Back to Journals » Journal of Pain Research » Volume 11
Practice and bias in intraoperative pain management: results of a cross-sectional patient study and a survey of anesthesiologists
Authors Ward S, Guest C, Goodall I, Bantel C
Received 12 October 2017
Accepted for publication 13 January 2018
Published 15 March 2018 Volume 2018:11 Pages 561—570
DOI https://doi.org/10.2147/JPR.S153857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Stephen Ward,1 Charlotte Guest,2 Ian Goodall,2 Carsten Bantel3,4
1Pain Service, Barts Health, St Bartholomew’s Hospital, London, UK; 2Pain Medicine, Chelsea and Westminster Hospital NHS Foundation Trust, London, UK; 3Section of Anaesthetics, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, London, UK; 4Department of Anesthesiology, Intensive Care Medicine, Emergency Medicine and Pain Management, Universität Oldenburg, Oldenburg, Germany
Background: Perioperative pain carries a considerable risk of becoming persistent; hence aggressive preventive approaches are advocated. Persistently high prevalence of postoperative pain, however, suggests anesthesiologists underuse these strategies. A prospective cross-sectional study of patients in the postanesthetic care unit (PACU) and a survey of anesthesiologists were thus conducted to evaluate practice and uncover bias in intraoperative pain management.
Methods: Notes of PACU patients were reviewed and information regarding surgical context, comorbidities, and analgesic administration was retrieved. Variables were analyzed for their predictive properties on pain and intraoperative analgesic management. Furthermore, clinical dose–effect estimates for intraoperative morphine were determined. Finally, anesthesiologists completed a questionnaire comprising statements regarding pain relating to surgical context and morphine administration.
Results: Data of 200 patients and 55 anesthesiologists were analyzed. Prevalence of pain in PACU was 28% and was predicted by local anesthetic (LA) and low-dose morphine administration. Additionally, when LA was used, little coanalgesics were employed. These results suggest a restrained approach by anesthesiologists toward intraoperative pain management. It is supported by their reluctance to administer more than 10 mg morphine, despite these individuals regarding this practice as insufficient. The hesitancy toward morphine also transpired in the dose–effect estimates with the average applied dose operating on an ED63 instead of an ED95 level.
Conclusion: This study confirmed a high prevalence of pain in PACU. It also indicated conservative intraoperative analgesic administration by anesthesiologists. The modest morphine usage and overreliance on LA application, which are not supported by published evidence, additionally suggest bias in current intraoperative pain management.
Keywords: intraoperative morphine, multimodal analgesia, local anesthetic infiltration, clinical decision making, effective-dose, postoperative pain
Introduction
With a prevalence of 30% after surgery, acute pain clearly remains an unresolved problem of modern medicine often impacting on patient outcome.1,2 Depending on the surgical procedure, it carries up to 80% risk of becoming persistent (persistent postsurgical pain [PPSP]).3,4
During surgery, peripheral nociceptors are mechanically stimulated or sensitized by mediators released from tissues and immune cells (peripheral sensitization).5 As a result, frequent bursts of afferent activity (nociceptive barrage) enter the central nervous system potentially altering neuronal function and signal transmission (central sensitization).6–8 Central sensitization has been suggested the key mechanism underlying PPSP.3 The goal of present-day perioperative analgesic management, therefore, is to prevent or limit its development through the employment of an aggressive analgesic therapy (preventive analgesia).5 Preventive analgesia can be applied any time perioperatively and usually comprises a multimodal strategy including opioids.9
Anesthesiologists are at the heart of perioperative analgesic management, both as prescribers and administrators. Consequently, it is feasible to suggest the repeatedly observed high prevalence of pain in the early postoperative period may be at least partially the result of a lack of implementation of sufficient pain management strategies.2,10 However, although recent research has identified some variables that predict severe postoperative pain and opioid consumption,11 limited data are available concerning the intricate relationship between intraoperative analgesic management including opioid dosing and pain on arrival in the postanesthetic care unit (PACU). Furthermore, in contrast to general medicine, very little research has been conducted to investigate anesthesiologists’ decision making in relation to analgesic administration. Particularly, scarce knowledge exists concerning the influence of base rates and cognitive errors on choice, dose, and number of intraoperatively employed analgesic strategies.12–14
Thus, this study was aimed to gain further insight by employing a two-step approach into the thought processes and potentially confounding biases of anesthesiologists regarding intraoperative analgesic management. First, current practice of anesthesiologists was observed and its outcomes established in a cross-sectional patient sample. Results were then related to anesthesiologists’ responses to a 6-item questionnaire evaluating their attitudes toward intraoperative pain management.
