Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Preadmission Insulin-Treated Type 2 Diabetes Mellitus Patients Had Increased Mortality in Intensive Care Units
Authors Fan R, Xie L, Peng X, Yu B, Zou H, Huang J, Yu X, Wang D, Yang Y
Received 8 April 2022
Accepted for publication 9 July 2022
Published 22 July 2022 Volume 2022:15 Pages 2135—2148
DOI https://doi.org/10.2147/DMSO.S369152
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Rongping Fan,1,2,* Lei Xie,1,2,* Xuemin Peng,1,2 Bo Yu,3 Huajie Zou,1,2,4 Jiaojiao Huang,1,2 Xuefeng Yu,1,2 Daowen Wang,3 Yan Yang1,2
1Division of Endocrinology, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China; 2Branch of National Clinical Research Center for Metabolic Diseases, Wuhan, 430030, People’s Republic of China; 3Division of Cardiology, Department of Internal Medicine and Hubei Key Laboratory of Genetics and Molecular Mechanisms of Cardiological Disorders, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China; 4Division of Endocrinology, Department of Internal Medicine, The Affiliated Hospital of Qinghai University, Xining, Qinghai, 810001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Yang; Daowen Wang, Division of Endocrinology, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China, Tel +86-27-83665513, Fax +86-27-83662883, Email [email protected]; [email protected]
Aim: To explore the clinical outcomes among preadmission insulin-treated type 2 diabetes mellitus (T2DM) in intensive care units (ICU).
Patients and Methods: In this retrospective observational study, 578 T2DM patients admitted to ICU were recruited from March 2011 to February 2021, which were composed of 528 patients treated with insulin after ICU admission (including 300 preadmission non-insulin-treated and 228 preadmission insulin-treated patients) and 50 patients treated without insulin before and after ICU admission. Clinical outcomes were compared between the groups. Variables of age (± 10 years), gender, blood glucose > 10 mmol/l on ICU admission, and original comorbidities were used for matching to get the 1:1 matched cohort. The Kaplan–Meier survival curves were graphed to describe the survival trend and Cox regression analysis was performed to get adjusted hazard ratio (HR).
Results: Compared with the preadmission non-insulin-treated T2DM patients, preadmission insulin-treated T2DM patients had higher incidence of hypoglycemia [14.5% (33/228) vs 8.7% (26/300); p = 0.036]. In the 1:1 matched cohort, the preadmission insulin-treated T2DM patients had significantly increased mortality rate [30.0% (45/150) vs (16.0% (24/150)); adjusted HR, 1.68 (1.01– 2.80)] than preadmission non-insulin-treated T2DM patients. Compared with T2DM patients treated without insulin before and after ICU admission, preadmission insulin-treated T2DM patients had higher mortality and longer length of ICU stay (all p < 0.05).
Conclusion: Preadmission insulin treatment was associated with increased mortality rate and longer length of ICU stay among T2DM patients in ICU. Preadmission insulin-treated T2DM patients might have worse clinical outcomes when they are critically ill.
Keywords: insulin treatment, intensive care units, type 2 diabetes mellitus, mortality, ICU stay
Introduction
Diabetes has the fastest increasing incidence of all diseases worldwide, and it poses a major threat to global health.1,2 The proportion of patients with T2DM admitted to the ICU is also growing.3 One study reported a mortality rate of 36.0% in ICU patients with T2DM, compared with 29.1% in those without diabetes, which indicated that critically ill patients with T2DM tend to have worse outcomes and prognoses.4 Thus, glycemic care of critically ill patients with T2DM is an important part of treatment besides regulating their homeostatic function and stress response.5,6 Suitable glucose control in critically ill patients was recommended for a better clinical outcome.
Insulin is widely used as a classic, direct, and effective anti-diabetic agent.7 Traditionally, insulin treatment was always applied in T2DM patients when the function of pancreatic islet beta cells fails to compensate for the ongoing insulin resistance8 and patients have complicated comorbidities such as chronic kidney disease.9 Along with the definition of “intensive insulin therapy”, the time of insulin initiation is getting earlier, even in newly diagnosed T2DM patients.10–12 Therefore, there is a trend that the population with insulin application is growing.
