Back to Journals » International Journal of Women's Health » Volume 6
Potential role of aromatase inhibitors in the treatment of endometriosis
Authors Abu Hashim H
Received 2 April 2014
Accepted for publication 9 May 2014
Published 21 July 2014 Volume 2014:6 Pages 671—680
DOI https://doi.org/10.2147/IJWH.S34684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Hatem Abu Hashim
Department of Obstetrics and Gynecology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Abstract: Endometriosis is an estrogen-dependent chronic inflammatory disease affecting 5%–10% of reproductive-age women, with a prevalence of 5%–50% in infertile women and >33% of women with chronic pelvic pain. Third-generation aromatase inhibitors (AIs) are approved adjuvants for the treatment of estrogen receptor-positive breast cancer. Molecular studies have revealed the presence of aromatase P450, the key enzyme in the biosynthesis of ovarian estradiol, inside the endometriotic tissue, indicating local synthesis of estradiol. Thereby, AIs represent an appealing medical option for the management of different aspects of this enigmatic disease, especially pelvic pain and infertility. Accordingly, this review aims to evaluate the potential role of AIs in the treatment of endometriosis-associated symptoms, mainly pain and infertility. Notably, several studies have demonstrated that the combination of AIs with conventional therapy as oral contraceptive pills, progestins, or gonadotropin-releasing hormone analogs can be used to control endometriosis-associated pain and pain recurrence in premenopausal women, particularly those with pain due to rectovaginal endometriosis refractory to other medical or surgical treatment. Some case reports have shown promising results in the treatment of postmenopausal endometriosis as first-line treatment, when surgery is contraindicated, or as second-line treatment in the case of postoperative recurrence. Third-generation AIs, especially letrozole, have challenged clomiphene citrate as an ovulation-induction agent in patients with polycystic ovary syndrome and in cases of unexplained infertility. However, few studies are available regarding the use of AIs to treat endometriosis-associated infertility. Therefore, larger multicenter randomized trials using AIs for the treatment of endometriosis-associated infertility are needed to clarify its effect. The safety of AIs for ovulation induction or superovulation has generated a lively discussion. Data from recent retrospective and prospective studies have supported its safety.
Keywords: endometriosis, aromatase inhibitors, letrozole, anastrozole, pelvic pain, infertility
Introduction
Endometriosis is an estrogen-dependent chronic inflammatory disease affecting the health and well-being of 5%–10% of women of reproductive age,1 with a prevalence of 5%–50% in infertile women and >33% of women with chronic pelvic pain.2,3 Endometriosis is characterized by the presence of ectopic endometrial implants typically occurring in the pelvis, most commonly in the ovaries, pelvic peritoneum, uterosacral ligaments, pouch of Douglas, and rectovaginal septum. Patients can present with a wide range of symptoms; however, the cardinal clinical features are infertility and chronic pelvic pain.4
The definitive pathogenesis of endometriosis is still unknown, but retrograde menstruation, proposed by Sampson in the 1920s, is still considered the most widely accepted theory, although many points remain poorly understood concerning this theory.5 Of note, the incidence of retrograde menstruation is similar in women with and without endometriosis, so the pathogenesis seems to be a multifactorial mechanism comprising functionally different endometrial tissue in addition to altered immunity and other molecular abnormalities allowing the survival of the regurgitated endometrial debris. Subsequently, these endometrial implants go through various sequential events for the disease to develop.5,6 As endometriosis is an estrogen-dependent disease, conventional treatments utilized for many years aimed to act indirectly to create an estrogen-deficient state, eg, gonadotropin-releasing hormone agonists (GnRHas) and combined oral contraceptive pills or counteracting estrogen action, eg, progestins, eventually resulting in inhibition of the endometrial implant growth and alleviation of symptoms.4
Aromatase P450 is the key enzyme for ovarian estrogen biosynthesis. It catalyzes the conversion of androstenedione and testosterone produced in the ovarian theca cells to estrone and estradiol (E2) in the ovarian granulose cells. Recently, there has accumulated a body of evidence demonstrating that endometriotic lesions express aromatase and are able to synthesize their own E2.5,7,8 In view of this observation, the use of aromatase inhibitors (AIs) for the management of endometriosis is an appealing concept. Accordingly, this review aims to evaluate the potential role of AIs in the treatment of endometriosis-associated symptoms, particularly pain and infertility.
Materials and methods
A PubMed search was carried out to identify all the published studies evaluating the efficacy of AIs in treating endometriosis-associated pain and endometriosis-associated infertility with the following keywords: endometriosis, aromatase inhibitors, letrozole, anastrozole, pelvic pain, and infertility. The last search update was on February 28, 2014. No search limits were used. All relevant articles were identified and assessed for quality, and their reference lists were checked in order to identify other studies for potential inclusion in this review. Relevant evidence was selected for inclusion in the following order: meta-analyses, systematic reviews, different guidelines, randomized controlled trials (RCTs), and prospective cohort studies, followed by other observational studies, nonsystematic reviews, case series, and reports.
