Back to Journals » Journal of Pain Research » Volume 12
Pre-emptive scalp infiltration with dexamethasone plus ropivacaine for postoperative pain after craniotomy: a protocol for a prospective, randomized controlled trial
Authors Jia Y , Zhao C , Ren H , Wang T, Luo F
Received 12 October 2018
Accepted for publication 18 March 2019
Published 24 May 2019 Volume 2019:12 Pages 1709—1719
DOI https://doi.org/10.2147/JPR.S190679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Erica Wegrzyn
Yitong Jia,1,* Chunmei Zhao,1,* Hao Ren,1,* Tao Wang,2,* Fang Luo3
1Department of Anesthesiology and Pain Management, Beijing Tiantan Hospital, Capital Medical University; 2Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University; 3Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Background: Approximately 55–87% of the patients undergoing craniotomy experience moderate to severe pain during the first 48 hrs after surgery, which negatively influences patients’ postoperative rehabilitation. Recently, local infiltration of analgesia (LIA) has been widely performed clinically as a promising analgesic method that could avoid the side effects of analgesics but only has a short pain-free duration; researchers have clarified that the addition of dexamethasone to LIA could provide significant analgesic effects and significantly prolong the duration of analgesic effects without obvious complications for various types of surgeries. To date, no studies have evaluated the addition of dexamethasone to LIA for patients receiving craniotomy. The aim of the study was to test the hypothesis that pre-emptive scalp infiltration with a steroid (dexamethasone) plus a local anesthetic (ropivacaine) could achieve superior postoperative analgesic effects to a local anesthetic (ropivacaine) alone in adult patients undergoing a craniotomy.
Study design and methods: This study is a randomized controlled trial that will include one intervention and one control group involving a total of 140 adults scheduled for elective craniotomy for resection of supratentorial tumors under general anesthesia and with an anticipated full recovery within 2 hrs postoperatively. The intervention will involve pre-emptive scalp infiltration with ropivacaine plus dexamethasone (the dexamethasone group) or ropivacaine alone (the control group), and the participants in both groups will complete a 6-month follow-up. The primary outcome will be the cumulative sufentanil consumption within 48 hrs postoperatively.
Discussion: The intervention, if effective, this study will provide clinically important information on the role of dexamethasone in scalp infiltration for post-craniotomy pain management.
Keywords: postoperative pain, craniotomy, pre-emptive scalp infiltration, dexamethasone, sufentanil consumption
Background
In the 1990s, conventional wisdom considered that neurosurgical patients did not experience obvious postoperative pain,1–3 and the reasons for that viewpoint may include the following three aspects. On one hand, most craniotomy incisions are in areas of reduced pain fiber density than other regions; on the other hand, the dura is not richly innervated with pain receptors; moreover, the brain parenchyma is insensitive to pain.3 However, a growing group of recent studies have reported that the incidence of pain still remains high. Approximately 55–87% of the patients undergoing craniotomy would experience moderate to severe pain during the first 48 hrs after surgery.4–7 More than 50% of neuroanesthesiologists judged that the postoperative pain of neurosurgical patients was undertreated.4 Inadequate postoperative analgesia for craniotomy patients could negatively influence their postoperative rehabilitation such as inducing distressed or depressed emotions, increasing stress reactions and leading to sympathetically mediated hypertension, which may have dangerous consequences including cerebral edema, cerebral hemorrhage, prolonged hospital stay, and mortality.8,9 Moreover, acute pain following craniotomy is associated with an increased risk of chronic pain, which may lead to central sensitization.10,11 Thus, postoperative pain management for craniotomy patients remains a significant challenge for anesthesiologists, and effective analgesia is essential for patients’ prognosis as well as their postoperative quality of life (QoL).