Methods
The study was approved as a service evaluation by the Clinical Governance Support Team at Chelsea and Westminster Hospital (CWH), London, UK (reference number 667). Hence, according to UK regulations, formal ethical review was not required. Results were reported according to the strengthening of the reporting of observational studies in epidemiology statement.15
Audit of pain on arrival in PCAU
Starting from May 2012, for a total of 50 days, clinical notes and anesthetic and observation charts of patients passing the adult PACU at CWH, London, UK, were reviewed, reflecting the practice of 76 anesthesiologists. The following variables were anonymously recorded: age; gender; history of chronic pain; history of previous surgery; history of psychiatric disease; surgical specialty; length of surgery; surgical context (emergency, elective, keyhole, open); type of anesthesia (general, epidural, spinal); analgesics administered (number, type, dose); number of morphine boluses given; and side effects of treatments.
Primary outcome was defined as “pain on arrival” in PACU and assessed using a 5-point verbal numeric rating scale (VNRS) from “0” (no pain) to “4” (worst pain imaginable).
Clinical dose–effect estimation for intraoperative morphine administration
Strong opioids, in particular morphine, are still at the core to most perioperative analgesic strategies despite some evidence that their injudicious use (too little or too much) might contribute to PPSP.16–18 Intraoperative dose–effect estimates can serve as indicators of how aggressively anesthesiologists intend to treat pain. Sufficient intraoperative morphine therapy should aim for effective doses (ED) that suppress pain in at least 95% of patients (ED95) similar to what is common practice for local anesthetics (LA) or muscle relaxants.19,20 To assess how anesthesiologists in our cohort treated pain in general, ED50 and ED95 dose–effect estimates were calculated as follows.
Morphine doses were log transformed and VNRS scores were translated into percent drug effect. Scores of “0” represented 100% morphine effect, “1” represented 75%, “2” represented 50%, “3” represented 25%, and “4” represented 0% effect, respectively.
Linear regression models were employed as described by Tallarida.21 First, a general model (model 1) was generated that included all cases where morphine was administered (n=183 from 200). As this model provided a poor fit of the data (r=0.03), three alternative models (models 2.1–2.3) were created.
Dose–effect relationships for analgesics usually suggest increasing drug efficacy with increasing doses. To fit this assumption, data inclusion into the models was refined. Low morphine doses that elicited no or mild effects were included, as were high doses that had near maximum or maximum effect. Conversely, low doses that elicited maximum or near maximum effect or high doses achieving minimal effect were excluded from the model. However, the cutoff dose for what constituted a high or low dose had to be determined. Three different morphine cutoff doses were chosen arbitrarily: 5 mg (model 2.1), 7 mg (model 2.2), and 10 mg (model 2.3) and linear regression lines fitted.21 The best-fit model was chosen for calculations of ED.