Previous studies demonstrated that insulin therapy for critical illness decreased mortality in certain patients,13,14 and some researchers reported that insulin treatment was related to an increased mortality rate.15,16 These studies provide inconsistent evidence on the outcome benefits of insulin treatment. However, insulin exposure before admission is rarely taken into account when clinical outcomes are being explored in these studies. Gamble et al took a unique approach to quantify the relationship between insulin exposure and mortality, and they found that increasing levels of insulin exposure were associated with higher mortality in older patients with T2DM.17 Clinically, critically ill patients with T2DM admitted to the ICU can be divided into those who have not used insulin before admission and those who have received insulin treatment. Even if insulin exposure during hospitalization plays an important role in clinical outcomes, the association of preadmission insulin treatments with the risk of mortality in critically ill patients should not be ignored.
When considering the preadmission glycemic control methods, we will gain new insights into the impact of insulin treatment on clinical outcomes in critically ill patients with T2DM. Accordingly, we conducted a retrospective study to assess the effect of preadmission insulin treatment on clinical outcomes in patients with T2DM admitted to the ICU.
Materials and Methods
Study Population
This retrospective study investigated 791 T2DM patients from 4005 patients admitted to the ICU between March 2011 and February 2021 in Tongji Hospital Wuhan, China. Inclusion criteria were: (1) age ≥18 years; (2) length of ICU stay ≥3 days; and the exclusion criteria were: (1) age ≥85 years; (2) previous glycemic control methods were unknown; (3) missing all or almost all data on laboratory characteristics and clinical characteristics; and (4) type 1 diabetes mellitus. After excluding the patients who met the exclusion criteria, further analysis was conducted on 578 critically ill patients with T2DM, in which 528 patients received treatments with insulin (including 228 with preadmission insulin treatment and 300 with preadmission non-insulin treatment) and 50 patients received treatments without insulin after ICU admission (Figure 1). The time distribution of these 528 patients admitted to the hospital is presented in Supplementary Figure 1 to show that the sample collection over the large time span was not biased towards one or a few years. The ethics committee of Tongji Hospital approved the study design (IRBID: TJ-IRB20200229). The written informed consent was waived by the Ethics Committee due to the retrospective and anonymous nature of the data. In addition, the present study complies with the Declaration of Helsinki.
 |
Figure 1 The flowchart of study design. |
Definitions
T2DM was diagnosed according to a self-reported medical history or the use of anti-diabetic agents or insulin as chronic medication. Alternatively, newly diagnosed T2DM was based on HbA1c ≥6.5% and/or random plasma glucose ≥11.1 mmol/L18 and classified as T2DM in the medical record system. The preadmission insulin-treated T2DM patients were referred to patients treated with insulin (intravenous and/or subcutaneous insulin) within 90 days before ICU admission. The preadmission non-insulin-treated T2DM patients were referred to patients receiving treatments without insulin (oral anti-diabetic agents, lifestyle intervention, or no intervention) before ICU admission.
Data Collection and Endpoints Definitions
The demographic data, medical history, laboratory results, and data clinical treatments were extracted through the electronic medical records. Two study investigators collected and checked these data independently. The glycemic control methods before and after ICU admission are shown in Supplementary Table 1. Data from laboratory tests included parameters from the routine blood test, blood biochemistry for liver, renal function, random blood glucose, coagulation function, and myocardial markers. All laboratory values were based upon the first measurement on ICU admission. If serum chemistry measurements were not tested on ICU admission, then the most recent values measured close to the first day of ICU admission were used.
Statistical Analysis
Categorical variables were calculated as n (%) and compared using Pearson’s chi-square or Fisher’s exact test. Continuous variables were described as mean (standard deviation) or median (interquartile range) and analyzed with the independent t-test or Mann–Whitney test. The main characteristics at ICU admission, treatments during ICU stay, and clinical outcomes were compared among preadmission insulin-treated T2DM patients and preadmission non-insulin-treated T2DM patients. Then, patients in these two groups were matched by age ± 10 years, gender, glucose >10 mmol/L on ICU admission, diabetic nephropathy, chronic kidney disease, coronary heart disease, and ALT > 41 U/L based on the result of the previous analysis. Continuous variables in 150 matched pairs were compared using a paired t-test or a paired samples Wilcoxon test. Kaplan–Meier survival curves were graphed to compare the 60-day survival rate for patients in the matched cohort by the Log rank test. We calculated the hazard ratios and 95% confidence interval by Cox proportional regression models and the proportional assumptions were examined by Schoenfeld’s global test. All statistical analyses were conducted with SPSS 26.0, Stata 15.0, and GraphPad Prism 8.0. A two-tailed p-value <0.05 was considered statistically significant.