Opioids, such as fentanyl, morphine, codeine, and remifentanil, have been commonly used for the treatment of postoperative pain after various types of surgery and are the mainstay of treatment for post-craniotomy pain which could achieve rapid recovery but it is limited by the potential for various side effects, such as sedation, miosis, hypercarbia, respiratory depression, postoperative nausea/vomiting (PONV), intracranial hypertension, masking of acute cerebral edema, neurologic changes, or interference with neurological assessments.12–16 Moreover, although remifentanil could achieve rapid analgesia, inappropriate use may be associated with excessive postoperative pain and pain sensitization.17,18 Nonsteroidal anti-inflammatory drugs (NSAIDs) are cyclooxygenase (COX)-1/COX-2 inhibitors that appear to be effective for patients undergoing craniotomy, and NSAIDS are superior to other analgesics because they are devoid of negative side effects, including sedation, respiratory depression and so on.15 Although the utilization of NSAIDs could reduce postoperative opioid consumption,19,20 the use of NSAIDs has been restricted for its potential to cause platelet dysfunction and even regarding intracerebral hemorrhaging.20,21 COX-2 inhibitors such as parecoxib may relieve postoperative pain following craniotomies without the properties of antiplatelet but it has been clinically restricted because this drug is likely to cause cardiovascular disease secondary to thrombotic events.22 In addition, although NSAIDs are considered to be advantageous agents for postoperative analgesia, it is unlikely to provide enough analgesic effects when used alone.15 Preoperative gabapentin in patients undergoing craniotomy could relieve acute postoperative pain to some degree;23 however, a randomized controlled trial (RCT) reported by Zeng et al, concluded that it decreased postoperative acute pain scores only within 24 hrs but did not have an effect at 48 hrs.24 Moreover, gabapentin use may contribute to delay extubation and increased postoperative sedation.25 In addition, this drug seems to be associated with several adverse side effects, such as dizziness, fatigue, drowsiness, peripheral edema, and ataxia.26 Ketamine is a common N-methyl-D-aspartate receptor antagonist that could produce comparable analgesic effects to opioids.27 Nonetheless, its use in neurosurgery patients is questioned on account of the adverse effects on intracranial pressure, seizure threshold, and mentation.19
In recent years, local infiltration of analgesia (LIA) has been widely performed clinically as a promising analgesic method that could avoid the side effects of analgesics, such as opioids.28 Batoz et al, evaluated the analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection and reported that patients who received an infiltration of the surgical site with 20 mL of 0.75% ropivacaine achieved lower visual analog scale (VAS) score within 2 months.28 Moreover, Jie et al, performed pre-emptive scalp infiltration with 0.5% ropivacaine and 1% lidocaine on patients undergoing craniotomy and revealed that LIA could provide effective postoperative analgesia after craniotomy.29 However, some researchers demonstrated that despite the addition of adrenaline, scalp infiltration with local anesthetics could only play relatively satisfactory analgesic effects that last for a short time after surgery.30
Recently, it is revealed that steroids might play a pivotal role in reducing postoperative pain because of their anti-inflammatory effects and analgesic properties. Several studies have evaluated the efficacy of the addition of steroids to local anesthetics in LIA of which dexamethasone is an inexpensive synthetic glucocorticoid and has a strong anti-inflammatory effect with a long half-life of 36–72 hrs.31–33 Bayram et al, have performed peritonsillar infiltration of a levobupivacaine hydrochloride and dexamethasone combination in tonsillectomy patients, and the results revealed that this combination could significantly decrease patients’ VAS scores within 3 days.33 In Ikeuchi’s study,34 the pain severity of patients who underwent total knee arthroplasty and received peri-articular injections of ropivacaine, dexamethasone, and isepamicin were significantly lower than that of the subjects in the control group, in which patients received a ropivacaine and isepamicin combination only on postoperative day 1 and 3.
To date, however, there are no studies that have evaluated the addition of dexamethasone to LIA on patients receiving craniotomy. Thus, the study is designed as a single-center, blinded, RCT involving local anesthetics as a control, and we hypothesize that pre-emptive scalp infiltration with a steroid (dexamethasone) plus a local anesthetic (ropivacaine) can achieve superior postoperative analgesic effects to a local anesthetic (ropivacaine) alone in adult patients undergoing a craniotomy. The cumulative sufentanil consumption within 48 hrs postoperatively will be the primary outcome.
Study design and methods
Study design
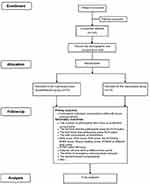
This is a prospective, single-center, blinded, randomized, controlled clinical study designed to compare the efficacy and safety of pre-emptive scalp infiltration with dexamethasone plus ropivacaine and ropivacaine alone for postoperative pain after craniotomy. One hundred and forty participants undergoing craniotomy will be randomly assigned to the dexamethasone group and the control group at a 1:1 ratio. The patient flow diagram of the study is presented in Figure 1, and the trial schedule is shown in Table 1.
 | Table 1 Trial schedule |
Study setting, recruitment, and ethics
This study will enroll patients undergoing supratentorial tumor surgery in Beijing Tiantan Hospital Affiliated to Capital Medical University. Patients’ recruitment will start in September 2018. Protocol modifications are not expected, and all researchers will be trained referring to the same training protocol. The study plan (protocol version 1.0) is approved by the Ethics Committee of Beijing Tiantan Hospital (KY2018-034-02). This study strategy has been registered at ClinicalTrials.Gov (NCT03618264) and is in accordance with the World Medical Association’s “Declaration of Helsinki”. All patients will sign written informed consent to participate in the study. All participants will have sufficient time to decide whether to participate in this study. Patients who participate in the study will have the right to obtain the relevant information, and they will be allowed to withdraw their consent or discontinue participation without restrictions at any time point of the study. The confidentiality of participant records will be protected.
