Back to Journals » OncoTargets and Therapy » Volume 9
Potential predictive role of chemotherapy-induced changes of soluble CD40 ligand in untreated advanced pancreatic ductal adenocarcinoma
Authors Azzariti A, Brunetti O , Porcelli L, Graziano G, Iacobazzi RM, Signorile M, Scarpa A, Lorusso V, Silvestris N
Received 16 February 2016
Accepted for publication 30 March 2016
Published 28 July 2016 Volume 2016:9 Pages 4681—4686
DOI https://doi.org/10.2147/OTT.S106496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Amalia Azzariti,1,* Oronzo Brunetti,2,* Letizia Porcelli,1 Giusi Graziano,3 Rosa Maria Iacobazzi,1 Michele Signorile,2 Aldo Scarpa,4 Vito Lorusso,2 Nicola Silvestris2
1Preclinical and Clinical Pharmacology Unit, 2Medical Oncology Unit, 3Scientific Direction, National Cancer Research Centre, Istituto Tumouri “Giovanni Paolo II”, Bari, 4ARC-NET Research Centre, University of Verona, Verona, Italy
*These authors contributed equally to this work
Abstract: Pancreas ductal adenocarcinoma lacks predictive biomarkers. CD40 is a member of the tumor necrosis factor superfamily. CD40–sCD40L interaction is considered to contribute to the promotion of tumor cell growth and angiogenesis. The aim of the present study was to investigate the role of serum sCD40L as a predictor in metastatic pancreatic cancer. We evaluated 27 consecutive pancreatic cancer patients treated with FOLFIRINOX (21 patients) or gemcitabine plus nab-paclitaxel combination (six patients). The sCD40L level was measured in serum by enzyme-linked immunosorbent assay at baseline, at first evaluation (all patients), and at time to progression (18 patients). The radiological response was evaluated according to the Response Evaluation Criteria in Solid Tumors, Version 1.1. The Wilcoxon signed-rank test was used to compare pre–post treatment sCD40L levels with respect to clinical response, while Pearson’s correlation coefficient was used for the correlation between sCD40L and CA19.9 pre- and post-treatment. The Kruskal–Wallis test was also conducted for further comparisons. We observed a statistically significant reduction in the sCD40L level after 3 months of treatment in patients with partial response (11,718.05±7,097.13 pg/mL vs 4,689.42±5,409.96 pg/mL; P<0.01). Conversely, in patients with progressive disease, the biomarker statistically increased in the same time (9,351.51±7,356.91 pg/mL vs 22,282.92±11,629.35 pg/mL; P<0.01). This trend of sCD40L was confirmed in 18 patients at time to progression after the first evaluation. No differences were recorded within the stable disease group. Moreover, there was a positive correlation between the sCD40L and CA19.9 pre–post treatment variation percentage (Pearson’s correlation coefficient =0.52; P<0.05). Our data suggest a possible predictive role of sCD40L in pancreatic cancer patients, similar to CA19.9.
Keywords: pancreatic ductal adenocarcinoma, FOLFIRINOX, gemcitabine plus nab-paclitaxel, predictive factor, soluble CD40 ligand
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the malignancies with poorer prognosis. It is projected to become the second leading cause of cancer death by 2030 with a 5-year overall survival (OS) rate of ~7%.1,2 At the first diagnosis, only 10%–20% of PDAC patients present primarily resectable disease.3 Moreover, even resected patients experience relapse soon after surgery. In locally advanced, metastatic, and relapsed settings, FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) and gemcitabine plus nab-paclitaxel chemotherapy regimens increase OS, even if prognosis still remains unfavorable.4,5 In particular, the four-drug combination regimen FOLFIRINOX has been shown to be superior to gemcitabine alone in patients with metastatic PDAC, in terms of median OS of 11.1 months in the FOLFIRINOX group as compared to 6.8 months in the gemcitabine group and a median progression-free survival (mPFS) of 6.4 months compared to 3.3 months of gemcitabine alone.4 Nab-paclitaxel in association with gemcitabine achieved a median OS and an mPFS of 8.5 months and 5.5 months, respectively.5 In this scenario, many researchers investigated the pathophysiologic mechanisms of tumorigenesis of PDAC6 with the aim to discover new potential diagnostic, prognostic, and therapeutic targets.7–9 Moreover, the absence of appropriate soluble biomarkers, with the exception of CA19.9,10 hinders both early detection and the course of chemotherapy in patients with unresectable disease.