Types of aromatase inhibitors
The aromatase P450 enzyme is present in high levels in the ovarian granulose cells of premenopausal women; meanwhile, adipose tissue is a major site of aromatase expression in postmenopausal women.9 AIs were first used for the treatment of postmenopausal estrogen receptor-positive advanced breast cancer owing to their ability to reduce estrogen production by inhibition of the aromatase P450 enzyme. Accordingly, they have the potential to treat estrogen-dependent conditions, such as endometriosis.8,10
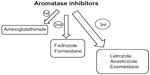
AIs are present in three generations (Figure 1). Aminoglutethimide, a first-generation inhibitor, induces a medical adrenalectomy that results in many side effects, such as lethargy, skin rashes, and nausea. Fadrozole and formestane are more selective second-generation inhibitors with fewer side effects; however, the only route of administration is intramuscular. Letrozole, anastrozole, and exemestane are the third generation of AIs. Letrozole and anastrozole are triazole derivatives characterized by being selective, reversible, and potent AIs. Given orally at doses of 1–5 mg/day, they inhibit estrogen levels by 97% to more than 99%;11–13 meanwhile, exemestane is a steroidal irreversible AI effectively working at a dose of 25 mg/day.12,14
  | Figure 1 Generations of aromatase inhibitors. |
Aromatase inhibitors from a pathophysiological perspective of endometriosis
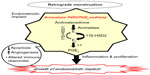
AIs appear to represent a new promising alternative for treatment of endometriosis through the alteration of mechanisms involved in the molecular development of endometriosis.5,7,8 Aromatase P450/prostaglandin E2 (PGE2) represents an important pathway involved in the development of endometriotic implants (Figure 2). Aromatase activity is not detectable in normal endometrium; however, it is expressed inappropriately in the eutopic endometrium from women with endometriosis. Accordingly, the eutopic endometrium represents an intracrine source of estrogen. Of note, estrogen is known to upregulate PGE2, which is a potent inducer of aromatase activity in endometriotic cells. Thereby, in view of this abnormal independent estrogen biosynthesis, a positive feedback loop is formed, leading to repeated proliferation and inflammation within endometriotic deposits, favoring endometriotic deposits survival in addition to other various mechanisms, such as altered immune responses, angiogenesis, and apoptosis.5,7,8 Importantly, the locally produced estrogen within these endometriotic deposits is mainly the potent E2 rather than the less biologically active estrone. This has been attributed to a defective function of stromal cells of endometriosis, resulting in failure to induce the expression of the epithelial 17β-hydroxysteroid dehydrogenase type 2 enzyme (17β-HSD2) responsible for the conversion of E2 to estrone.5,15,16
Aromatase inhibitors for endometriosis-associated pain
Being an estrogen-dependent chronic inflammatory disease, endometriosis requires a life-long management plan with the goal of maximizing the use of medical treatment and avoiding repeated surgical procedures. Additionally, it is vexing to both clinicians and patients to treat cases with recurrent or pain resistant to standard regimes.17 The hormonal drugs investigated – combined oral contraceptives, danazol, gestrinone, medroxyprogesterone acetate and GnRHas – are equally effective, but their adverse effects and cost profiles differ.18
The proof that AIs can be effective in alleviating chronic pelvic pain associated with endometriosis in premenopausal women stems from its molecular basis. Since endometriotic implants respond not only to ovarian E2 but also to extraovarian E2 synthesized by aromatase pathways, standard management, such as GnRHas, which effectively downregulates ovarian E2 biosynthesis, has little impact on extraovarian E2 production. In this context, AIs will downregulate extraovarian E2 synthesis, which in turn stimulates ovarian E2 production through initiation of the follicle-stimulating hormone (FSH) surge. Accordingly, AIs were combined with standard regimes to suppress both ovarian and extraovarian E2.19–21
There are two systematic reviews in the literature that examined the potential of AIs for the treatment of endometriosis-associated pain22,23 (Table 1). Nawathe et al22 looked at eight studies (137 women in total).20,21,24–29 Of these, seven studies were case series/reports of only 40 women,20,24–29 and only one was an RCT of 97 women.21 Analysis of the case series/reports showed that AIs combined with progestins or the oral contraceptive pill or GnRHas reduced mean pain scores and lesion size and improved quality of life. Results from the RCT demonstrated that AIs in combination with GnRHas for 6 months’ duration (3.6 mg goserelin + 1 mg anastrozole) significantly improved pain (P<0.0001) compared with GnRHas (3.6 mg goserelin) alone, together with significant improvement in multidimensional patient scores (P<0.0001). Of note, there was no significant reduction in spine or hip-bone densities. The authors concluded that AIs appear to have a promising effect on pain associated with endometriosis, but they admitted the poor quality of evidence included in their review.22
The second systematic review was conducted recently by Ferrero et al.23 They identified ten studies with a total of 251 women.19–21,28,29,30–34 At least ten patients in each study were considered for inclusion. Five studies were prospective noncomparative,20,28–31 four were RCTs,21,32–34 and one was a prospective patient-preference trial.19 Notably, seven studies looked at the efficacy of AIs in improving endometriosis-related pain symptoms, while three RCTs examined the use of AIs as postoperative therapy in preventing the recurrence of pain symptoms after surgery for endometriosis.