Patient population
Inclusion criteria
- Patients scheduled for elective craniotomy for resection of a supratentorial tumor under general anesthesia from September 2018 to December 2020;
- American Society of Anaesthesiologists (ASA) physical status of I–II;
- Age 18–64 years;
- Participates required to fix their head in a head clamp intraoperatively;
- Participates with an anticipated full recovery within 2 hrs postoperatively.
Exclusion criteria
- History of craniotomy;
- Expected delayed extubation or no plan to extubate;
- Participants who cannot use a patient-controlled analgesia (PCA) device;
- Participants who cannot understand the instructions of a numeral rating scale (NRS)35 before surgery;
- Extreme body mass index (BMI) (<15 or >35);
- Allergy to opioids, dexamethasone, or ropivacaine;
- History of excessive alcohol or drug abuse, chronic opioid use (more than 2 weeks), or use of drugs with confirmed or suspected sedative or analgesic effects;
- History of psychiatric disorders, uncontrolled epilepsy or chronic headache;
- Pregnant or at breastfeeding;
- Symptomatic cardiopulmonary, renal, or liver dysfunction or history of diabetes;
- Preoperative Glasgow Coma Scale <15;
- Suspicion of intracranial hypertension;
- Peri-incisional infection;
- Participants who have received radiation therapy and chemotherapy preoperatively or with a high probability to require a postoperative radiation therapy and chemotherapy according to the preoperative imaging.
Randomization and blinding
The randomization will be performed by a computerized random-number generator list; participants will be randomly assigned to the dexamethasone group or control group. Opaque sealed envelopes with the participants’ screening order outside and their assigned group inside will be used to ensure the allocation concealment. The participants and surgeons responsible for the operation will be blinded to the group assignment. Before the surgery, an envelope will be opened by the study investigator in charge of the operation, and the patient will be assigned to undergo the corresponding local infiltration. The study investigator will also be responsible for preparing the respective drugs to be used for scalp infiltration in the two groups: a 50 mL syringe containing 30 mL of miscible liquids consisting of 10 mg dexamethasone, 150 mg ropivacaine and normal saline or a 50 mL syringe containing 30 mL of miscible liquids consisting of 150 mg ropivacaine and normal saline. Both syringes will contain clear fluid, appear identical and be labeled as “study drug”. Only the anesthesiologist in charge of the craniotomy will be not blinded, while the patient, nurses, surgeons, and anesthesiologists in charge of the postoperative period and pain evaluation will be blinded.
Interventions and comparison
Anesthesia induction and management
During preoperative visit, the study protocol and the NRS score (0 indicates no pain, 10 indicates the most severe pain imaginable), PONV scores,29 Ramsay sedation scale (RSS) scores,36 patient satisfaction scale (PSS) scores,37 World Health Organization QoL abbreviated version (WHOQOL-BREF) scores,38 wound healing scores,39 and Patient and Observer Scar Assessment Scale (POSAS) scores40 will be explained to the participants. In addition, patients will be guided on how to use a PCA device.
Before the surgery, patient randomization will be conducted, and the patients will be assigned to either the dexamethasone group or the control group. Standard monitoring including blood pressure (BP), heart rate (HR), electrocardiography, pulse oximetry (SpO2), and bispectral index (BIS system, Covidien/Medtronic, USA) will be continuously applied. General anesthesia will be induced with intravenous (IV) midazolam at 0.05 mg/kg, sufentanil at 0.3–0.5 μg/kg, propofol at 1.5–3 mg/kg, and cis-atracurium at 0.2 mg/kg.
After tracheal intubation, arterial radial puncture will be performed to monitor invasive arterial BP. The lungs will be ventilated with 60% oxygen and 40% air, and ventilation will be adjusted to maintain normocapnia. Anesthesia will be maintained with IV propofol, and remifentanil and muscle relaxation will be maintained with IV cis-atracurium. Before head fixation, LIA will be performed by the neurosurgeons in charge of the surgery.
Sufentanil will be administered to attenuate potent stress responses induced by noxious stimuli at certain time points such as scalp incision or skull drilling to maintain the mean arterial blood pressure (MAP) and HR fluctuations within 20% of the baseline. No additional analgesics will be administered intraoperatively. Additional doses of hypotensive drugs or vasoactive drugs will be administered by the anesthesiologists in charge of the surgery. Crystalloid and colloid infusions will be used as needed. The comorbidities, dosage of all drugs, intraoperative physical parameters and intraoperative fluid input and output will be closely monitored and recorded by the investigator.
Pre-emptive scalp infiltration
Before head fixation, the local infiltration solution will be infiltrated with a 22-gauge needle introduced to the skin at a 45° angle along the incision and throughout the entire thickness of the scalp as well as the head clamp points by the surgeon in charge of the craniotomy. The local infiltration solution in the dexamethasone group will consist of dexamethasone (0.33 mg/mL) and ropivacaine (5 mg/mL). The local infiltration solution in the control group will consist of ropivacaine (5 mg/mL) alone. The volume of local infiltration solution will be determined by the surgeon in charge of the surgery according to the incision length, and the capacity of the solution used will be recorded by the investigator.