CD40L is a cell surface interaction molecule, belonging to the tumor necrosis factor family, expressed by T-cells. Its interaction with CD40 plays a key role in adaptive immune response.11,12 This molecule has been detected in a soluble form (sCD40L), produced not only by activated T-lymphocytes but also by platelets with proinflammatory and prothrombotic activities.13,14 sCD40L seems to be elevated in autoimmune diseases, cardiovascular disorders, and cancer.15,16 Furthermore, elevated serum sCD40L levels in cancer patients may play an immunosuppressive role.17 In fact, since myeloid-derived suppressor cells from cancer patients express higher levels of CD40, sCD40L acts on these cells, allowing an expansion of regulatory T-cells as well as an induction of cytokines, such as interleukin-10 and interleukin-6. Moreover, sCD40L induces a higher expression of PD-1 on T-cells, resulting in an activation of the immunosuppression system. A previous study reported that oxidative stress induces CD40 activation in PDAC followed by the enhancement of the production of inflammation cytokines and chemokines influencing the tumor microenvironment.17 The clinical significance of sCD40L has been recently evaluated in the PDAC patients with respect to healthy controls and patients with chronic pancreatitis, suggesting serum sCD40L level as a potential prognostic biomarker.18 Herein, we evaluated the potential predictive role of basal and posttreatment sCD40L serum levels in the metastatic PDAC patients receiving first-line chemotherapy with FOLFIRINOX or gemcitabine plus nab-paclitaxel.
Patients and methods
Study population
This is a prospective study enrolling patients from the National Cancer Research Centre, Istituto Tumouri “Giovanni Paolo II”, Bari, Italy. We considered 27 consecutive chemonaive subjects with histologically diagnosed PDAC. The patients were treated with FOLFIRINOX (21 patients) or gemcitabine plus nab-paclitaxel (six patients) as the first-line chemotherapy. The serum levels of CA19.9 and sCD40L were analyzed before therapy (baseline) and after 3 months at first evaluation (for all patients). For 18 patients with partial response (PR) or stable disease (SD) at first evaluation, the sCD40L serum levels were also available and therefore analyzed at time to progression. A correlation between the serum levels of sCD40L and CA19.9 was also performed. The study was approved by the ethics committee of the National Cancer Research Centre, Istituto Tumouri “Giovanni Paolo II”, Bari, Italy, and written informed consent was obtained from all the patients enrolled in the study.
All the records were reviewed, and the following data were collected: sex, age, grade of tumor differentiation (G1, G2, G3), Eastern Cooperative Oncology Group performance status (0–2), chemotherapy regimen (FOLFIRINOX or gemcitabine plus nab-paclitaxel), and response to chemotherapy according to the Response Evaluation Criteria in Solid Tumors, Version 1.1.19
Blood sample collection
Venous blood samples were drawn before therapy (baseline), after 3 months (all patients), and at progression. Blood samples were centrifuged after 30 minutes at 4°C, and serum fractions were divided into aliquots, frozen, and stored at −80°C until assayed.
Serum sCD40L and CA19.9 detection
sCD40L serum levels were measured by the Quantikine Human CD40 Ligand Immunoassay (R&D Systems, Inc., Minneapolis, MN, USA) following the manufacturer’s instructions. Optical density was determined using the multilabel plate reader Victor 3 (PerkinElmer Inc., Waltham, MA, USA) set to 450 nm, with a wavelength correction set to 540 nm. The concentration of sCD40L, read from the standard curve obtained in each experiment and multiplied by the dilution factor (1:4), is reported in pg/mL. CA19.9 serum levels were tested in certified laboratories (International Organization for Standardization 9001:2008) according to the manufacturer’s instructions.