Results
Characteristics of T2DM Patients at ICU Admission and Treatments During ICU Stay
The basic characteristics of 528 T2DM patients who received insulin treatment after ICU admission, including 300 (56.8%) preadmission non-insulin-treated T2DM patients and 228 (43.2%) preadmission insulin-treated T2DM patients are shown in Table 1. Among these patients, 331 (62.7%) were male and 197 (37.3%) were female. The average age was 62.4 years. In the preadmission insulin-treated T2DM patients, the proportions of patients with blood sugar over 10 mmol/L, prevalence of diabetic nephropathy, chronic kidney disease, and coronary heart disease were significantly higher than those in the preadmission non-insulin-treated T2DM patients (p < 0.05 for both). Laboratory parameters showed that compared with preadmission non-insulin-treated T2DM patients, preadmission insulin-treated T2DM patients had higher levels of creatinine, blood urea nitrogen N-terminal pro-B-type natriuretic peptide (NT-ProBNP), potassium, erythrocyte sedimentation rate (ESR) and lower levels of estimated glomerular filtration rate (eGFR), hemoglobin, alanine aminotransferase (ALT), aspartate aminotransferase (AST) (all p < 0.05). Treatments during ICU stay are presented at the bottom of Table 1. Preadmission insulin-treated T2DM patients were more frequently treated with continuous renal replacement therapy (CRRT) and received higher average daily insulin consumption compared with preadmission non-insulin-treated T2DM patients (all p < 0.05). When comparing the matched cohorts, the baseline characteristics were comparable and there was no significant difference in terms of clinical treatments including the average daily insulin consumption in ICU among the two groups (all p > 0.05) (Table 1).
 |
Table 1 Comparison of Main Characteristics at ICU Admission and Treatments During ICU Stay Between Preadmission Non-Insulin-Treated and Preadmission Insulin-Treated T2DM Patients |
Compared with Preadmission Non-Insulin-Treated T2DM Patients, Preadmission Insulin-Treated T2DM Patients Had Higher Mortality
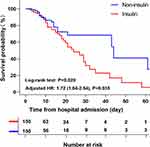
The clinical outcomes are shown in Table 2. Before matching, the incidence of hypoglycemia was higher in the preadmission insulin-treated T2DM patients than in the preadmission non-insulin-treated T2DM patients [14.5% (33/228) vs 8.7% (26/300); p = 0.036]. Preadmission insulin-treated T2DM patients had a trend of higher mortality than preadmission non-insulin-treated T2DM patients [24.6% (56/228) vs.20.0% (60/300); p = 0.210]. No significant difference was observed in the length of ICU stay between the two groups. Among 150 matched pairs, compared with the preadmission non-insulin-treated T2DM patients, the preadmission insulin-treated T2DM patients had significantly increased mortality rates [30.0% (45/150) vs (16.0% (24/150)); p = 0.004]. In Figure 2, the Kaplan–Meier survival analysis presented a significantly poorer survival in preadmission insulin-treated T2DM patients compared with preadmission non-insulin-treated T2DM patients (log-rank p < 0.05). The Schoenfeld’s global test showed that the preadmission insulin-treated T2DM patients did not violate the proportional hazard assumption (p = 0.065). Thus, according to the Cox regression, the risk of mortality in the preadmission insulin-treated T2DM patients was higher (crude HR, 1.77; 95% CI, 1.10–2.84; p = 0.020). After further adjustments for confounding variables, the survival rate of preadmission insulin-treated T2DM patients was still lower than those in preadmission non-insulin-treated T2DM patients (adjusted HR, 1.68; 95% CI, 1.01–2.80; p = 0.045) (Table 3). These results indicated that the preadmission insulin-treated T2DM patients had worse clinical outcomes than preadmission non-insulin-treated T2DM patients.