Survey of anesthesiologists
In parallel to the patient audit, all 76 anesthesiologists (trainees and consultants) of the anesthetic department at CWH were approached to anonymously complete a questionnaire. Returning a questionnaire was deemed as consent to participate in the study. Questionnaire contents were based on outcomes of a pilot patient audit and subsequent discussions with anesthesiologists. Four topics were thus identified that considerably influenced clinicians’ considerations about intraoperative pain management: surgical approach (open versus keyhole surgery), elderly patients, effects of opioids on time-to-waking, and 10 mg as upper dose limit for morphine. First, a set of preliminary statements was generated. Subsequently, n=5 pain nurses, pain specialists, and anesthesiologists impartial to the study were asked to rate face and content validity of statements using a scoring sheet and to make comments and suggestions where appropriate. After eliminating or rewording of poorly performing items, the questionnaire was once again reviewed by the same n=5 pain nurses, pain specialists, and anesthesiologists. Lastly, remaining issues were solved by discussion among the authors of the study.
The final questionnaire, hence, comprised the following six statements: 1) Open surgery influences my choice of the number of intraoperative analgesics I administer compared to keyhole surgery. 2) Keyhole surgery is generally less painful than open surgery. 3) When administering or prescribing opioids, I am more cautious in elderly patients. 4) Ten milligrams of morphine is usually enough for most patients. 5) If I had >10 mg morphine drawn up and available to me, I would use it. 6) I think higher intraoperative opioid doses prolong time-to-waking.
Participants were asked to indicate their level of agreement on a horizontal 5-point Likert scale ranging from “strongly agree” to “strongly disagree”.
Statistical analysis
Audit data analysis was benchmarked against the Royal College of Anaesthetists’ recommendation that patients should experience “no more than mild pain” on arrival in PACU.22 VNRS data were converted into binary scores with ratings of 0–1 representing “acceptable (none to mild) pain” and ratings of 2–4 “unacceptable (moderate to severe) pain”.23 Likewise, “age”, “total morphine dose”, “total number of administered morphine boluses”, and “total number of administered analgesics” were converted into categorical variables.23
Logistic regression was used to 1) analyze whether the employed variables predicted “unacceptable pain” (primary outcome) and 2) to determine what variables predicted intraoperative morphine and analgesic use (secondary outcome).
Further, Pearson’s correlation analysis was used to determine the association between length of surgery and the time that elapsed from last morphine administration to time-to-waking.
Likert scale data were treated as ordinal, and nonparametric (Fisher’s exact test) tests were employed where appropriate.
Statistical analysis was performed with SPSS 21 (IBM UK, Portsmouth, UK) and Prism 5 for Mac (GraphPad Software, La Jolla, CA, USA). Data are presented as mean with 95% confidence intervals (95% CI) where appropriate. A p<0.05 was considered significant. However, for predictor analysis, a more liberal significance level was employed (p<0.15) as suggested by Kalkman et al.24
Results
A summary of patient demographics and characteristics, pain on arrival in PACU, type of surgery, anesthetic technique, and analgesic administration is shown in Table 1.
Side effects were recorded 209 times. Drowsiness was most common (n=154; 74%), followed by nausea (n=41; 20%) and vomiting (n=12; 6%). There was one case each of respiratory depression (0.5%) and pruritus (0.5%).
Predictors of pain on arrival in PACU
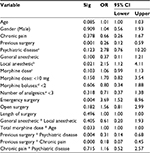
Results of regression analysis for unacceptable pain on arrival in PACU are shown in Table 2. When considering p<0.15 as statistically significant as suggested by Kalkman et al,24 “age”, “emergency surgery”, “history of psychiatric disease”, and “previous surgery” were predictors of pain. Interestingly, variables that directly reflected the therapeutic decisions of anesthesiologists (“general anesthesia”, “LA administration”, “total morphine dose”, and “total morphine dose of <10 mg”) also predicted pain. Hence, these results strongly suggest a considerable contribution of anesthesiologists to the unacceptable pain 28% of patients experience in the early postoperative period.
Predictors of analgesic administration
The results of the predictor analysis for intraoperative analgesic administrations (Table 3) provide insight into what factors influence anesthesiologists’ therapeutic decisions. For example, surgical context (“elective surgery” and “length of surgery”) predicted administration of at least 10 mg of morphine. Not surprisingly, patient factors also influenced anesthesiologists’ treatment strategies. For example, female patients were more likely to receive higher morphine doses while patients with chronic pain received less. However, most strikingly, LA administration predicted the cautious use of morphine and coanalgesics, indicating a strong reliance of anesthesiologists on this treatment.