Additional interventions
The infusion of propofol and remifentanil will be removed when the sutured of the scalp is finished. Additionally, 4 mg of ondansetron will be administered to prevent PONV. Atropine and neostigmine will be administered to antagonize any residual muscle relaxation. The trachea will be extubated when the patient’s hemodynamic, respiratory, and neurologic evaluations remain satisfactory, and the patient will be transferred to the post-anesthetic care unit (PACU). A PCA device, which will contain 200 µg of sufentanil and 16 mg of ondansetron diluted to a total volume of 100 mL in 0.9% saline, will be administrated during 48-hr postoperative period. The PCA device will have a bolus dose of sufentanil set as 2 μg with a lockout interval of 10 mins, and the maximum dose will be limited to 8 μg per hour. The patients could push the PCA button by themselves when they feel pain and to repeat it until the pain was relieved. If the participants experience inadequate analgesia five times after sufentanil bolus, the bolus dose will be increased to 3 μg, and the maximum dose will be increased to 12 μg per hour. Insufficient postoperative analgesia will be defined as NRS score exceeds 4 after receiving the maximum dose of sufentanil with the PCA device. Patients with insufficient analgesia will be treated with paracetamol at a dose of 500–1,000 mg intramuscularly or 50 mg intravenously. Total doses and the frequency of rescue analgesic will be recorded. All other aspects of the rehabilitation process will be identical between the two groups.
Follow-up
The participants in both groups will complete a 6-month follow-up. The follow-up evaluations will include the cumulative sufentanil consumption with the PCA device, NRS scores, PONV scores, RSS scores, PSS scores, WHOQOL-BREF scores, wound healing scores, POSAS scores, and adverse events (AEs). The follow-up will be conducted by experienced research members who are blinded to the study. The participants who would also require radiation therapy or chemotherapy postoperatively on the basis of intraoperative and postoperative pathology or other conditions will be followed up to the day before receiving radiation therapy or chemotherapy. In addition, the patients who will undergo early revision within the first 48 hrs for other than wound-healing problems such as hematoma or brain swelling will be excluded from the study.
Outcome measures
Baseline data
The pre-enrollment evaluation will be collected including age (years), gender (male or female), BMI, pre-existing pain and duration, length of the scalp incision, length of surgery and anesthesia, time from the end of surgery to tracheal extubation and so on.
Primary outcome
The primary outcome will be the total amount of sufentanil consumption (μg) by the PCA pump during the post-operative period. Both the initial dose and background infusion of the PCA pump in this study will be set at 0, and the bolus dose of sufentanil will be set at 2 μg with a lockout interval of 10 mins. Participants will be advised to push the analgesic demand button if they feel pain.
Secondary outcomes
Number of participants with no sufentanil consumption within 48 hrs postoperatively;
The first time that the participants press the PCA button within 48 hrs after the operation;
Total number of times that participants press the PCA button including effective presses and ineffective presses within 48 hrs postoperatively;
The total consumption of anesthetics including opioids during the intraoperative period;
Duration of hospitalization after the operation;
NRS scores at 2 hrs, 4 hrs, 8 hrs, 24 hrs, 48 hrs, 72 hrs, 1 week, 2 weeks, 1 month, 3 months, and 6 months after surgery;
PONV within 48 hrs after surgery, which was rated by participants as follows: 0, absent; 1, nausea not requiring treatment; 2, nausea requiring treatment; and 3, vomiting;
Patients’ HR and MAP before anesthetic induction, after anesthetic induction, after scalp infiltration, during skull drilling, mater cutting, at skin closure and at 2 hrs, 4 hrs, 8 hrs, 24 hrs, 48 hrs after surgery;
The times of emergency reducing BP within 48 hrs after the operation. The criteria for treatment determined by the participant’s surgeon in charge; The times of emergency reducing BP will be recorded by the investigator;
RSS scores at 2, 4, 8, 24, and 48 hrs after the operation;
PSS scores at 48 hrs, 1 week, 1 month, 3 months, and 6 months after surgery (0 is unsatisfactory, and 10 is very satisfactory);
WHOQOL-BREF scores at 1 month, 3 months, and 6 months after surgery; The WHOQOL-BREF is a self-reporting questionnaire that contains 26 items and addresses four organization QoL domains including physical health (7 items), psychological health (6 items), social relationships (3 items), and environment (8 items). Two other items measure overall QoL and general health. Each domain’s mean score can range between 4 and 20 with a higher score indicating a better QoL;
Wound healing score at 3 weeks and 6 weeks after surgery;
POSAS scores at 6 months postoperatively; The POSAS includes subjective symptoms of pain and pruritus and consists of two numerical scales, namely the Patient Scar Assessment Scale and the Observer Scar Assessment Scale;
The steroid-induced complications such as wound infection, wound hematoma, impaired wound healing,chest infection or gastric ulcers during hospitalization.