Statistical analysis
Baseline characteristics of the study population were calculated, and the results were expressed as frequencies and percentages. To assess the comparison between pre–post treatment levels of sCD40L and CA19.9, the Wilcoxon signed-rank test was used, separately for each clinical response. In the smaller group of 18 patients, the Kruskal–Wallis test was considered to test the differences among sCD40L levels in correspondence to three evaluation moments. The analysis was followed by pairwise comparisons. The correlation between sCD40L and CA19.9 in terms of pre–post treatment variation was tested using Pearson’s correlation coefficient (PCC). The pre–post treatment variation – calculated as (value of marker at evaluation – value of marker at baseline)/value of marker at baseline ×100 – of both clinical markers was also used to identify the patients with increasing values and the ones with decreasing values of sCD40L and CA19.9. These two groups of interest were compared in terms of PFS by using the log-rank test. The results are graphically presented with Kaplan–Meier curves. The multivariate Cox regression model was used to investigate the effect of some confounding factors on the relationship between sCD40L and CA19.9 levels and clinical response. PFS was defined as the interval between the date of diagnosis and disease progression. Receiver operating characteristic (ROC) curves were performed, for both sCD40L and CA19.9, to find the best cutoff value to predict PFS at 6 months. Statistical significance was achieved at a P-value of <0.05. All the analyses were performed using the R statistical software (Version 3.1.2; R Core Team [2015]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Twenty-seven consecutive patients with metastatic PDAC receiving first-line chemotherapy were evaluated for their serum basal and posttreatment sCD40L and CA19.9 levels. We considered 19 (70%) males and eight (30%) females with a mean age of 63 years. The majority of patients had a poorly differentiated (56%) or moderately differentiated (41%) adenocarcinoma with a well-performing Eastern Cooperative Oncology Group performance status (93% of patients with 0–1). Twenty-one patients (78%) received FOLFIRINOX and six (22%) received gemcitabine plus nab-paclitaxel. PR, SD, and PD were observed in 30%, 37%, and 33% patients, respectively (Table 1).
  | Table 1 Patient characteristics |
Chemotherapy-induced changes in sCD40L and CA19.9 levels and response rate
We compared pre- and post-chemotherapy sCD40L and CA19.9 levels with clinical response. At a posttreatment assessment (first evaluation at 3 months), we observed a statistically significant reduction in the mean pretreatment values of serum sCD40L in patients with PR (11,718.05±7,097.13 pg/mL vs 4,689.42±5,409.96 pg/mL, P<0.01). Moreover, there was a statistically significant increase in the mean pretreatment values of serum sCD40L in patients with PD (9,351.51±7,356.91 pg/mL vs 22,282.92±11,629.35 pg/mL, P<0.01). There was no statistical difference in sCD40L among the patients with SD (Table 2). Analysis of serum CA19.9 variation was performed, and the same trend as in sCD40L variation was detected. It showed that the mean values of CA19.9 levels had a significant decrease (831.5±984.38 UI/mL vs 355.75±312.09 UI/mL, P<0.01) in the PR group and a significant decrease (521.4±694.3 UI/mL vs 292.7±514.6 UI/mL, P<0.05) in the PD group. In the smaller group of 18 patients with an initial PR or SD and with a blood test performed at time to progression, the Kruskal–Wallis test showed a significant difference (P<0.01) among the three evaluations of sCD40L mean levels (Table 3). In particular, the pairwise comparisons, calculated on the same data set, revealed significant increasing mean values of this biomarker at time to progression, considering the mean levels of both the baseline (P<0.01) and the first evaluation (P<0.0001; Table 4).
  | Table 3 Results of Kruskal–Wallis test: sCD40L levels in baseline, first evaluation, and progression |
  | Table 4 Pairwise comparisons: evaluation of differences in sCD40L levels at baseline, first evaluation, and progression |
Correlation between sCD40L and CA19.9 levels
We examined a possible relationship between sCD40L and CA19.9 levels by conducting a correlation analysis. There was a significant positive correlation (PCC =0.52; P<0.01) between the pre- and posttreatment variation of these biomarkers (Figure 1).
  | Figure 1 Correlation between the sCD40L and CA19.9 pre–post treatment variations (%). |
Pretreatment sCD40L and CA19.9 levels and progression-free survival
In univariate survival analysis, sCD40L had a significant prognostic impact on PFS. mPFS was 5 months and 8 months for patients with increasing and decreasing values of sCD40L, respectively (log-rank P<0.05; Figure 2A). In order to clarify these results, multivariate Cox regression was performed and the model was adjusted for tumor grade, performance status, and number of metastatic sites, considering they are clinically relevant in the evaluation of response to chemotherapy treatment.