All the prospective noncomparative studies found that AIs combined with either progestins or the oral contraceptive pill reduce the severity of pain symptoms and improve quality of life.20,28–31 The patient-preference study reported that letrozole plus norethisterone acetate is more effective in reducing pain and deep dyspareunia than norethisterone acetate alone. Adverse effects of treatment were significantly more frequent in patients treated with the double-drug regimen than in those receiving norethisterone acetate alone (43.2% versus 18.4%, respectively; P=0.020). At the completion of treatment, no significant difference was observed in patient satisfaction between the two study groups (56.1% of those receiving the double-drug regimen and 63.4% of the patients receiving norethisterone acetate alone were either satisfied or very satisfied with their treatment). Pain symptoms quickly recurred at 6- and 12-month follow-up after the discontinuation of treatment without significant differences between the two study groups.19
Treatment with letrozole plus norethisterone acetate resulted in a lower incidence of side effects and lower discontinuation rate than letrozole plus triptorelin (a GnRHa), as demonstrated in one RCT.34 Two RCTs reported that the use of postoperative AIs plus GnRHas for 6 months reduced the risk of recurrence of endometriosis when compared with GnRHas alone.21,33 Ferrero et al23 concluded that AIs effectively reduce the severity of endometriosis-related pain symptoms. Additionally, they recommended more trials to investigate whether the long-term administration of AIs is superior to the currently available endocrine therapies in terms of improvement of pain, adverse effects, and patient satisfaction.
More recently, the endometriosis guideline published by the European Society of Human Reproduction and Embryology demonstrated that the existing evidence from the aforementioned two systematic reviews was of moderate quality. Owing to the severe side effects identified, including vaginal dryness, hot flushes, and diminished bone mineral density (BMD), the society recommended that
“In women with pain from rectovaginal endometriosis refractory to other medical or surgical treatment, clinicians can consider prescribing aromatase inhibitors in combination with oral contraceptive pills, progestagens, or GnRH analogues, as they reduce endometriosis-associated pain”
(grade B recommendation).35,36
The use of AIs for the treatment of postmenopausal endometriosis
It is noteworthy that evidence regarding the use of AIs in postmenopausal endometriosis is rather limited compared to premenopausal patients, mainly due to the lower prevalence of the disease among postmenopausal patients. In a review article, Oxholm et al37 demonstrated that only Punnonen et al,38 Henriksen,39 and Ranney40 investigated the prevalence of endometriosis in postmenopausal women, and reported prevalences of 2.2%, 3.7%, and 4.8%, respectively, in populations of woman diagnosed with endometriosis. Accordingly, Oxholm et al37 estimated that endometriosis can affect between 2% and 5% of postmenopausal patients. In this subset of patients, surgery is preferred as first-line treatment for possible risk of malignancy or malignant transformation.41,42 However, recurrence rates are increased after surgery,25 and not all patients are suitable candidates for surgery. Therefore, medical treatment is worth trying in these circumstances.
In view of their ability to downregulate estrogen production via inhibition of aromatase P450, AIs were first used for the treatment of postmenopausal estrogen receptor-positive advanced breast cancer.10 In this context, estrogen activity in postmenopausal women is related only to the production from extraovarian sources, mainly adipose tissue via aromatization of androgens produced from the adrenal glands,43 or from exogenous administration as hormonal replacement therapy.44,45 Consequently, AIs appear to be effective in treating postmenopausal endometriosis through the blockade of extraovarian estrogen production compared to traditional treatments with GnRHas, progestins, and danazol.25,27
At present, there are no RCTs available in the literature assessing the use of AIs in postmenopausal patients. Currently available evidence is limited to case reports only. Polyzos et al12 reviewed five case reports looking at the potential of AIs for the treatment of postmenopausal endometriosis in which patients’ age ranged from 47 to 61 years.25,27,46–48 Of note, all patients had either surgical (ie, underwent total abdominal hysterectomy and bilateral oophorectomy at an earlier age)25,27,46,47 or natural menopause.48 In addition, most of them received hormonal replacement therapy and had been previously treated for endometriosis with either surgery, GnRHas, or progestins.