Clinical dose–effect estimation for intraoperative morphine administration
From the dose–effect models generated, model 2.2 showed the best fit and was subsequently used for dose calculations (Table 4).
  | Table 4 Clinical dose–effect estimates for intraoperative morphine application Abbreviations: ED, effective dose; n/a, not applicable. |
The ED95 estimate obtained with model 2.2 for intraoperative morphine for our patient cohort was 12.8 mg. Although this indicates doses of at least 10 mg morphine would have been needed in the majority of patients to achieve acceptable levels of pain, in reality only 38% (62/183) of patients received similar amounts. In addition, only n=14/183 (8%) received >10 mg.
If the mean morphine dose of our patient population (6.9 mg) is substituted for x in Equation 2.2 (Table 4), the result showed this dose to be an ED63. Therefore, applying the average morphine dose to our patients, only 63% could be expected to arrive comfortably in PACU, leaving 37% with inadequate pain relief. This calculated failure rate was statistically not different from the observed 28% of this study (p=0.227; Fisher’s exact test) adding credibility to our results. In addition, in 40% (n=73/183) of patients, the administered dose was even less than the calculated ED50 (5.4 mg, 95% CI: 4.6–6.2 mg; Table 4).
Survey of anesthesiologists
Fifty-five out of 76 (72%) anesthesiologists (n=23 trainees; n=32 consultants) returned the questionnaire. A Cronbach’s alpha of 0.96 suggested it had an excellent reliability. Frequency distributions of responses are shown in Figure 1.
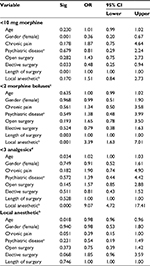
Eighty-two percent of all anesthesiologists agreed with the statement open compared to keyhole surgery influenced the number of analgesics they administered intraoperatively. Level of seniority influenced answers (p=0.034; odds ratio [OR]: 8.6; 95% CI: 1.0–73.4) as significantly more trainees (96%) compared to consultants (72%) favored this statement (statement 1; Figure 1A).
Fifty-eight percent of anesthesiologists (70% trainees; 50% consultants) were also more likely to agree with the notion of keyhole generally being less painful than open surgery (statement 2; Figure 1B). Although more trainees agreed (OR: 2.3; 95% CI: 0.7–7.1), statistically, seniority did not influence answers (p=0.175).
Nearly all anesthesiologists (96% trainees; 97% consultants) agreed they were more cautious administering or prescribing opioids in the elderly (statement 3) with level of seniority not influencing results (OR: 0.7; 95% CI: 0–12; p=1.000; Figure 1C).
Only 16% of all anesthesiologists approved that “10 mg of morphine is usually enough for most patients” (statement 4; Figure 1D). Although 22% trainees compared to 13% consultants agreed, group differences were statistically nonsignificant (OR: 1.9; 95% CI: 0.5–8.2; p=0.467).
However, only 33% anesthesiologists would use >10 mg morphine intraoperatively if available to them (statement 5). Here fewer trainees (26%) than consultants (37%) would use the additional dose (OR: 0.6; 95% CI: 0.2–1.9; Figure 1E) without this difference being statistically significant (p=0.402).
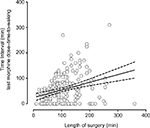
Interestingly, 38% agreed higher intraoperative opioid doses prolong time-to-waking (statement 6), although fewer trainees (30%) than consultants (44%) approved the notion (OR: 0.6; 95% CI: 0.2–17). These differences did not reach significance level (p=0.403; Figure 1F). However, responses to this statement are in agreement with results depicted in Figure 2. Here the length of the surgical procedure was plotted against the difference between the time elapsed after morphine was last administered and patients’ first waking. Results showed a positive correlation (r=0.404; p=0.000), indicating morphine was usually given at an early stage of the procedure.