Safety assessments
The details of all AEs, which are defined as negative or unintended clinical manifestations following the treatment and adverse device effects throughout the treatment and follow-up period, will be monitored and recorded in a case report form (CRF). The report will include the time of occurrence, severity, relationship with the treatment or study intervention, possible causes, and patient outcomes. All of the AEs will be treated and closely monitored without delay by the researchers until they are healed. Severe AEs or adverse device effects must be reported to the research ethics committee as soon as possible. The research ethics committee will review the AEs and determine whether termination of the study is necessary.
Sample size
Based on the previous studies and combined with our clinical experience,29 we estimate that the dose of sufentanil after surgery in participants who receive pre-emptive scalp infiltration with 0.5% ropivacaine will be approximately 100±50 μg, and the postoperative pain intensity or analgesic requirements will be decreased by 50–70% with the addition of dexamethasone.32–34,41 Thus, we hypothesize that the dose of sufentanil will be 70±50 μg within 48 hrs operatively in the dexamethasone group. PASS V.11 software (NCSS, Kaysville, Utah, USA) will be used. Based on 90% power to detect a significant difference (α=0.05, two sided), 62 participants will be required in each group. Considering a 10% withdrawal rate, the sample size will be 70 in each group.
Statistical analysis
All statistical analyses will be performed by statisticians who are blinded to the entire allocation and intervention process. Data analysis will be performed using the Statistical Product and Service Solutions (SPSS) version 22.0 (International Business Machines Inc., USA). The Shapiro–Wilk test will be used to test whether all data follow a normal distribution. The efficacy analyses will be performed based on the intention-to-treat (ITT) principle. The sensitivity and consistency analysis will be used by the per-protocol (PP) analysis. For patients who drop out of the study, the initial data or the last follow-up data will be utilized in accordance with specific conditions. Two-tailed analyses will be conducted, and a P-value of ≤0.05 will be considered statistically significant.
Demographic characteristics and baseline information will be analyzed. The Shapiro–Wilk test will be used to test the normal distribution of continuous variables. Normally distributed variables will be presented as mean ± SD and compared by independent samples Student’s t-test. Abnormally distributed variables will be presented as medians (interquartile ranges, IQRs) and compared by the Mann–Whitney U test.
Categorical variables will be presented as counts (percentages) and compared by Chi-square test or Fisher’s exact test.
The total amount of sufentanil consumption (µg) by the PCA pump during the 48 hr postoperative period will be the primary outcome of this study. The primary analysis was performed on a modified intention-to-treat principle, including only patients who attain postoperative recovery within 2 hrs. The Shapiro–Wilk test will be used to test the normal distribution of continuous variables. Continuous variables will be presented as mean ± SD and analyzed using independent samples Student’s t-test if they are normally distributed. The measurement data will be presented as medians (IQRs) and analyzed using the Mann–Whitney U test if they are not normally distributed.
The secondary outcomes will include the specific usage of PCA devices, times of emergency reducing BP, total consumption of anesthetics, NRS scores, PONV scores, HR and MAP, RSS scores, PSS scores, WHOQOL-BREF scores, wound healing scores, and POSAS scores at different time points. The secondary outcome measures will be compared using the same methods as the demographic characteristics and baseline information.
Safety analyses will be compared in the safety data set with the incidence of AEs or adverse device effects using the Chi-square test or Fisher’s exact test.
Discussion
This trial is designed as a randomized, single centered, parallel-group, blinded study aiming to test the hypothesis that pre-emptive scalp infiltration with ropivacaine plus dexamethasone can achieve superior postoperative analgesic effects than a local anesthetic (ropivacaine) alone in adult patients undergoing a craniotomy. Analgesic medication after craniotomy includes systemic treatments and topical treatments. Systemic medication as one of the modalities of pain management is associated with corresponding disadvantages, such as sedation, miosis, respiratory depression, increased intracranial pressure, causing platelet dysfunction and increased risk of cardiovascular disease.21 In recent years, scalp infiltration has been reported to be an effective and safe analgesic method but with a short pain-free duration.30 Researchers have clarified that the addition of dexamethasone to local anesthetics during LIA could provide significant analgesic effects and significantly prolong the duration of analgesic effects for various types of surgeries, such as tonsillectomies,33 total knee arthroplasties34, and cesarean section.41 To the best of our knowledge, this is the first study to evaluate the effects of the addition of dexamethasone to ropivacaine in scalp infiltration for postoperative pain after craniotomy.