Polyzos et al12 demonstrated that the use of letrozole or anastrozole in all treated patients resulted in improvement of endometriosis-associated pain over a treatment period of 4–15 months. Additionally, letrozole appeared to improve urinary tract and bowel symptoms related to endometriosis. Notably, letrozole but not exemestane had significant beneficial effect on symptom relief in one patient; however, the latter was utilized for only 15 days.46 Additionally, AIs significantly reduced the size of the endometriotic lesions, as measured by imaging techniques. Only one case with ureteral endometriosis did not respond to a 15-month course of anastrozole therapy, due to extensive ureteral fibrosis necessitating further surgery.47
In this review, Polyzos et al12 identified only one patient who suffered from hot flushes after 4 months of letrozole administration, who improved after coadministration of micronized E2 0.5 mg daily without pain recurrence. Two patients received bisphosphonates, and one of them reported a mild reduction in BMD following 9 months’ treatment with 1 mg anastrozole. The authors concluded that AIs may represent a promising new treatment for postmenopausal endometriosis, either as first-line treatment, when surgery is contraindicated, or as a second line in the case of postoperative recurrence. However, they admitted careful assessment of patients’ risk profiles and further research to clarify the long-term effects and side effects of these agents.12
Aromatase inhibitors for endometriosis-associated infertility
In advanced stages of endometriosis (revised American Society for Reproduction Medicine [ASRM] stage III/IV endometriosis), infertility could be attributed to extensive adhesions with distorted pelvic anatomy. However, the mechanisms of infertility associated with minimal–mild endometriosis (ASRM stage I/II endometriosis) remain controversial. Many factors have been raised, including abnormal folliculogenesis, elevated oxidative stress, altered both immune functions as well as hormonal milieu in the follicular and peritoneal environments, and reduced endometrial receptivity. These factors lead to poor oocyte quality, impaired fertilization, and implantation.49,50 Accordingly, infertility associated with minimal–mild endometriosis represents a therapeutic dilemma.51 Evidence to date indicates that ovulation suppression with medical therapy is not beneficial for endometriosis-associated infertility and should not be offered.35,36,52,53 On the other hand, ablation of endometriotic lesions plus adhesiolysis improves fertility in minimal–mild endometriosis.18,35,36,53,54
There is some evidence from RCTs that intrauterine insemination together with controlled ovarian stimulation instead of expectant management may be effective in improving live-birth rates in patients with minimal–mild endometriosis.35,36,55,56 In a retrospective controlled study, Werbrouck et al57 concluded that performing intrauterine insemination with controlled ovarian stimulation within 6 months after surgical treatment in infertile women with minimal–mild endometriosis may be beneficial, since pregnancy rates were similar to those achieved in unexplained infertility. Notably, the effect appears to be predominantly due to ovarian stimulation, since intrauterine insemination alone may not be beneficial.35,36,58
Third-generation AIs, especially letrozole, have been introduced as a new treatment modality in the armamentarium of ovulation-inducing agents for anovulatory infertile women in 2001.59 Letrozole’s ovulation-inducing effects have been attributed to the inhibition of androgen–estrogen conversion, thus releasing the hypothalamus–pituitary axis from negative feedback and increasing secretion of pituitary FSH. Additionally, increased follicular sensitivity to FSH owing to increased intrafollicular androgens was reported as a peripheral working mechanism.60,61 Letrozole is usually given orally at doses of 2.5–7.5 mg/day for 5 days, from the third to the seventh day of the menstrual cycle.62
Compared with clomiphene citrate (CC), letrozole downregulates E2 synthesis rather than competitively inhibiting its action. Additionally, letrozole has a relatively shorter half-life (~2 days versus ~2 weeks in CC), thereby avoiding the peripheral antiestrogenic effects commonly associated with CC on the endometrium and cervical mucus. With this background, it was postulated that AIs may have superior ovulation-induction properties in terms of follicular growth and endometrium development, which are important for embryo implantation.60,61 Accordingly, letrozole has challenged CC as an ovulation-induction agent in patients with polycystic ovary syndrome,62,63–66 and in cases of unexplained infertility.62,66,67
According to the present state of knowledge, several studies have reported that the combination of AIs with conventional therapy may result in improvement of endometriosis-associated pain, as declared earlier. However, few studies are available regarding the use of AIs to treat endometriosis-associated infertility (Table 2).
In a prospective RCT, Alborzi et al33 compared the pregnancy rate and recurrence of symptoms and signs in 144 endometriotic patients who received medical therapy for 2 months after laparoscopic surgery, with a 12-month follow-up period after restoration of the regular cycle. Patients were divided into three groups: group 1 (47 cases), who received letrozole 2.5 mg/day; group 2 (40 patients), who had triptorelin (GnRHa) 3.75 mg intramuscularly every 4 weeks; and group 3 (57 patients), who received no medication. There were no statistically significant differences reported among the three groups with regard to the pregnancy rate (23.4% in group 1 versus 27.5% and 28.1% in groups 2 and 3, respectively) or disease-recurrence rate (6.4% in group 1 versus 5% and 5.3% in groups 2 and 3, respectively). Notably, laparoscopy with histological confirmation of endometriosis was carried out for all patients; meanwhile, recurrence was defined by recurrent symptoms only (in the form of dysmenorrhea, dyspareunia, and pelvic pain). The authors found that functional cysts developed in 24.3% in the letrozole group compared with only 2.5% in the triptorelin group and none of the third group, and the difference was statistically significant (P<0.001). Overall, the authors concluded that after considering the cost and side effects of those short-term medications, their prescription was not recommended.
In a recent RCT, Abu Hashim et al68 evaluated pregnancy rates following superovulation with either letrozole or CC (combined with intrauterine insemination) in 136 women with primary infertility who had not achieved pregnancy 6–12 months after laparoscopic treatment for minimal–mild endometriosis. Women were randomized to 5 mg/day letrozole (69 women, 220 cycles) or 100 mg/day CC (67 women, 213 cycles) for 5 days, combined with intrauterine insemination for up to four cycles. Although the total number of follicles and serum E2 on the day of human chorionic gonadotropin administration were significantly higher in the CC group, the clinical pregnancy rate per cycle and the cumulative pregnancy rate after four cycles were comparable in both groups (15.9% versus 14.5% and 64.7% versus 57.2% in the letrozole and CC groups, respectively). Two twin pregnancies occurred in the CC group. In addition, miscarriage and live-birth rates were also comparable between both groups (11.4% versus 12.9% and 44.9% versus 40.3% in the letrozole and CC groups, respectively). From this study, we concluded that superovulation with letrozole is not more effective than CC combined with intrauterine insemination for women with minimal–mild endometriosis who have not achieved pregnancy after 6–12 months following laparoscopic treatment.