 |
Table 2 Comparison of Clinical Outcomes Between Preadmission Insulin-Treated and Preadmission Non-Insulin Treated T2DM Patients in ICU |
 |
Table 3 Univariable and Multivariable Cox Proportional Hazards Model for 60-Day Mortality of Critically Ill Patients with T2DM in the Matched Cohort |
Stratified Analyses of Clinical Outcomes Among T2DM Patients with Critical Ill States
As illustrated in Figure 3, T2DM patients with critical ill states were stratified by original comorbidities and treatments listed in Table 1. The mortality risk of preadmission insulin-treated T2DM patients in several subgroups was consistent with the overall findings. The increased risk of mortality was pronounced in patients with blood glucose >10 mmol/L on ICU admission (HR, 1.85; 95% CI, 1.04–3.29; p = 0.036). There were no significant differences among patients aged ≥60 years and those with a history of diabetic nephropathy, hypertension, chronic kidney disease, chronic liver disease, sepsis, but the association was stronger in patients with a history of coronary heart disease (HR, 3.40; 95% CI, 1.19–9.71; p = 0.023). No significant association was found between preadmission insulin treatment and mortality risk in patients who received CRRT, mechanical ventilation, and glucocorticoids treatment. However, the estimates for this association were significant in individuals treated with antibacterial drugs (HR, 1.68; 95% CI, 1.01–2.80; p = 0.046), which have inflammation regulation effects (Figure 3).
Compared with T2DM Patients Who Received Treatments Without Insulin Before and After ICU Admission, Preadmission Insulin-Treated T2DM Patients Had Higher Mortality
The basic characteristics of 50 T2DM patients who received treatments without insulin before and after ICU admission are shown in Table 4. These patients were defined as group N-N. In this section, the previously described preadmission non-insulin-treated T2DM patients and preadmission insulin-treated T2DM patients were defined as group N-I and group I-I, respectively. Compared to group N-N, group N-I had a higher prevalence of sepsis and lower proportions of males, coronary heart disease, and AMI (all p < 0.05). Group I-I had higher proportions of patients with blood glucose >10 mmol/L, diabetic nephropathy, chronic kidney disease, sepsis, and lower proportions of males, coronary heart disease, and AMI than those in the group N-N (all p < 0.05). Both patients in groups N-I and I-I received more frequent treatments of CRRT, mechanical ventilation, glucocorticoids, and antibacterial drugs than those in group N-N (all p < 0.05). Many biochemical values in groups N-I and I-I were significantly different from those in group N-N (most p < 0.05) (Table 1 and Table 4). The clinical outcomes of patients among these three groups are presented in Table 5 and Table 2. Compared to group N-N, group N-I had higher mortality [20.0 (60/300) vs (0.0 (0/50)); p = 0.001] and longer length of ICU stay [8.5 (5–14) vs 6 (4–8); p < 0.001]. Similar results were observed in group I-I; the mortality was significantly higher [24.6 (56/228) vs (0.0 (0/50)); p < 0.001] and the length of ICU stay was longer [9 (5–15.8) vs 6 (4–8); p < 0.001] in group I-I than those in group N-N. Besides, group I-I had a significantly higher incidence of hypoglycemia than group N-N [14.5 (33/228) vs 2.0 (1/50); p = 0.015].
As shown in Table 4, in the 1:1 matched cohort. The baseline characteristics were comparable, while several laboratory test results still differed among the matched groups (details are presented in Table 4), which indicated that groups N-I and I-I had more complex disease conditions when compared to the matched group N-N. Accordingly, it was observed that significantly more patients need mechanical ventilation support in group N-I compared to patients in group N-N (p < 0.05). In group I-I, the proportions of patients who received CRRT, mechanical ventilation, glucocorticoids treatment, and antibacterial treatment were significantly higher than patients in group N-N (all p < 0.05). In Table 5, the clinical outcomes were compared under the condition of comparable original comorbidities with different severity. There was no significant difference in mortality and the length of ICU stay between group N-I and N-N (all p > 0.05). However, patients in group I-I had significant higher mortality [39.3 (11/28) vs (0.0 (0/28)); p < 0.001] and relatively longer length of ICU stay [5.0 (4.0–8.0) vs 9.5 (5.3–17.0); p = 0.030] than those in group N-N. The incidence of severe hypoglycemia was slightly higher in group I-I than in group N-N [14.3 (4/28) vs (0.0 (0/28)); p = 0.111].
Discussion
In this study, we included 528 insulin-treated critically ill patients with T2DM including 300 preadmission non-insulin-treated T2DM patients and 228 preadmission insulin-treated T2DM patients to explore the impact of preadmission insulin treatment on clinical outcomes. Our study found that preadmission insulin treatment was not only associated with increased mortality but also related to a longer length of ICU stay. These results suggested that preadmission insulin-treated T2DM patients might have worse clinical outcomes when they are critically ill.