It is reported that postoperative pain after craniotomy is mainly originated from the damage to scalp, including the soft tissues, muscles, and dura mater, and releasing chemical mediators and activating nociceptors as a result of causing an action potential to be transmitted along a series of neurons.42 The whole process is regulated by various inflammatory mediators and neural pathways in the peripheral and central regions.43 Dexamethasone is an inexpensive long-acting glucocorticoid with potent anti-inflammatory properties both locally and systemically due to alteration of inflammatory mediators and afferent nociceptive signalling mechanisms.44 The reason that we choose the addition of dexamethasone for local infiltration rather thanIV administration is because researchers have revealed that LIA of dexamethasone is more effective than IV dexamethasone for decreasing postoperative pain.41 Whether the addition of dexamethasone to local anesthetics in scalp infiltration can provide strong topical anti-inflammatory effects and improve the analgesic effects after craniotomy remains to be further investigated. Moreover, dexamethasone also has the potential of antiemetic effects,34 so differences in PONV of both groups will be the secondary outcomes in this study.
The use of steroids may be related to several risks, such as impaired wound healing45 or wound infections;46 However, previous studies have not reported severe AEs related to the addition of dexamethasone to LIA in patients undergoing cesarean section, joint replacement surgery, and tonsillectomy34,41,47 and studies who added corticosteroids to local anesthetics in whom the CSF space was opened have not been reported. As a result, we choose the lowest concentration of dexamethasone for LIA according to the results of previous studies.34 Furthermore, careful consideration will be given in patients with a high risk of complications associated with steroid treatment because the wound healing score and POSAS score will be secondary outcomes in this study. We will set up an independent panel monitor consisting of neurosurgeons and pain specialists to monitor for AEs including, but not limited to, wound healing problems and number of patients undergoing surgical revision after each ten patients at. If AEs are identified, it will first be evaluated and treated by the independent panel and must be immediately reported to the Institutional Review Board (IRB). And the IRB will review the negative outcomes and determine whether termination of the study is necessary.
Notably, complications of craniotomy including postoperative bleeding, increased intracranial pressure, cerebral infarction, epilepsy, hypertension, air embolism, cranial nerve injury and cerebral edema which may increase the complexity of postoperative analgesia in patients undergoing craniotomy,21 and some patients can suffer from transient confusion after neurosurgery.28 All of the above aspects may increase the difficulty in diagnosing incision pain. Hence, experienced neurosurgeons in charge of the participants will be needed to make an accurate differential diagnosis and observe postoperative pain after craniotomy caused by the craniotomy incision.
In summary, this is a prospective, randomized, single center, parallel-group, blinded trial that aims to evaluate the efficacy and safety of pre-emptive scalp infiltration with dexamethasone plus ropivacaine for postoperative pain in patients undergoing supratentorial tumor surgery. If the results reveal that dexamethasone plus ropivacaine can significantly decrease sufentanil consumption postoperatively, this trial will provide clinical guidance for post-craniotomy pain management.
Study limitations
There are several limitations to be noted regarding this study. First, the half-life and anti-inflammatory effects have significant differences among various steroids, so the results of this trial cannot be applied to other steroids, such as methylprednisolone and betamethasone. Second, this study is a single-center, RCT with a small sample size, and multicenter RCTs with larger sample sizes are needed to provide a higher level of evidence of the analgesic effect of scalp infiltration with dexamethasone plus ropivacaine. Last but not least, although the analgesic strategy for the participants will be strictly controlled at the hospital, analgesics used at home cannot be closely observed, which may affect our results at the end of the follow-up period. Nevertheless, this study will provide clinically important information on the role of dexamethasone in scalp infiltration for patients undergoing a craniotomy.
Abbreviations
QoL, quality of life; PONV, postoperative nausea/vomiting; NSAIDs, Nonsteroidal anti-inflammatory drugs; COX, cyclooxygenase; RCT, randomized controlled trial; NMDA, N-methyl-D-aspartate; LIA, local infiltration of analgesia; VAS, visual analogue scale; ASA, American Society of Anaesthesiologists; PCA, patient-controlled analgesia; BMI, body mass index; RSS, Ramsay Sedation Scale; PSS, patient satisfaction scale; WHOQOL-BREF, World Health Organization Quality of Life Abbreviated Version; POSAS, Patient and Observer Scar Assessment Scale; BP, blood pressure; HR, heart rate; SpO2, pulse oximetry; IV, intravenous; PACU, post-anaesthetic care unit; AEs, adverse events; MAP, mean arterial pressure; CRF, case report form; SPSS, Statistical Product and Service Solutions; ITT, intention-to-treat; PP, per-protocol; IQRs, interquartile ranges.
Acknowledgment
This research was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. XMLX201707).
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. Yitong Jia, Chunmei Zhao, Hao Ren and Tao Wang contributed equally to this work and should be considered co-first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dunbar PJ, Visco E, Lam AM. Craniotomy procedures are associated with less analgesic requirements than other surgical procedures. Anesth Analg. 1999;88(2):335–340.