In a prospective pilot study of 20 infertile patients with endometriomas undergoing in vitro fertilization (IVF)/intracytoplasmic sperm injection, Lossl et al69 tested the effect of prolonged combined downregulation by anastrozole and GnRHas prior to IVF based on the concept that this combined regimen may efficiently suppress estrogen biosynthesis through a combined pituitary, ovarian, peripheral, and in situ action. Patients received goserelin 3.6 mg subcutaneously on treatment days 1, 28, and 56, and one daily tablet of anastrozole 1 mg from day 1 to day 69. Controlled ovarian stimulation was initiated from day 70. The primary outcome measure was signs of inactivation of the endometriosis during downregulation, such as decreased endometriomal volume and serum CA125 level. Secondary outcomes were evaluation of standard IVF parameters, including pregnancy and delivery rates, as well as endocrine response.
Interestingly, the authors found that prolonged combined anastrozole and goserelin downregulation significantly reduced endometriomal volume and serum CA125 level. The median change in endometriomal volume was minus 29% (95% confidence interval: 3–39%, P=0.007) and the CA125 level was reduced by 61% (95% confidence interval: 21–74%, P=0.001) from day 1 to day 70. In the IVF/intracytoplasmic sperm-injection cycle, the median number of oocytes retrieved was 7.5 (range 6.0–10.0) and the fertilization rate was 0.78 (range 0.38–1.0). Notably, nine of the 20 patients (45%) conceived. Four of them were biochemical pregnancies, another two patients had early spontaneous abortions, and only three patients (15%) delivered healthy children (two singletons and one twin). The observed high pregnancy loss (six patients) could have been accidental, but impaired quality of the oocytes and/or the endometrium following prolonged combined downregulation should be considered. The authors admitted the need for further adequately powered RCTs to test possible enhanced endometriosis inactivation and signs of improved IVF-cycle outcome by this combined anastrozole and goserelin downregulation compared to downregulation by the agonist alone.69
No serious adverse events were reported in this study. Attacks of flare-up in the form of vaginal spotting/bleeding occurred in 16 women (80%), two women (10%), and one woman (5%) after the first, second, and third injections of goserelin 3.6 mg, respectively, with functional ovarian cysts demonstrated by ultrasound. This could be attributed to the profound suppression of E2 during the combined treatment, which may have activated pituitary FSH secretion to a higher extent than treatment with the agonist alone.69
Side effects of aromatase inhibitors
The side effects associated with the use of third-generation AIs (letrozole, anastrozole) are mostly hypoestrogenic in nature, and include vaginal dryness, hot flushes, headache, back pain, leg cramps, and arthralgia. However, its long-term use is associated with increased risks of osteoporosis and fracture rate owing to diminished BMD.11,12 Fracture rates of 2%–11% have been reported in some studies in which AIs were utilized as adjuvant therapy for hormone receptor-positive breast cancer patients.70,71 Notably, the American Society of Clinical Oncologists recommends that BMD screening should be repeated annually for breast cancer patients using AI adjuvant therapy, and bisphosphonate therapy should be administered when measurement of the BMD in terms of T-score are -2.5 or lower.72
Other studies evaluated the use of add-back therapy with progestins and oral contraceptive pills in premenopausal women, and demonstrated no significant changes in BMD during their use.20,28 However, another trial showed that the use of goserelin (GnRHa) plus anastrozole resulted in significant bone loss after 6 months of treatment compared to goserelin-alone therapy, and that this effect persisted even after cessation of treatment. However, none of these patients became osteopenic or osteoporotic.21
Safety of aromatase inhibitors
The safety of AIs for ovulation induction or superovulation has generated a lively discussion. A poorly done abstract published by the ASRM in 2005,73 which never resulted in a paper, raised concerns of increased cardiac and bone malformations in newborns of letrozole-treated pregnancies compared with controls. However, there were numerous issues regarding the methodology of this report. The control group was strongly criticized, being chosen from a database of normal deliveries (36,050 deliveries) that would have had a lower risk of pregnancy complications and congenital malformations than an infertile population. Moreover, there was an apparent underrepresentation of congenital anomalies in this control group, since babies identified as abnormal on prenatal ultrasound were delivered at a tertiary care hospital rather than a community hospital.74
Subsequently, two retrospective studies did not confirm the initial findings of increased teratogenicity in pregnancies achieved with AIs.74,75 In a large multicenter retrospective study, Tulandi et al75 reviewed 911 newborns conceived after infertility treatment. Notably, they found the rate of congenital malformations and chromosomal abnormalities was not significantly different, but slightly higher, in newborns from CC-treated compared with letrozole-treated women (4.8% versus 2.4%, respectively). Particularly, the major cardiac congenital abnormality rate (eg, ventricular septal defect, transposition of great vessels, right ventricle atresia, pulmonary valve atresia) was significantly higher with CC than with letrozole (1.8% versus 0.2%, respectively; P=0.02).75 Another retrospective study by Forman et al74 looked at 477 newborns, and reported fewer malformations with letrozole (0) than with CC (2.6%) or spontaneous pregnancy (3.2%). Notably, a recent randomized trial assessing pregnancy outcomes after treatment with AIs in 796 infertile women confirmed the positive results for the safety for newborns from the previous two retrospective studies.76 Taken together, the previous concerns for the safety of AIs appear to be vanishing. Notably, their shorter half-life virtually ensures elimination from the body before implantation, thereby raising their safety margin compared with the relatively slowly eliminated CC.62
Monofollicular growth has been regarded as an advantage after using letrozole for ovulation induction. This could be attributed to its rapid elimination from the body in view of its shorter half-life, thereby modulating excessive FSH discharge.61,77 However, a comparable twin-pregnancy rate between CC and letrozole (8.3% versus 9.1%) in women with unexplained infertility was reported in an RCT.76 In addition, a triplet pregnancy after using letrozole for ovulation induction in an infertile woman with polycystic ovary syndrome was published as a case report.78 Therefore, although AIs are associated with a higher rate of monofollicular growth, pregnancies with increased rates of twins or higher-order multiples may be a potential complication of this treatment.