Our findings are consistent with Bolliger et al,19 whose study showed that the mortality events were more frequent in patients receiving insulin treatment before admission. Compelling evidence indicated that there was a link between insulin treatment and increased risk of mortality and other adverse clinical outcomes. A study that included 7401 patients with diabetes has suggested that insulin-treated diabetes was associated with a higher mortality rate and a longer length of hospital stay.20 Another study has found that patients having insulin-treated diabetes mellitus had an increased risk of major adverse cardiac events and worse outcomes.21 Meanwhile, our previous study also observed a similar increased in-hospital mortality in patients treated with insulin during hospitalization.16 The explanation for the increased risk of mortality of preadmission insulin-treated T2DM patients might be as follows. First, hyperglycemia and insulin resistance are hallmarks of an altered metabolism from the release of cortisol induced by the stress response in critically ill patients,22,23 which leads to the control of insulin doses becoming more complicated. Insulin treatment has a limited range of adjustments based on blood glucose because insulin therapy will more or less likely increase the risk of widely fluctuating blood glucose levels.24 In critically ill patients with T2DM, the preadmission insulin treatment perhaps makes it more difficult to regulate the insulin dose to maintain a suitable blood glucose range to achieve the real need of the body. Second, the present study also found that a higher incidence of hypoglycemia was observed in preadmission insulin-treated T2DM patients, we speculated that insulin-treatment-related hypoglycemia may play a crucial role in the increased mortality in this study. This is consistent with some recent research findings that hypoglycemia is associated with the risk of mortality.25–27
Additionally, the stratified analyses of clinical outcomes among critically ill patients with T2DM showed that the association between preadmission insulin treatment and mortality was significant in patients treated with antibacterial drugs and patients with coronary heart disease. First, to our knowledge, antibacterial treatment plays a pivotal role in the regulation of inflammation and immunity. In addition to glucose control, insulin is involved in various metabolisms in the body such as the promotion of protein synthesis, de novo lipogenesis, and inhibition of lipolysis.28 Moreover, insulin is a modulator of inflammation,29,30 and one of the factors which facilitate trained immunity through non-pathogenic signals.31 At the onset of critical illness, organs in the body experience drastic pathophysiological changes, and insulin may be involved in the regulation mechanism of this process as a pro-inflammatory factor.32 However, preadmission insulin-treated T2DM patients are not on the same starting line compared to preadmission non-insulin-treated T2DM patients, which suggested that preadmission insulin treatment might associate with a more complicated internal environment. Besides, it was reported that in insulin-treated T2DM patients, the incidence of cardiovascular death was increased.33 Insulin has a direct effect on the heart muscle, and its signal transduction pathways are modified under pathological conditions such as T2DM and myocardial ischemia.34 The above evidence may provide a clue for the increased mortality of preadmission insulin-treated T2DM patients in T2DM patients with coronary heart disease.
Compared with 50 patients who received treatment without insulin before and after ICU admission, preadmission non-insulin-treated T2DM patients and preadmission insulin-treated T2DM patients had more complicated disease conditions and higher mortality. The worse outcomes in preadmission non-insulin-treated T2DM patients and preadmission insulin-treated T2DM patients may be due to the complicated state of illness. However, other potential risks should not be excluded. For instance, an investigation reported that the quality-adjusted life years index of patients using insulin was reported as being lower than that of those using metformin, and this was evident in subjects who switched from metformin to insulin.35,36 The proportion of patients who used metformin before admission and then changed to insulin after admission in our study was 18.0% (54/300), and 29.6% (16/54) of these patients died. These data may indicate that changing glycemic control methods is a potential risk factor for mortality in T2DM patients with critically ill states. Therefore, initiating insulin therapy in preadmission non-insulin-treated T2DM patients must be done with caution.
This study has some limitations. First, the sample size was relatively small for the T2DM patients who received treatments without insulin before and after ICU admission because ICU patients generally received insulin treatment after admission, and oral medication was often replaced. Second, as the dynamic observation of biochemical values including inflammation indicators was limited, we failed to evaluate the development of clinical conditions. Third, this study was a retrospective study, and clinical data of some variables were missing, making it unable to further stratify patients into subgroups according to different preadmission insulin treatment protocols and compare their clinical outcomes. Besides, an improved prognosis of non-insulin treatment such as metformin cannot be excluded and further studies are required.