The multivariate survival analysis confirmed that sCD40L was an independent prognostic factor. In particular, the patients with increasing values of this biomarker had a hazard ratio of 2.39 (95% confidence interval [CI]: 1.03–5.56; P<0.05; Table 5). The ROC curve analysis showed a cutoff point of 10,840 pg/mL with the area under the curve equal to 0.65 for PD at 6 months.
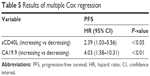  | Table 5 Results of multiple Cox regression |
The Kaplan–Meier curves and the relative log-rank test also showed a significant prognostic impact of CA19.9 on PFS. In particular, the median survival time for the groups with increasing and decreasing values of this antigen was 4.5 months and 8 months, respectively (log-rank P<0.05; Figure 2B). The multivariate Cox regression confirmed that CA19.9 was also an independent prognostic factor. The patients with increasing values of the biomarker had a hazard ratio of 4.03 (95% CI: 1.58–10.31; P<0.01; Table 5). The confounding factors considered in the model were tumor grade, PS, and number of metastatic sites. The ROC curve analysis showed a cutoff point of 378 UI/mL with the area under the curve equal to 0.52 for PD at 6 months.
Discussion
sCD40L is an 18-kDa trimer produced by activated platelets and T-lymphocytes that binds CD40.20 Literature data report that a strong interaction between sCD40L and CD40 expressed on neoplastic cells induces apoptosis, whereas the low ligation signal of sCD40L could increase proliferation and angiogenesis.21 Moreover, this ligand appears to have diagnostic relevance in cancer. In fact, its serum levels have been found to be more elevated in advanced breast, colorectal, and prostate cancer patients with respect to healthy donors from ~10 ng/mL to ~0 ng/mL.22 In addition, sCD40L levels were significantly lowered in hormone refractory prostate cancer after treatment with ketoconazole and alendronate. This suggests a potential predictive role of sCD40L in this malignancy.16–18,20,21,23
In vitro and in vivo studies display a crucial involvement of the CD40 pathway in regulating the immune reaction and fibrosis in PDAC, a tumor with a strong interaction between cancer cells and microenvironment.24 The clinical significance of serum sCD40L as a potential diagnostic and prognostic biomarker in patients with PDAC has been recently reported.17 Chung and Lim demonstrated that the serum sCD40L levels were significantly increased in the PDAC group compared with the healthy and chronic pancreatitis groups. Moreover, they demonstrated that patients with high serum sCD40L had a poorer prognosis with respect to low serum sCD40L. The authors showed a potential diagnostic/prognostic role of sCD40L.18 On the other hand, we focused on the predictive role of this biomarker.
In the present study, we prospectively analyzed the trend of sCD40L at baseline, after 3 months of therapy, and at progression in 27 metastatic PDAC patients treated with FOLFIRINOX or gemcitabine plus nab-paclitaxel chemotherapy regimens. We demonstrated a significant relation between sCD40L serum-level reduction and response to therapy in the absence of statistical differences in patients with SD at the first evaluation. Furthermore, the serum levels of sCD40L were significantly related to progression of disease in both the nine chemoresistant patients and the 18 initially responsive patients. The trend of increase in progression was significantly confirmed in the group of 18 patients by means of Kruskal–Wallis and subsequent pairwise comparisons. According to these results, we suggest that serum sCD40L levels could play a predictive role regarding the response to the first line treatment in advanced PDAC.
At the same time, evaluation of level changes of serum CA19.9 confirmed its predictive role in patients with both PD and PR.10 Moreover, PCC showed a statistically significant correlation between serum-level variations of these two molecules, demonstrating that sCD40L might have the same predictive value as CA19.9.