Conclusion
Endometriosis is an estrogen-dependent chronic inflammatory disease characterized by the presence of active endometrial tissue outside the uterus. Infertility and chronic pelvic pain are considered the cardinal clinical features of this enigmatic disease. The aromatase P450 enzyme catalyzes the final and rate-limiting step in the biosynthesis of estrogen, and is present in several human tissues, including the ovaries and adipose tissue. Notably, molecular studies revealed that this key enzyme in the biosynthesis of E2 has been demonstrated inside endometriotic tissue, indicating local synthesis of E2. Therefore, AIs, especially the potent and highly selective third generation, were utilized for the treatment of endometriosis.
Notably, several studies have found that the combination of AIs with conventional therapy, such as oral contraceptive pills, progestins, or GnRHas, can be used to control endometriosis-associated pain and pain recurrence in premenopausal women, particularly those with pain due to rectovaginal endometriosis refractory to other medical or surgical treatment. Additionally, the use of progestins or oral contraceptive pills with AIs will act as add-back therapy to prevent reduction in BMD and risks of osteoporosis associated with prolonged use of AIs alone. Some case reports have shown promising results in the treatment of postmenopausal endometriosis as first-line treatment, when surgery is contraindicated, or as a second line in the case of postoperative recurrence. This point warrants validation in larger prospective trials.
Due to the downregulation of estrogen synthesis and reduced pituitary feedback, AIs stimulate endogenous gonadotropin secretion. AIs, especially letrozole, have challenged CC as an ovulation-induction agent in patients with polycystic ovary syndrome and in cases of unexplained infertility. However, few studies are available regarding the use of AIs to treat endometriosis-associated infertility. Therefore, larger multicenter randomized trials using AIs for the treatment of endometriosis-associated infertility are needed to clarify its effect. The safety of AIs for ovulation induction or superovulation is a major concern. Data from recent retrospective and prospective studies support their safety.
Disclosure
The author reports no conflicts of interest in this work.
References
Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. | |
McLeod BS, Retzloff MG. Epidemiology of endometriosis: an assessment of risk factors. Clin Obstet Gynecol. 2010;53(2):389–396. | |
Guo SW, Wang Y. The prevalence of endometriosis in women with chronic pelvic pain. Gynecol Obstet Invest. 2006;62(3):121–130. | |
Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. | |
Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. | |
Carvalho L, Podgaec S, Bellodi-Privato M, Falcone T, Abrão MS. Role of eutopic endometrium in pelvic endometriosis. J Minim Invasive Gynecol. 2011;18(4):419–227. | |
Bulun S, Zeitoun K, Takayama K, Sasano H. Molecular basis for treating endometriosis with aromatase inhibitors. Hum Reprod Update. 2000;6(5):413–418. | |
Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87. | |
Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril. 2006;85(5):1307–1318. | |
Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98(18):1285–1291. | |
Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertil Steril. 2012;98(6):1370–1379. | |
Polyzos NP, Fatemi HM, Zavos A, et al. Aromatase inhibitors in post-menopausal endometriosis. Reprod Biol Endocrinol. 2011;9:90. | |
Ferrero S, Venturini PL, Ragni N, Camerini G, Remorgida V. Pharmacological treatment of endometriosis: experience with aromatase inhibitors. Drugs. 2009;69(8):943–952. | |
Geisler J, King N, Anker G, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4(9):2089–2093. | |
Bulun SE, Cheng YH, Pavone ME, et al. 17β-Hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):44–50. | |
Cheng YH, Imir A, Fenkci V, Yilmaz MB, Bulun SE. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17β-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. Am J Obstet Gynecol. 2007;196(4):391. e1–e7; discussion 391. e7–e8. | |
Practice Committee of American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril. 2008;90(Suppl 5):S260–S269. | |
Royal College of Obstetricians and Gynaecologists. The investigation and management of endometriosis. 2006. Available from: http://www.rcog.org.uk/files/rcog-corp/GTG2410022011.pdf. Accessed June 4, 2014. | |
Ferrero S, Camerini G, Seracchioli R, Ragni N, Venturini PL, Remorgida V. Letrozole combined with norethisterone acetate compared with norethisterone acetate alone in the treatment of pain symptoms caused by endometriosis. Hum Reprod. 2009;24(12):3033–3041. | |