2. Williams J, Craen R, Novick T, Komar W. The efficacy of intramuscular (IM) codeine for post-craniotomy pain. Can J Anaesth. 1997;44(Supplement 1):A28B. doi:10.1007/BF03015372
3. Bonica JJ. The Management of Pain.
4. Suksompong S, Chaikittisilpa N, Rutchadawong T, Chankaew E, von Bormann B. Pain after major craniotomy in a University Hospital: a prospective cohort study. J Med Assoc Thai. 2016;99(5):539–548.
5. Gottschalk A, Berkow LC, Stevens RD, et al. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg. 2007;106(2):210–216. doi:10.3171/jns.2007.106.2.210
6. Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: a retrospective study. Can J Anaesth. 2007;54(7):544–548. doi:10.1007/BF03022318
7. Hansen MS, Brennum J, Moltke FB, Dahl JB. Pain treatment after craniotomy: where is the (procedure-specific) evidence? A qualitative systematic review. Eur J Anaesthesiol. 2011;28(12):821–829. doi:10.1097/EJA.0b013e32834a0255
8. Titsworth WL, Abram J, Guin P, et al. 110 implementation of a standardized multimodal postoperative pain protocol reduces postoperative pain among neurosurgical patients. Neurosurgery. 2014;61:195. doi:10.1227/01.neu.0000452384.25705.d8
9. Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000;93(1):48–54.
10. Flexman AM, Ng JL, Gelb AW. Acute and chronic pain following craniotomy. Curr Opin Anaesthesiol. 2010;23(5):551–557. doi:10.1097/ACO.0b013e32833e15b9
11. De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38(3):
12. Verchere E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14(2):96–101.
13. Leslie K, Williams DL. Postoperative pain, nausea and vomiting in neurosurgical patients. Curr Opin Anaesthesiol. 2005;18(5):461–465. doi:10.1097/01.aco.0000182564.25057.fa
14. Peng K, Jin XH, Liu SL, Ji FH. Effect of intraoperative dexmedetomidine on post-craniotomy pain. Clin Ther. 2015;37(5):1114–1121.e1111. doi:10.1016/j.clinthera.2015.02.011
15. Dunn LK, Naik BI, Nemergut EC, Durieux ME. Post-craniotomy pain management: beyond opioids. Curr Neurol Neurosci Rep. 2016;16(10):93. doi:10.1007/s11910-016-0693-y
16. Durieux ME, Himmelseher S. Pain control after craniotomy: off balance on the tightrope? J Neurosurg. 2007;106(2):207–209. doi:10.3171/jns.2007.106.2.207
17. Zhang L, Shu R, Zhao Q, Li Y, Yu Y, Wang G. Preoperative butorphanol and flurbiprofen axetil therapy attenuates remifentanil-induced hyperalgesia after laparoscopic gynaecological surgery: a randomized double-blind controlled trial. Br J Anaesth. 2016;117(4):504–511. doi:10.1093/bja/aew248
18. van der Zwan T, Baerts WD, Perez RS, de Lange JJ. Postoperative condition after the use of remifentanil with a small dose of piritramide compared with a fentanyl-based protocol in patients undergoing craniotomy. Eur J Anaesthesiol. 2005;22(6):438–441.
19. Vacas S, Van de Wiele B. Designing a pain management protocol for craniotomy: a narrative review and consideration of promising practices. Surg Neurol Int. 2017;8:291. doi:10.4103/sni.sni_301_17
20. Schug SA, Manopas A. Update on the role of non-opioids for postoperative pain treatment. Best Pract Res Clin Anaesthesiol. 2007;21(1):15–30. doi:10.1016/j.bpa.2006.12.002
21. Vadivelu N, Kai AM, Tran D, Kodumudi G, Legler A, Ayrian E. Options for perioperative pain management in neurosurgery. J Pain Res. 2016;9:37–47. doi:10.2147/JPR.S85782
22. Pawlosky N. Cardiovascular risk: are all NSAIDs alike? Can Pharm J. 2013;146(2):80–83. doi:10.1177/1715163513481569
23. Misra S, Koshy T, Unnikrishnan KP, Suneel PR, Chatterjee N. Gabapentin premedication decreases the hemodynamic response to skull pin insertion in patients undergoing craniotomy. J Neurosurg Anesthesiol. 2011;23(2):110–117. doi:10.1097/ANA.0b013e3181da3c3b
24. Zeng M, Dong J, Lin N, et al. Preoperative gabapentin administration improves acute postoperative analgesia in patients undergoing craniotomy: a randomized controlled trial. J Neurosurg Anesthesiol. 2018. doi:10.1097/ANA.0000000000000533
25. Ture H, Sayin M, Karlikaya G, Bingol CA, Aykac B, Ture U. The analgesic effect of gabapentin as a prophylactic anticonvulsant drug on postcraniotomy pain: a prospective randomized study. Anesth Analg. 2009;109(5):1625–1631. doi:10.1213/ane.0b013e3181b0f18b
26. Dahl JB, Mathiesen O, Moiniche S. ‘Protective premedication’: an option with gabapentin and related drugs? A review of gabapentin and pregabalin in in the treatment of post-operative pain. Acta Anaesthesiol Scand. 2004;48(9):1130–1136. doi:10.1111/j.1399-6576.2004.00484.x
27. Mintz DC. Anesthetic pharmacology: basic principles and clinical practice, 2nd ed. Anesthesiology. 2012;117(4). doi:10.1097/ALN.0b013e31825a310c
28. Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg. 2009;109(1):240–244. doi:10.1213/ane.0b013e3181a4928d
29. Song J, Li L, Yu P, Gao T, Liu K. Preemptive scalp infiltration with 0.5% ropivacaine and 1% lidocaine reduces postoperative pain after craniotomy. Acta Neurochir (Wien). 2015;157(6):993–998. doi:10.1007/s00701-015-2394-8
30. Saringcarinkul A, Boonsri S. Effect of scalp infiltration on postoperative pain relief in elective supratentorial craniotomy with 0.5% bupivacaine with adrenaline 1:400,000. J Med Assoc Thai. 2008;91(10):1518–1523.
