anterior subcapsular cataract and 11 eyes had a poorly dilated pupil. Five eyes had a white cataract, eight eyes had brunescent lenses (1 eye had a poorly dilated pupil and 1 eye had corneal opacity) and four eyes had corneal opacity (1 eye had a poorly dilated pupil). The mean nuclear opalescence grade was 4.20, based on the LOCS III criteria. The anterior chamber depth ranged from 2.26 mm to 4.52 mm.
 |
Table 1 Demographics of the patients |
Intraoperative observations
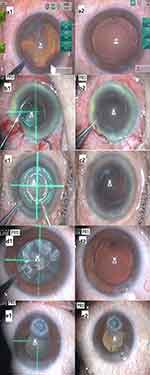
No anterior capsule tears or tags occurred among the 43 eyes. All eyes received a round, 360-degree, free-floating, and appropriately sized capsulotomy without any intraoperative complications. The surgeries were completed with standard phacoemulsification and capsular bag IOL fixation. Figure 2 shows digital photographs of the anterior segment before and after creating the anterior capsulotomy with the PPC device at the time of surgery (a1 and a2: anterior subcapsular cataract; b1 and b2: poorly dilated pupil; c1 and c2: white cataract; d1 and d2: brunescent cataract; e1 and e2: corneal opacity with poorly dilated pupil).
For eyes with a poorly dilated pupil, no pupil expanders or iris hooks were necessary. Precise capsulotomies were performed in all patients by placing the PPC device under the iris through the small pupil. Video 1 shows an example of using the PPC device in an eye with a poorly dilated pupil.
One patient had a poorly dilated pupil along with cornea opacity and thick iridocorneal adhesion (Figures 3 and 4). In this situation, if the pupil were to be dilated forcibly by an iris hook or pupil expander, it would likely create corneal thinning or perforation. To enlarge the pupillary aperture, the pupil was stretched by instruments before inserting the PPC device. An anterior capsulotomy was subsequently performed with the PPC device. Video 2 shows the surgery of this patient.
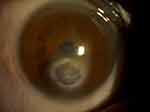 |
Figure 3 Digital photograph of the anterior segment of a patient with a poorly dilated pupil, corneal opacity, and iridocorneal adhesion. |
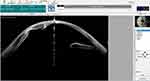 |
Figure 4 Anterior segment optical coherence tomography (OCT) of a patient with a poorly dilated pupil, corneal opacity, and iridocorneal adhesion. |
Video 3 shows an eye with a white cataract. All patients with white cataracts received a round strong capsulotomy without the use of dry trypan blue to visualize the anterior capsule, as is used in creating manual CCC.
Postoperative observations
The IOL was well-centered in all patients. In all patients, no other postoperative complications occurred during 2 months of follow up. Table 2 presents the corneal endothelial cell count preoperatively and at 2 months postoperatively. The endothelial cell loss was approximately 10.7%. Figure 5 shows the change in the BCVA preoperatively and at 1 day postoperatively and 2 months postoperatively; the BCVA was 1.16±1.04 logMAR, 0.31±0.48 logMAR, and 0.16±0.27 logMAR, respectively. A statistically significant improvement occurred over time (p<0.001 between preoperative and postoperative 1 day and p<0.001 between postoperative 1 day and 2 months). The spherical equivalent of preoperative targeted refraction, and the results of manual refraction at postoperative 1 day and at postoperative 2 months, were −0.80±3.78D, −0.38±2.34D, and 0.51±2.27D, respectively. The data were not significantly different. Intraocular pressures measured preoperatively and at 2 months postoperatively were 15.02±3.27 and 14.30±3.40 mmHg, respectively. Hence, intraocular pressure was significantly decreased after cataract surgery (p=0.018).