Amsterdam LL, Gentry W, Jobanputra S, Wolf M, Rubin SD, Bulun SE. Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertil Steril. 2005;84(2):300–304. | |
Soysal S, Soysal ME, Ozer S, Gul N, Gezgin T. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: a prospective randomized trial. Hum Reprod. 2004;19(1):160–167. | |
Nawathe A, Patwardhan S, Yates D, Harrison GR, Khan KS. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG. 2008;115(7):818–822. | |
Ferrero S, Gillott DJ, Venturini PL, Remorgida V. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod Biol Endocrinol. 2011;9:89. | |
Razzi S, Fava A, Sartini A, De Simone S, Cobellis L, Petraglia F. Treatment of severe recurrent endometriosis with an aromatase inhibitor in a young ovariectomised woman. BJOG. 2004;111(2):182–184. | |
Takayama K, Zeitun K, Gunby RT, Sasano H, Carr BR, Bulun SE. Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertil Steril. 1998;69(4):709–713. | |
Shippen ER, West WJ Jr. Successful treatment of severe endometriosis in two premenopausal women with an aromatase inhibitor. Fertil Steril. 2004;81(5):1395–1398. | |
Fatemi HM, Al-Turki HA, Papanikolaou EG, Kosmas L, De Sutter P, Devroey P. Successful treatment of an aggressive recurrent postmenopausal endometriosis with an aromatase inhibitor. Reprod Biomed Online. 2005;11(4):455–457. | |
Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril. 2004;81(2):290–296. | |
Hefler L, Grimm C, van Trotsenburg M, Nagele F. Role of the vaginally administered aromatase inhibitor anastrozole in women with rectovaginal endometriosis: a pilot study. Fertil Steril. 2005;84(4):1033–1036. | |
Remorgida V, Abbamonte LH, Ragni N, Fulcheri E, Ferrero S. Letrozole and desogestrel-only contraceptive pill for the treatment of stage IV endometriosis. Aust N Z J Obstet Gynaecol. 2007;47(3):222–225. | |
Remorgida V, Abbamonte HL, Ragni N, Fulcheri E, Ferrero S. Letrozole and norethisterone acetate in rectovaginal endometriosis. Fertil Steril. 2007;88(3):724–726. | |
Roghaei MA, Tehrany HG, Taherian A, Koleini N. Effects of letrozole compared with danazol on patients with confirmed endometriosis: a randomized clinical trial. Int J Fertil Steril. 2010;4(2):67–72. | |
Alborzi S, Hamedi B, Omidvar A, Dehbashi S, Alborzi S, Alborzi M. A comparison of the effect of short-term aromatase inhibitor (letrozole) and GnRH agonist (triptorelin) versus case control on pregnancy rate and symptom and sign recurrence after laparoscopic treatment of endometriosis. Arch Gynecol Obstet. 2011;284(1):105–110. | |
Ferrero S, Venturini PL, Gillott DJ, Remorgida V. Letrozole and norethisterone acetate versus letrozole and triptorelin in the treatment of endometriosis related pain symptoms: a randomized controlled trial. Reprod Biol Endocrinol. 2011;9:88. | |
ESHRE Endometriosis Guideline Development Group. Management of women with endometriosis. 2013. Available from: http://endometriosis.eshre.eu/docs/ESHRE%20guideline%20on%20endometriosis%202013_3.pdf. Accessed June 4, 2014. | |
Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014; 29(3):400–412. | |
Oxholm D, Knudsen UB, Kryger-Baggesen N, Ravn P. Postmenopausal endometriosis. Acta Obstet Gynecol Scand. 2007;86(10):1158–1164. | |
Punnonen R, Klemi PJ, Nikkanen V. Postmenopausal endometriosis. Eur J Obstet Gynecol Reprod Biol. 1980;11(3):195–200. | |
Henriksen E. Endometriosis. Am J Surg. 1955;90(2):331–337. | |
Ranney B. Endometriosis. 3. Complete operations. Reasons, sequelae, treatment. Am J Obstet Gynecol. 1971;109(8):1137–1144. | |
Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176(3):572–579. | |
Borgfeldt C, Andolf E. Cancer risk after hospital discharge diagnosis of benign ovarian cysts and endometriosis. Acta Obstet Gynecol Scand. 2004;83(4):395–400. | |
Ackerman GE, Smith ME, Mendelson CR, MacDonald PC, Simpson ER. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab. 1981;53(2):412–417. | |
Goumenou AG, Chow C, Taylor A, Magos A. Endometriosis arising during estrogen and testosterone treatment 17 years after abdominal hysterectomy: a case report. Maturitas. 2003;46(3):239–241. | |
Sesti F, Vettraino G, Pietropolli A, Marziali M, Piccione E. Vesical and vaginal recurrent endometriosis in postmenopause following estrogen replacement therapy. Eur J Obstet Gynecol Reprod Biol. 2005;118(2):265–266. | |
Mousa NA, Bedaiwy MA, Casper RF. Aromatase inhibitors in the treatment of severe endometriosis. Obstet Gynecol. 2007;109(6):1421–1423. | |
Bohrer J, Chen CC, Falcone T. Persistent bilateral ureteral obstruction secondary to endometriosis despite treatment with an aromatase inhibitor. Fertil Steril. 2008;90(5):2004. e7–e9. | |