31. Basuni AS, Ezz HA, Albirmawy OA. Preoperative peritonsillar infiltration of dexamethasone and levobupivacaine reduces pediatric post-tonsillectomy pain: a double-blind prospective randomized clinical trial. J Anesth. 2013;27(6):844–849. doi:10.1007/s00540-013-1638-0
32. Shantiaee Y, Mahjour F, Dianat O. Efficacy comparison of periapical infiltration injection of dexamethasone, morphine and placebo for postoperative endodontic pain. Int Dent J. 2012;62(2):74–78. doi:10.1111/j.1875-595X.2011.00092.x
33. Bayram A, Dogan M, Cihan C, Karatas D, Gokahmetoglu G, Ozcan I. The efficacy of levobupivacaine hydrochloride-dexamethasone infiltration for post-tonsillectomy pain in adults. J Craniofac Surg. 2015;26(7):e651–653. doi:10.1097/SCS.0000000000001975
34. Ikeuchi M, Kamimoto Y, Izumi M, et al. Effects of dexamethasone on local infiltration analgesia in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(7):1638–1643. doi:10.1007/s00167-013-2367-5
35. Chien CW, Bagraith KS, Khan A, Deen M, Strong J. Comparative responsiveness of verbal and numerical rating scales to measure pain intensity in patients with chronic pain. J Pain. 2013;14(12):1653–1662. doi:10.1016/j.jpain.2013.08.006
36. Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656–659.
37. Erdine S, Ozyalcin NS, Cimen A, Celik M, Talu GK, Disci R. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007;11(3):309–313. doi:10.1016/j.ejpain.2006.04.001
38. Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual life res. 2004;13(2):299–310. doi:10.1023/B:QURE.0000018486.91360.00
39. Langford P, Wolfe R, Danks RA. Wound healing after craniotomy: a randomized trial comparing scalp clips to artery forceps for scalp hemostasis. J Neurosurg. 2009;111(6):1175–1178. doi:10.3171/2009.5.JNS081481
40. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
41. Maged AM, Deeb WS, Elbaradie S, et al. Comparison of local and intra venous dexamethasone on postoperative pain and recovery after caeseream section. A randomized controlled trial. Taiwan J Obstet Gynecol. 2018;57(3):346–350. doi:10.1016/j.tjog.2018.04.004
42. Puntis M, Garner A. Management of pain following craniotomy. Br J Nurs. 2015;24(14):740–744. doi:10.12968/bjon.2015.24.14.740
43. Lai LT, Ortiz-Cardona JR, Bendo AA. Perioperative pain management in the neurosurgical patient. Anesthesiol Clin. 2012;30(2):347–367. doi:10.1016/j.anclin.2012.05.004
44. Hauritz RW, Hannig KE, Henriksen CW, Børglum J, Bjørn S, Bendtsen TF. The effect of perineural dexamethasone on duration of sciatic nerve blockade: a randomized, double-blind study. Acta Anaesthesiol Scand. 2017;62:4.
45. Wicke C, Halliday B, Allen D, et al. Effects of steroids and retinoids on wound healing. Arch Surg. 2000;135(11):1265–1270.
46. Cutolo M, Seriolo B, Pizzorni C, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153–155. doi:10.1016/j.autrev.2008.07.010
47. Naja Z, Kanawati S, Al Khatib R, et al. The effect of IV dexamethasone versus local anesthetic infiltration technique in postoperative nausea and vomiting after tonsillectomy in children: a randomized double-blind clinical trial. Int J Pediatr Otorhinolaryngol. 2017;92:21–26. doi:10.1016/j.ijporl.2016.10.030
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.