 |
Table 2 Preoperative and postoperative endothelial cell count results |
 |
Figure 5 Best corrected visual acuity at preoperation, postoperative 1 day, and postoperative 2 months. |
Discussion
In this study, we evaluated use of a PPC device in challenging cases of cataract surgery when using the phacoemulsification technique and IOL implantation. We found the PPC device facilitated the creation of a round, appropriately sized anterior capsulotomy in challenging cases of cataract surgery. An anterior capsular tear can create hazardous complications such as posterior capsular rupture, vitreous loss, dropped nucleus, IOL displacement, choroidal hemorrhage, and iris trauma.14 Previous studies have reported the incidence of anterior capsule tears as between 0.79% and 5.55% for manual CCC,15–17 and between 0.0% and 5.3% for FLACS.18–21 In the current study, no patient had intraoperative anterior capsule tears when the PPC device was used. Thompson et al8 previously demonstrated that the strength of the PPC capsulotomy edge was two to four times greater than that produced by the femtosecond laser or manual CCC in cadaver eyes. However, other studies have reported the incidence of anterior capsulotomy tears after using the PPC device as between 4.0% and 6.0%.9,10,22 The rate of anterior capsule tears was less in our experience with using the PPC device than the rate reported in the literature for manual CCC, especially in difficult cases.
In this study, no cases of corneal touch occurred, even in patients with a shallow anterior chamber. The smallest anterior chamber was 2.26 mm in this study. No corneal thermal damage was observed. Endothelial cell loss was approximately 10.7% at 2 months after surgery, compared to the preoperative measurement. In conventional cataract surgery, the reported endothelial cell loss data varies between 7.8% and 18.4%.23–26 Based on the grade of nucleosclerosis in this study, the PPC device may not cause additional damage to the corneal endothelium.
Postoperative intraocular pressure is influenced by the position of the IOL with respect to the iris and the angle of the anterior chamber. For example, if the IOL is placed in the sulcus anteriorly, aqueous blockage could occur if the iris is touching the IOL. In this study, there was a significant decrease in intraocular pressure following cataract surgery, which could reflect appropriate IOL positioning achieved using the PPC device.
In cases of cataract with anterior subcapsular opacity, particularly when the opacity extends toward the periphery and is thick, manual CCC creation is difficult and often results in radial tears. However, the PPC device created a round, free-floating anterior capsulotomy with a decreased risk of radial tear.
White cataracts are associated with an increased risk of incomplete anterior capsulotomy and with a posterior capsule tear rate of up to 11%.27 Creating an anterior capsulotomy is more difficult owing to a poor red fundus reflex and a larger capsule tension, which makes it difficult to identify the anterior lens capsule. High intralenticular pressure can create an immediate and extensive radial tear that increases the risk of posterior capsule tear and dropped nucleus. The release of the milky fluid can make it difficult to complete a capsulorhexis or treat attached tags in FLACS.28,29 With FLACS, the liquefied cortex is liberated, which can interfere with subsequent incoming laser pulses. However, the PPC device simultaneously creates a 360-degree mechanical cleavage; therefore, the liquified cortex does not interfere with the creation of a capsulotomy.
In cases of poorly dilated pupils with or without other ocular conditions such as corectopia, pseudoexfoliative syndrome, and posterior synechia, obtaining a good surgical view for the creation of a manual CCC can be difficult. Surgeons have used intraoperative intracameral epinephrine injection, manual pupil stretching with an instrument, and iris hook or pupil expender insertion to increase the intraoperative pupil size to facilitate the creation of manual CCC. If the pupil size is smaller than the set-up size of the capsulotomy, then FLACS cannot be used. However, with the PPC device, the suction cup is soft and insulated against heat; therefore, it can be placed under the iris and create a complete capsulotomy without heat energy damage.
In cases of corneal opacity, the femtosecond laser in FLACS passes through the cornea; therefore, the energy is irregularly transferred to the anterior capsule to create an incomplete capsulotomy. With the PPC device, the heat energy is transferred to the anterior capsule directly within the anterior camber; therefore, it creates a precise capsulotomy independent of corneal clarity.