Discussion
Anesthesiologists’ mental models relevant to their decision making are increasingly addressed in research.12,25 However, the little evidence available is mostly concerned with improvement of patient safety rather than postoperative pain.14,26 This occurs (the fact that research in decision making in pain management is virtually non existent) despite the presence of evidence that suggests perioperative pain management significantly contributes to the development of PPSP.3,4
Central to decision making is the employment of heuristics (mental shortcuts). This is “a strategy that ignores part of available information with the goal of making decisions more frugally and quickly.”27 Yet, disregarding information such as base rates risks the introduction of error into clinical practice.12,28
The high prevalence of pain in PACU observed here is at least in part the result of suboptimal pain management. Analysis of intraoperative analgesic use and results from the anesthesiologists’ survey indicate considerable bias in current anesthetic practice as a probable contributor. This bias seems to be common, as our findings not only confirmed previous reports concerning the prevalence of pain in PACU, but likewise found a similar routine of intraoperative coanalgesic use as reported recently in a French survey.23,29
Contrary to the French, anesthesiologists in our cohort relied considerably on the employment of intraoperative morphine.29,30 Therefore, understanding decision making in relation to opioid use may help to improve perioperative pain management.
In this study, intraoperative opioid use was cautious with <10% patients receiving >10 mg morphine. However, support for the requirement of higher doses was gained from ED95 estimates. In this study, to achieve comfort in 95% patients, 12.8 mg morphine would have been needed. This result was strikingly similar to the dose Aubrun et al calculated for patients in PACU.30,31
Problems with low-dose morphine administration were further emphasized through the finding that the average dose for our study population equaled an ED63, potentially resulting in 37% of patients waking in pain. However, the actual observed prevalence of pain in PACU was slightly less highlighting the concept of pain as a complex phenomenon. This notion was also supported here by the surprising protective effect of previous surgery on pain in PACU, which we find currently difficult to explain.
Nevertheless, the impact of applying low morphine doses was certainly detrimental in our study, and contrary to the increasing evidence suggesting aggressive dosing to ensure success of preventive analgesic strategies.5,32 Although morphine administration was too cautious in our cohort, it must be emphasized that overtreatment might also contribute to the development of PPSP.18 Therefore, currently, careful titration of opioids in the context of a multimodal preventive analgesic approach possibly offers the best treatment option.33 However, more research is needed to allow clinicians in the future to determine individual patients’ opioid needs.
Prescribing and administering opioids have been acknowledged to be challenging for health care professionals, especially in the community.34 However, our survey results suggest similar barriers on part of anesthesiologists as well. While half of the participants disagreed 10 mg morphine would be enough for most patients, two-thirds would not give more, even if available to them.
Barriers to therapies often result from cognitive errors which are a consequence of a neglect of base rates (base rate fallacy).26 Base rate fallacies can also be hypothesized for this study. Anesthesiologists, for instance, were concerned about opioid-induced side effects with prolonged sedation and altered pharmacokinetics, particularly in the elderly. There were also concerns over morphine-induced sedation, which indeed were translated into clinical practice as anesthesiologists tended to administer opioids early during surgery. Evidence, however, does not support this practice.17,35 In fact, 0.15 mg/kg morphine immediately before the end of surgery reduced pain scores and opioid requirements in PACU, but did not prolong time-to-waking.17
Furthermore, previous reports indicated that patients after emergency procedures or with a history of psychiatric diseases suffered increasing pain postoperatively.11 However, this base rate was neglected here and both conditions predicted unacceptable pain in PACU.
Most clinicians would agree LAs are capable of completely suppressing painful stimuli. In addition, there is a plethora of data demonstrating beneficial effects of LAs on postoperative pain and opioid consumption.36,37 Therefore, identifying “administration of LAs” as a predictor of pain in PACU was surprising.