Conclusion
In critically ill patients with T2DM, preadmission insulin treatment is associated with an increased mortality rate and longer length of ICU stay. The history of preadmission insulin treatment may be a marker of poor prognosis for T2DM patients with critically ill states and may help clinicians identify patients who need more aggressive treatments to prevent poor clinical outcomes. Clinicians should be more concerned about preadmission insulin-treated T2DM patients in ICU.
Prior Presentations
An old version of this original work has been preprinted and posted to Research Square. The DOI is: 10.21203/rs.3.rs-674359/v1. Posted 12 Jul, 2021.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the institutional review board of Tongji Hospital (IRBID: TJ-IRB20200229), the consent to participate was not applicable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by projects from the National Nature Science Foundation of China (grant numbers 81670754, 81974114, 81630010, 81790624, C-0052), Ministry of Science and Technology of China (grant number 2020YFC0844500), Major Projects of the Technological Innovation of Hubei province (grant number 2017ACA170), and funds from the Jie Chu Jing Ying foundation (grant number 2018076).
Disclosure
All authors declared no conflict of interest.
References
1. Zhou B, Lu Y, Hajifathalian K, et al. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet. 2016;387(10027):1513–1530.
2. Krug EG. Trends in diabetes: sounding the alarm. Lancet. 2016;387(10027):1485–1486. doi:10.1016/S0140-6736(16)30163-5
3. Siegelaar SE, Hickmann M, Hoekstra JB, Holleman F, DeVries JH. The effect of diabetes on mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2011;15(5):R205. doi:10.1186/cc10440
4. Christiansen CF, Johansen MB, Christensen S, O’Brien JM, Tonnesen E, Sorensen HT. Type 2 diabetes and 1-year mortality in intensive care unit patients. Eur J Clin Invest. 2013;43(3):238–247. doi:10.1111/eci.12036
5. Chase JG, Desaive T, Bohe J, et al. Improving glycemic control in critically ill patients: personalized care to mimic the endocrine pancreas. Crit Care. 2018;22(1):182. doi:10.1186/s13054-018-2110-1
6. Preiser JC, van Zanten AR, Berger MM, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care. 2015;19:35. doi:10.1186/s13054-015-0737-8
7. McDonnell ME, Umpierrez GE. Insulin therapy for the management of hyperglycemia in hospitalized patients. Endocrinol Metab Clin North Am. 2012;41(1):175–201. doi:10.1016/j.ecl.2012.01.001
8. Rachdaoui N. Insulin: the friend and the foe in the development of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(5):1770. doi:10.3390/ijms21051770
9. Fonseca VA, Haggar MA. Achieving glycaemic targets with basal insulin in T2DM by individualizing treatment. Nat Rev Endocrinol. 2014;10(5):276–281. doi:10.1038/nrendo.2014.17
10. Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371(9626):1753–1760. doi:10.1016/S0140-6736(08)60762-X
11. Weng J. Short-term intensive insulin therapy could be the preferred option for new onset type 2 diabetes mellitus patients with HbA1c > 9. J Diabetes. 2017;9(10):890–893. doi:10.1111/1753-0407.12581
12. Hanefeld M, Bramlage P. Insulin use early in the course of type 2 diabetes mellitus: the ORIGIN trial. Curr Diab Rep. 2013;13(3):342–349. doi:10.1007/s11892-013-0366-z
13. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi:10.1056/NEJMoa011300
14. Pittas AG, Siegel RD, Lau J. Insulin therapy and in-hospital mortality in critically ill patients: systematic review and meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2006;30(2):164–172. doi:10.1177/0148607106030002164
15. van Steen SC, Rijkenberg S, van der Voort PHJ, DeVries JH. The association of intravenous insulin and glucose infusion with intensive care unit and hospital mortality: a retrospective study. Ann Intensive Care. 2019;9(1):29. doi:10.1186/s13613-019-0507-x
16. Yu B, Li C, Sun Y, Wang DW. Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes. Cell Metab. 2021;33(1):65–77 e2. doi:10.1016/j.cmet.2020.11.014
17. Gamble JM, Simpson SH, Eurich DT, Majumdar SR, Johnson JA. Insulin use and increased risk of mortality in type 2 diabetes: a cohort study. Diabetes Obes Metab. 2010;12(1):47–53. doi:10.1111/j.1463-1326.2009.01125.x
18. China Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition) (in Chinese). Chin J Endocrinol Metabol. 2021;13(4):315–409.