The limitations of this study are the small number of patients, the fact that data are based on the findings of a single center, and one experienced surgeon having performed all surgeries (thus, the surgeon’s experience may have impacted the results).
Conclusion
This report, to the best of our knowledge, is the first paper to specifically discuss challenging cataract surgery cases. In our surgical experience, the PPC device provided an effective, precise, round, appropriately sized anterior capsulotomy, even in challenging cases of cataract surgery such as poorly dilated pupils, corneal opacity, anterior subcapsular opacity, white cataract, and brunescent cataract. Further investigations are required to understand the long-term safety and efficacy of the PPC device.
Abbreviation list
BCVA, best corrected visual acuity; CCC, continuous curvilinear capsulorhexis; FLACS, femtosecond laser-assisted cataract surgery; IOL, intraocular lens; OCT, optical coherence tomography; PPC, precision pulse capsulotomy.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Palanker DV, Blumenkranz MS, Andersen D, et al. Femtosecond laser-assisted cataract surgery with integrated optical coherence tomography. Sci Transl Med. 2010;2(58):58ra85. doi:10.1126/scitranslmed.3001305
2. Friedman NJ, Palanker DV, Schuele G, et al. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011;37(7):1189–1198. doi:10.1016/j.jcrs.2011.04.022
3. Auffarth GU, Reddy KP, Ritter R, Holzer MP, Rabsilber TM. Comparison of the maximum applicable stretch force after femtosecond laser-assisted and manual anterior capsulotomy. J Cataract Refract Surg. 2013;39(1):105–109. doi:10.1016/j.jcrs.2012.08.065
4. Nagy ZZ, McAlinden C. Femtosecond laser cataract surgery. Eye Vis (Lond). 2015;2:11. doi:10.1186/s40662-015-0021-7
5. Grewal DS, Schultz T, Basti S, Dick HB. Femtosecond laser-assisted cataract surgery–current status and future directions. Surv Ophthalmol. 2016;61(2):103–131. doi:10.1016/j.survophthal.2015.09.002
6. Donaldson KE, Braga-Mele R, Cabot F, et al; ASCRS Refractive Cataract Surgery Subcommittee. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753–1763. doi:10.1016/j.jcrs.2013.09.002
7. Chang DF, Mamalis N, Werner L. Precision pulse capsulotomy: preclinical safety and performance of a new capsulotomy technology. Ophthalmology. 2016;123(2):255–264. doi:10.1016/j.ophtha.2015.10.008
8. Thompson VM, Berdahl JP, Solano JM, Chang DF. Comparison of manual, femtosecond laser, and precision pulse capsulotomy edge tear strength in paired human cadaver eyes. Ophthalmology. 2016;123(2):265–274. doi:10.1016/j.ophtha.2015.10.019
9. Hooshmand J, Abell RG, Allen P, Vote BJ. Thermal capsulotomy: initial clinical experience, intraoperative performance, safety, and early postoperative outcomes of precision pulse capsulotomy technology. J Cataract Refract Surg. 2018;44(3):355–361. doi:10.1016/j.jcrs.2017.12.027
10. Kelkar JA, Mehta HM, Kelkar AS, et al. Precision pulse capsulotomy in phacoemulsification: clinical experience in Indian eyes. Indian J Ophthalmol. 2018;66(9):1272–1277. doi:10.4103/ijo.IJO_286_18
11. Pandey SK, Sharma V. Zepto-rhexis: a new surgical technique of capsulorhexis using precision nano-pulse technology in difficult cataract cases. Indian J Ophthalmol. 2018;66(8):1165–1168. doi:10.4103/ijo.IJO_286_18
12. Waltz K, Thompson VM, Quesada G. Precision pulse capsulotomy: initial clinical experience in simple and challenging cataract surgery cases. J Cataract Refract Surg. 2017;43(5):606–614. doi:10.1016/j.jcrs.2017.01.023