Sasson IE, Taylor HS. Aromatase inhibitor for treatment of a recurrent abdominal wall endometrioma in a postmenopausal woman. Fertil Steril. 2009;92(3):1170. e1–e4. | |
[No authors listed]. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. | |
Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90(2):247–257. | |
Rossmanith WG. Minimal endometriosis: a therapeutic dilemma? Gynecol Endocrinol. 2009;25(11):762–764. | |
Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P. Ovulation suppression for endometriosis for women with subfertility. Cochrane Database Syst Rev. 2007;(3):CD000155. | |
[No authors listed]. Practice bulletin no 114: management of endometriosis. Obstet Gynecol. 2010;116(1):223–236. | |
Jacobson TZ, Duffy JM, Barlow D, Farquhar C, Koninckx PR, Olive D. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev. 2010;(1):CD001398. | |
Deaton JL, Gibson M, Blackmer KM, Nakajima ST, Badger GJ, Brumsted JR. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990;54(6):1083–1088. | |
Tummon IS, Asher LJ, Mar tin JS, Tulandi T. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertil Steril. 1997;68(1):8–12. | |
Werbrouck E, Spiessens C, Meuleman C, D’Hooghe T. No difference in cycle pregnancy rate and in cumulative live-birth rate between women with surgically treated minimal to mild endometriosis and women with unexplained infertility after controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril. 2006;86(3):566–571. | |
Nulsen JC, Walsh S, Dumez S, Metzger DA. A randomized and longitudinal study of human menopausal gonadotropin with intrauterine insemination in the treatment of infertility. Obstet Gynecol. 1993;82(5):780–786. | |
Mitwally FM, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75(2):305–309. | |
Holzer H, Casper R, Tulandi T. A new era in ovulation induction. Fertil Steril. 2006;85(2):277–284. | |
Casper RF, Mitwally MF. Review: aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91(3):760–771. | |
Pritts EA. Letrozole for ovulation induction and controlled ovarian hyperstimulation. Curr Opin Obstet Gynecol. 2010;22(4):289–294. | |
Casper RF. Letrozole versus clomiphene citrate: which is better for ovulation induction? Fertil Steril. 2009;92(3):858–859. | |
Abu Hashim H. Clomiphene citrate alternatives for the initial management of polycystic ovary syndrome: an evidence-based approach. Arch Gynecol Obstet. 2012;285(6):1737–1745. | |
Misso ML, Wong JL, Teede HJ, et al. Aromatase inhibitors for PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(3):301–312. | |
Pavone ME, Bulun SE. The use of aromatase inhibitors for ovulation induction and superovulation. J Clin Endocrinol Metab. 2013;98(5):1838–1844. | |
Fouda UM, Sayed AM. Extended letrozole regimen versus clomiphene citrate for superovulation in patients with unexplained infertility undergoing intrauterine insemination: a randomized controlled trial. Reprod Biol Endocrinol. 2011;9:84. | |
Abu Hashim H, El Rakhawy M, Abd Elaal I. Randomized comparison of superovulation with letrozole vs. clomiphene citrate in an IUI program for women with recently surgically treated minimal to mild endometriosis. Acta Obstet Gynecol Scand. 2012;91(3):338–345. | |
Lossl K, Loft A, Freiesleben NL, et al. Combined down-regulation by aromatase inhibitor and GnRH-agonist in IVF patients with endometriomas – a pilot study. Eur J Obstet Gynecol Reprod Biol. 2009;144(1):48–53. | |
Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366(9484):455–462. | |
Buzdar AU. The ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial: an update. Clin Breast Cancer. 2004;5 Suppl 1:S6–S12. | |
Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21(21):4042–4057. | |
Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins. Fertil Steril. 2005;84 Suppl 1:S95 | |
Forman R, Gill S, Moretti M, Tulandi T, Koren G, Casper R. Fetal safety of letrozole and clomiphene citrate for ovulation induction. J Obstet Gynaecol Can. 2007;29(8):668–671. | |
Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85(6):1761–1765. | |
Badawy A, Shokeir T, Allam AF, Abdelhady H. Pregnancy outcome after ovulation induction with aromatase inhibitors or clomiphene citrate in unexplained infertility. Acta Obstet Gynecol Scand. 2009;88(2):187–191. | |
Mitwally MF, Biljan MM, Casper RF. Pregnancy outcome after the use of an aromatase inhibitor for ovarian stimulation. Am J Obstet Gynecol. 2005;192(2):381–386. | |
Dicken CL, Nakhuda GS, Guarnaccia MM, Sauer MV, Lobo RA. Triplet pregnancy after ovulation induction with an aromatase inhibitor. Fertil Steril. 2008;90(4):1199. e9–e11. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.