19. Bolliger D, Seeberger MD, Lurati Buse G, et al. The influence of pre-admission hypoglycaemic therapy on cardiac morbidity and mortality in type 2 diabetic patients undergoing major non-cardiac surgery: a prospective observational study. Anaesthesia. 2012;67(2):149–157. doi:10.1111/j.1365-2044.2011.06963.x
20. Haltmeier T, Benjamin E, Beale E, Inaba K, Demetriades D. Insulin-treated patients with diabetes mellitus undergoing emergency abdominal surgery have worse outcomes than patients treated with oral agents. World J Surg. 2016;40(7):1575–1582. doi:10.1007/s00268-016-3469-2
21. Reinstadler SJ, Stiermaier T, Eitel C, et al. Relationship between diabetes and ischaemic injury among patients with revascularized ST-elevation myocardial infarction. Diabetes Obes Metab. 2017;19(12):1706–1713. doi:10.1111/dom.13002
22. Xiu F, Stanojcic M, Diao L, Jeschke MG. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol. 2014;2014:486403. doi:10.1155/2014/486403
23. Li L, Messina JL. Acute insulin resistance following injury. Trends Endocrinol Metab. 2009;20(9):429–435. doi:10.1016/j.tem.2009.06.004
24. Kodner C, Anderson L, Pohlgeers K. Glucose management in hospitalized patients. Am Fam Physician. 2017;96(10):648–654.
25. Nuzzo A, Brignoli A, Ponziani M, et al. Aging and comorbidities influence the risk of hospitalization and mortality in diabetic patients experiencing severe hypoglycemia. Nutr Metab Cardiovasc Dis. 2022;32(1):160–166. doi:10.1016/j.numecd.2021.09.016
26. McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med. 2016;176(7):969–978. doi:10.1001/jamainternmed.2016.2275
27. Standl E, Stevens SR, Lokhnygina Y, et al. Confirming the bidirectional nature of the association between severe hypoglycemic and cardiovascular events in type 2 diabetes: insights from EX SCEL. Diabetes Care. 2020;43(3):643–652. doi:10.2337/dc19-1079
28. Kolb H, Kempf K, Rohling M, Martin S. Insulin: too much of a good thing is bad. BMC Med. 2020;18(1):224. doi:10.1186/s12916-020-01688-6
29. Brundage SI, Kirilcuk NN, Lam JC, Spain DA, Zautke NA. Insulin increases the release of proinflammatory mediators. J Trauma. 2008;65(2):367–372. doi:10.1097/TA.0b013e3181801cc0
30. Filgueiras LR, Capelozzi VL, Martins JO, Jancar S. Sepsis-induced lung inflammation is modulated by insulin. BMC Pulm Med. 2014;14:177. doi:10.1186/1471-2466-14-177
31. Ieronymaki E, Daskalaki MG, Lyroni K, Tsatsanis C. Insulin signaling and insulin resistance facilitate trained immunity in macrophages through metabolic and epigenetic changes. Front Immunol. 2019;10:1330. doi:10.3389/fimmu.2019.01330
32. Preiser JC, Ichai C, Orban JC, Groeneveld AB. Metabolic response to the stress of critical illness. Br J Anaesth. 2014;113(6):945–954. doi:10.1093/bja/aeu187
33. Schwartz GG, Nicholls SJ, Toth PP, et al. Relation of insulin treatment for type 2 diabetes to the risk of major adverse cardiovascular events after acute coronary syndrome: an analysis of the BETonMACE randomized clinical trial. Cardiovasc Diabetol. 2021;20(1):125. doi:10.1186/s12933-021-01311-9
34. Iliadis F, Kadoglou N, Didangelos T. Insulin and the heart. Diabetes Res Clin Pract. 2011;93(Suppl 1):S86–S91. doi:10.1016/S0168-8227(11)70019-5
35. Bondy SC, Wu M, Prasad KN. Alternatives to insulin for the regulation of blood sugar levels in type 2 diabetes. Int J Mol Sci. 2020;21(21):8302. doi:10.3390/ijms21218302
36. Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174(8):1227–1234. doi:10.1001/jamainternmed.2014.2894
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.