One possible reason might have been the inappropriate application of base rates about LAs by anesthesiologists in this study and consequently an overreliance on this analgesic principle. This notion is based on the further finding of LA use as a predictor of cautious administration of morphine and other analgesics (Table 3). It is also supported by failure rates of 10%–30% for neuraxial and 30% for peripheral nerve blocks, respectively.36,38,39 Recently, doubts have even been raised regarding the effectiveness of LA infiltration for pain after hip and spinal surgery.40,41 Finally, when employing LA drugs dosing, duration of action and wound coverage also need to be considered.37 Based on our findings, anesthesiologists should employ a more proactive, aggressive strategy to facilitate smooth transition from local to systemic analgesic administration in the postoperative period. Base rates, however, were not always neglected in this study. Although most anesthesiologists stated they were more cautious administering morphine in the elderly, age, nevertheless, was not a meaningful predictor of analgesic administration. Concerns over age are substantiated by reports of altered opioid pharmacokinetics and pharmacodynamics in the elderly.42 However, these considerations are not supported by clinical evidence as perioperative pain in the elderly is often underestimated and their analgesic needs have shown to be similar to that of younger patients.42,43 Furthermore, women are known to experience more pain and require additional analgesics postoperatively compared with men.44 This base rate was translated into practice here with female patients receiving higher doses of morphine. Finally, although anesthesiologists agreed open surgery is more painful than keyhole surgery (item 2), and that they would adapt management accordingly (item 1), observed practice was different. We could not identify the surgical method as a predictor of opioid administration. This is in accordance with recent evidence, which indicates both surgical approaches can cause considerable pain.1
Study limitations
The inclusion of a restricted number of variables was one of the limitations of the present study as the employed regression models could explain only 25% of the observed variability of this study. However, since we aimed to identify predictors for intraoperative analgesic management with reasonably good study power,45 we a priori included only variables we thought most important.11 Ethnicity, American Society of Anesthesiologists status, and severity of surgery have been excluded, but should be addressed in future work.
Moreover, it could be argued the data presented here, especially the minimal usage of regional anesthesia, represent only an isolated practice. Yet, limited employment of local anesthesia is not exclusive to our hospital. Gerbershagen et al, Sommer et al, and Fletcher et al described similar findings for German, Dutch, and French cohorts, respectively.1,2,29
Furthermore, the dose–effect estimates conducted here could be criticized for not being adjusted for age, gender, coanalgesics, or weight. However, a recent study investigating morphine titration in PACU employing 3000 patients not only used a similar method, but also found similar results.31
It might be argued that the dose analysis was not procedure specific and therefore did not take into account the potentially varying degrees of surgical stimulation and resulting different analgesic needs. However, the intention of this analysis was not to develop strict dose recommendations but instead act as an indicator for the intended “aggressiveness” of anesthetists in treating perioperative pain. As such, it indeed highlighted a rather cautious practice of opioid usage in our patient cohort.
Ideally, each drug administration should be individualized according to each patient’s specific circumstances. As this approach should help to prevent critical opioid overdoses, it should equally limit unnecessary pain experiences. However, currently, no markers exist that could help assess pain and morphine requirements objectively.46
Finally, the questionnaire could have been more thoroughly validated and included questions regarding the use of regional anesthesia. However, it was designed to predominantly evaluate attitudes surrounding the administration of intraoperative morphine. Therefore, more detailed work is needed in the future to address anesthesiologists’ thought processes in this regard.
Conclusion
This study provides first evidence for considerable heuristics and base rate fallacies influencing the practice of anesthesiologists’ intraoperative pain management.
The high prevalence of pain in PACU resulted from cautious use of morphine and coanalgesics, as well as overreliance on the success of LA infiltration. This practice was likely the consequence of important base rate fallacies as part of the employed heuristics in anesthesiologists’ decision-making processes.
In the future, decision making relevant to pain management needs further investigation, in addition to the development of strategies to help anesthesiologists avoid cognitive errors.