13. Chylack LT
14. Carifi G, Miller MH, Pitsas C, et al. Complications and outcomes of phacoemulsification cataract surgery complicated by anterior capsule tear. Am J Ophthalmol. 2015;159(3):463–469. doi:10.1016/j.ajo.2014.11.027
15. Marques FF, Marques DM, Osher RH, Osher JM. Fate of anterior capsule tears during cataract surgery. J Cataract Refract Surg. 2006;32(10):1638–1642. doi:10.1016/j.jcrs.2006.05.013
16. Olali CA, Ahmed S, Gupta M. Surgical outcome following breach rhexis. Eur J Ophthalmol. 2007;17(4):565–570. doi:10.1177/112067210701700414
17. Unal M, Yucel I, Sarici A, et al. Phacoemulsification with topical anesthesia: resident experience. J Cataract Refract Surg. 2006;32(8):1361–1365. doi:10.1016/j.jcrs.2006.02.063
18. Day AC, Gartry DS, Maurino V, Allan BD, Stevens JD. Efficacy of anterior capsulotomy creation in femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2014;40(12):2031–2034. doi:10.1016/j.jcrs.2014.07.027
19. Abell RG, Davies PEJ, Phelan D, Goemann K, McPherson ZE, Vote BJ. Anterior capsulotomy integrity after femtosecond laser-assisted cataract surgery. Ophthalmology. 2014;121(1):17–24. doi:10.1016/j.ophtha.2013.08.013
20. Nagy ZZ, Takacs AI, Filkorn T, et al. Complications of femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2014;40(1):20–28. doi:10.1016/j.jcrs.2013.08.046
21. Chang JS, Chen IN, Chan WM, Ng JC, Chan VK, Law AK. Initial evaluation of a femtosecond laser system in cataract surgery. J Cataract Refract Surg. 2014;40(1):29–36. doi:10.1016/j.jcrs.2013.08.045
22. Kucukevcilioglu M, Koylu MT, Mutlu FM. A novel capsulorhexis technique in white cataract surgery. Semin Ophthalmol. 2017;32(5):661. doi:10.3109/08820538.2015.1123736
23. Gogate P, Ambardekar P, Kulkarni S, Deshpande R, Joshi S, Deshpande M. Comparison of endothelial cell loss after cataract surgery: phacoemulsification versus manual small-incision cataract surgery: six-week results of a randomized control trial. J Cataract Refract Surg. 2010;36(2):247–253. doi:10.1016/j.jcrs.2009.09.023
24. Bamdad S, Bolkheir A, Sedaghat MR, Motamed M. Changes in corneal thickness and corneal endothelial cell density after phacoemulsification cataract surgery: a double-blind randomized trial. Electron Physician. 2018;10(4):6616–6623. doi:10.19082/6616
25. Krarup T, Holm LM, la Cour M, Kjaerbo H. Endothelial cell loss and refractive predictability in femtosecond laser-assisted cataract surgery compared with conventional cataract surgery. Acta Ophthalmol. 2014;92(7):617–622. doi:10.1111/aos.12406
26. El-Zankalony Y, Elewa L, Saleh S. Corneal endothelial cell count following femtosecond laser-assisted cataract surgery versus conventional phacoemulsification. J Egypt Ophthalmol Soc. 2016;109(1):21–25. doi:10.4103/2090-0686.192747
27. Vasavada A, Singh R, Desai J. Phacoemulsification of white mature cataracts. J Cataract Refract Surg. 1998;24(2):270–277. doi:10.1016/S0886-3350(98)80210-1
28. Titiyal JS, Kaur M, Singh A, Arora T, Sharma N. Comparative evaluation of femtosecond laser-assisted cataract surgery and conventional phacoemulsification in white cataract. Clin Ophthalmol. 2016;10:1357–1364. doi:10.2147/OPTH.S108243
29. Taravella MJ, Meghpara B, Frank G, Gensheimer W, Davidson R. Femtosecond laser-assisted cataract surgery in complex cases. J Cataract Refract Surg. 2016;42(6):813–816. doi:10.1016/j.jcrs.2016.02.049
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.