The univariate analysis confirmed that a decrease in serum sCD40L is predictive of a longer mPFS (8 months) despite the increase that predicts a short mPFS (5 months). The multivariate analysis showed that this biomarker is an independent factor.
If we consider these results and the crucial role of sCD40L in cancer microenvironment, this biomarker could be useful in a stromal tumor such as PDAC, not forgetting that CA19.9 is negative in ~20% of PDAC patients,24 as found in six of our patients.
In addition, other roles for CD40L are described in the literature, such as its immunosuppressive role (ie, HIV infection).10 In particular, Beatty et al25 demonstrated the role of the CD40 pathway in regulating the immune reaction in PDAC by recruitment of tumor-associated macrophages. They showed that the CD40 pathway can be therapeutically targeted to restore tumor immune surveillance by stromal tumor-associated macrophages. According to preclinical data, a Phase I trial of an agonist CD40 monoclonal antibody combination with gemcitabine reported a good safety profile with a PR for four of seven patients.26 An immunosuppressive effect of sCD40L, similar to an immunocheckpoint, has been hypothesized.
Conclusion
Although the small number of patients considered limits the interpretation of our data, we suggest that sCD40L may be a reliable predictive biomarker, potentially useful in the composition of the complex biological scenario of this malignancy.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65(1):5–29. | ||
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. | ||
Silvestris N, Longo V, Cellini F, et al. Neoadjuvant multimodal treatment of pancreatic ductal adenocarcinoma. Crit Rev Oncol Hematol. 2016;98:309–324. | ||
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. | ||
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. | ||
Gnoni A, Licchetta A, Scarpa A, et al. Carcinogenesis of pancreatic adenocarcinoma: precursor lesions. Int J Mol Sci. 2013;14(10):19731–19762. | ||
Brunetti O, Russo A, Scarpa A, et al. MicroRNA in pancreatic adenocarcinoma: predictive/prognostic biomarkers or therapeutic targets? Oncotarget. 2015;6(27):23323–23341. | ||
Xu X, Strimpakos AS, Saif MW. Biomarkers and pharmacogenetics in pancreatic cancer. Highlights from the “2011 ASCO Annual Meeting”. Chicago, IL, USA. JOP. 2011;12(14):325–329. | ||
Silvestris N, Gnoni A, Brunetti AE, et al. Target therapies in pancreatic carcinoma. Curr Med Chem. 2014;21(8):948–965. | ||
Huang Z, Liu F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35(8):7459–7465. | ||
van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. | ||
Noelle RJ. CD40 and its ligand in host defense. Immunity. 1996;4(5):415–419. | ||
Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–172. | ||
Henn V, Slupsky JR, Gräfe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–594. | ||
Tamura N, Kobayashi S, Kato K, et al. Soluble CD154 in rheumatoid arthritis: elevated plasma levels in cases with vasculitis. J Rheum. 2001;28(12):2583–2590. | ||
Huang J, Jochems C, Talaie T, et al. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120(15):3030–3038. | ||
Klein D, Timoneri F, Ichii H, Ricordi C, Pastori RL. CD40 activation in human pancreatic islets and ductal cells. Diabetologia. 2008;51(10):1853–1861. | ||
Chung HW, Lim JB. Clinical significance of elevated serum soluble CD40 ligand levels as a diagnostic and prognostic tumour marker for pancreatic ductal adenocarcinoma. J Transl Med. 2014;12:102. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
Pietravalle F, Lecoanet-Henchoz S, Blasey H, et al. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J Biol Chem. 1996;271(11):5965–5967. | ||
Alexandroff AB, Jackson AM, Paterson T, et al. Role for CD40-CD40 ligand interactions in the immune response to solid tumours. Mol Immunol. 2000;37(9):515–526. | ||
Schonbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104(19):2266–2268. | ||
Figg WD, Liu Y, Arlen P, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol. 2005;173(3):790–796. | ||
Miller EA, Gopal R, Valdes V, Berger JS, Bhardwaj N, O’Brien MP. Soluble CD40 ligand contributes to dendritic cell-mediated T-cell dysfunction in HIV-1 infection. AIDS. 2015;29(11):1287–1296. | ||
Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumour stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. | ||
Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19(22):6286–6295. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