Acknowledgments
The authors would like to thank Dushyanth Gnanappiragasam for his help in the data acquisition process. This work was supported by the Higher Education Funding Council for England (HEFC-E). Part of this work was presented at the British Pain Society Annual Scientific Meeting, April 16–19, 2013, in Bournemouth, UK.
Disclosure
Dr Carsten Bantel has received an educational grant from TEVA UK and consultancy fees from NAPP pharmaceuticals as well as Mundipharma. The authors report no other conflicts of interest in this work.
References
Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. | ||
Sommer M, de Rijke JM, van Kleer M, et al. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur J Anaesthesiol. 2008;25(4):267–274. | ||
Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. 2013;257(3):403–412. | ||
Van de Ven TJ, John Hsia HL. Causes and prevention of chronic postsurgical pain. Curr Opin Crit Care. 2012;18(4):366–371. | ||
Dahl JB, Kehlet H. Preventive analgesia. Curr Opin Anaesthesiol. 2011;24(3):331–338. | ||
Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. | ||
Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3):S2–S15. | ||
Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. | ||
Vadivelu N, Mitra S, Schermer E, Kodumudi V, Kaye AD, Urman RD. Preventive analgesia for postoperative pain control: a broader concept. Local Reg Anesth. 2014;7:17–22. | ||
Tufano R, Puntillo F, Draisci G, et al. ITalian observational study of the management of mild-to-moderate post-operative pain (ITOSPOP). Minerva Anestesiol. 2012;78(1):15–25. | ||
Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111(3):657–677. | ||
Stiegler MP, Neelankavil JP, Canales C, Dhillon A. Cognitive errors detected in anaesthesiology: a literature review and pilot study. Br J Anaesth. 2012;108(2):229–235. | ||
Woolever DR. The art and science of clinical decision making. Fam Pract Manag. 2008;15(5):31–36. | ||
Stiegler MP, Ruskin KJ. Decision-making and safety in anesthesiology. Curr Opin Anaesthesiol. 2012;25(6):724–729. | ||
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. | ||
Ho KY, Tay W, Yeo MC, et al. Duloxetine reduces morphine requirements after knee replacement surgery. Br J Anaesth. 2010;105(3):371–376. | ||
Aubrun F, Amour J, Rosenthal D, Coriat P, Riou B. Effects of a loading dose of morphine before i.v. morphine titration for postoperative pain relief: a randomized, double-blind, placebo-control study. Br J Anaesth. 2007;98(1):124–130. | ||
Lavand’homme P, Steyaert A. Opioid free anaesthesia. Opioid side effects: tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol. Epub 2017 May 17. | ||
Dahaba AA, Perelman SI, Moskowitz DM, et al. Influence of acute normovolaemic haemodilution on the dose-response relationship, time-course of action and pharmacokinetics of rocuronium bromide. Br J Anaesth. 2006;97(4):482–488. | ||
Kant A, Gupta PK, Zohar S, Chevret S, Hopkins PM. Application of the continual reassessment method to dose-finding studies in regional anesthesia: an estimate of the ED95 dose for 0.5% bupivacaine for ultrasound-guided supraclavicular block. Anesthesiology. 2013;119(1):29–35. | ||
Tallarida RJ. Dose-response analysis. In: Drug Synergism and Dose-Effect Data Analysis. Boca Raton, London, New York, Washington, DC: Chapman & Hall/CRC; 2000:21–39. | ||
Vickers A. Section 11: acute pain services. In: Colvin JR, editor. Rising the Standard–a Compendium of Audit Recipes for Continuous Quality Improvement in Anaesthesia. 2nd ed. London: Royal College of Anaesthetists; 2006:229–250. | ||
Dahmani S, Dupont H, Mantz J, Desmonts JM, Keita H. Predictive factors of early morphine requirements in the post-anaesthesia care unit (PACU). Br J Anaesth. 2001;87(3):385–389. | ||
Kalkman CJ, Visser K, Moen J, Bonsel GJ, Grobbee DE, Moons KG. Preoperative prediction of severe postoperative pain. Pain. 2003;105(3):415–423. | ||
Yentis SM. Decision analysis in anaesthesia: a tool for developing and analysing clinical management plans. Anaesthesia. 2006;61(7):651–658. | ||
Stiegler MP, Tung A. Cognitive processes in anesthesiology decision making. Anesthesiology. 2014;120(1):204–217. | ||
Gigerenzer G, Gaissmaier W. Heuristic decision making. Annu Rev Psychol. 2011;62:451–482. | ||
De Neys W, Cromheeke S, Osman M. Biased but in doubt: conflict and decision confidence. PLoS One. 2011;6(1):e15954. | ||
Fletcher D, Fermanian C, Mardaye A, Aegerter P. A patient-based national survey on postoperative pain management in France reveals significant achievements and persistent challenges. Pain. 2008;137(2):441–451. | ||
Aubrun F, Valade N, Coriat P, Riou B. Predictive factors of severe postoperative pain in the postanesthesia care unit. Anesth Analg. 2008;106(5):1535–1541. | ||
Aubrun F, Langeron O, Quesnel C, Coriat P, Riou B. Relationships between measurement of pain using visual analog score and morphine requirements during postoperative intravenous morphine titration. Anesthesiology. 2003;98(6):1415–1421. | ||
Pogatzki-Zahn EM, Zahn PK. From preemptive to preventive analgesia. Curr Opin Anaesthesiol. 2006;19(5):551–555. | ||
Anwar S, O’Brien B. The role of intraoperative interventions to minimise chronic postsurgical pain. Br J Pain. 2017;11(4):186–191. | ||
Gardiner C, Gott M, Ingleton C, Hughes P, Winslow M, Bennett MI. Attitudes of health care professionals to opioid prescribing in end-of-life care: a qualitative focus group study. J Pain Symptom Manage. 2012;44(2):206–214. | ||
Katoh T, Suguro Y, Kimura T, Ikeda K. Morphine does not affect the awakening concentration of sevoflurane. Can J Anaesth. 1993;40(9):825–828. | ||
Ng K, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev. 2004;(2):CD003765. | ||
Barreveld A, Witte J, Chahal H, Durieux ME, Strichartz G. Preventive analgesia by local anesthetics: the reduction of postoperative pain by peripheral nerve blocks and intravenous drugs. Anesth Analg. 2013;116(5):1141–1161. | ||
Sites BD, Beach ML, Spence BC, et al. Ultrasound guidance improves the success rate of a perivascular axillary plexus block. Acta Anaesthesiol Scand. 2006;50(6):678–684. | ||
Ready LB. Acute pain: lessons learned from 25,000 patients. Reg Anesth Pain Med. 1999;24(6):499–505. | ||
Kjaergaard M, Moiniche S, Olsen KS. Wound infiltration with local anesthetics for post-operative pain relief in lumbar spine surgery: a systematic review. Acta Anaesthesiol Scand. 2012;56(3):282–290. | ||
Zoric L, Cuvillon P, Alonso S, et al. Single-shot intraoperative local anaesthetic infiltration does not reduce morphine consumption after total hip arthroplasty: a double-blinded placebo-controlled randomized study. Br J Anaesth. 2014;112(4):722–728. | ||
Aubrun F, Bunge D, Langeron O, Saillant G, Coriat P, Riou B. Postoperative morphine consumption in the elderly patient. Anesthesiology. 2003;99(1):160–165. | ||
Wilkinson K, Martin IC, Gough MJ, et al. An Age Old Problem: A Review of Care Received by Elderly Patients Undergoing Surgery. London: National Confidential Enquiry into Patient Outcome and Death; 2010:63–95. | ||
Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97(5):1464–1468. | ||
Field A. Regression. Discovering Statistics Using SPSS. 3rd ed. London: Sage Publications Ltd.; 2009:197–263. | ||
Bantel C, Laycock H, Nagy I. The potential use of biomarkers and new diagnostic tools in the management of acute pain. Pain Manage. 2012;2(3):1–4. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.