Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 13
Potential of Quinine Sulfate for COVID-19 Treatment and Its Safety Profile: Review
Authors Latarissa IR, Barliana MI , Meiliana A, Lestari K
Received 13 August 2021
Accepted for publication 19 November 2021
Published 6 December 2021 Volume 2021:13 Pages 225—234
DOI https://doi.org/10.2147/CPAA.S331660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Irma Rahayu Latarissa,1 Melisa Intan Barliana,2,3 Anna Meiliana,4 Keri Lestari1,3
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 2Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 3Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia; 4Prodia Clinical Laboratory, Central Jakarta, Indonesia
Correspondence: Keri Lestari
Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Jl. Raya Bandung Sumedang KM. 21, Jatinangor, Bandung, 45363, Indonesia
Email [email protected]
Abstract: The coronavirus disease 2019 (COVID-19) pandemic is currently the largest and most serious health crisis in the world. There is no definitive treatment for COVID-19. Vaccine administration has begun in various countries, but no vaccine is 100% effective. Some people are not protected after vaccination, and there are some groups of people who cannot be vaccinated therefore, research on COVID-19 treatment still needs to be done. Of the several drugs under study, chloroquine (CQ) and hydroxychloroquine (HCQ) are quite controversial, although they have good activity against SARS-CoV-2, both drugs have serious side effects. Indonesia with its wealth of natural ingredients has one potential compound, quinine sulfate (QS), which has the same structure and activity as CQ and HCQ and a better safety profile. The aim of this article was to review the potential of QS against the SARS-Cov-2 virus and outline its safety profile. We conclude that QS has the potential to be developed as a COVID-19 treatment with a better safety profile than that of CQ and HCQ.
Keywords: SARS-CoV-2, quinine, Cinchona bark, chloroquine, hydroxychloroquine
Introduction
The coronavirus disease 2019 (COVID-19) is currently a very serious health problem in the world. The World Health Organization (WHO) categorized the disease as a pandemic on March 11, 2020. The infection, which is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is spreading rapidly to various countries.1,2
Currently, there is no definitive treatment for COVID-19. Various efforts are being made on a global and national scale to deal with the spread of this infection. Research on drugs that can be used to treat COVID-19 continues, among which are Chloroquine (CQ) and Hydroxychloroquine (HCQ). However, the use of these two drugs caused serious side effects such that on November 13, 2020, the Indonesia Food and Drug Administration issued a notification letter regarding the revocation of the Emergency Use Authorization (EUA) for CQ and HCQ. Previously, the United States Food and Drug Administration (US-FDA) had revoked the EUA for CQ and HCQ on June 15, 2020. Subsequently, the WHO stopped the clinical trial (Solidarity Trial) of HCQ because the drug’s potential benefits for such use do not outweigh its known and potential risks.3
Indonesia, with its wealth of natural ingredients, has one natural compound, Quinine Sulfate (QS), which is an active compound from quinine extract used to treat malaria subjects. This compound has been used for 80 years under the category of a limited over-the-counter drug so that the profile of these drugs is well understood.4 QS compounds have a structure and mechanism similar to those of CQ and HCQ. These three compounds can bind to the Lys353 residue in the peptide domain of the Angiotensin Converting Enzyme 2 (ACE-2) receptor to prevent binding between the virus and the ACE-2 receptor in humans and prevent viral fusion with cells; thus, they have the potential to treat COVID-19.5 Based on its characteristic which is the main component of natural compounds, it is hoped that QS can be an alternative therapy for COVID-19 that has effectiveness rivaling that of CQ and HCQ, but with a better safety profile.
A recent study by Große et al indicated that QS is a potential treatment option for SARS-CoV-2 infection, which is well tolerated with a toxicological profile that is predictable and significantly better than that of HCQ and CQ.6 However, it is undeniable that QS also has several side effects that affect hematology, kidney function, liver function and cardiovascular function.7 Hematological disorders that are often experienced with the use of QS include: thrombocytopenia, microangiopathic hemolytic anemia, neutropenia, disseminated intravascular coagulation, eosinophilia, autoimmune hemolytic anemia, lymphopenia, and methemoglobinemia, as well as impaired kidney function, characterized by increased creatinine levels. Liver disorders are characterized by elevated serum transaminases. Meanwhile, the cardiovascular system usually manifests chest pain, T wave inversion and pericarditis.8 Apart from these side effects, quinine has been used for more than 70 years and shows a good safety profile as long as it is used as prescribed, and the therapeutic dose is not exceeded. The side effects that occur due to the use of quinine are also reversible, can be cured and are overcome by discontinuing quinine.9
Method
Scientific data searches were carried out online on open access articles. This review includes research articles from the PubMed, Science Direct and Google Scholar journal databases for the period 2011–2021 with the keywords “Quinine Sulfate (QS) for COVID-19”, “QS safety profile”, “chloroquine and hydroxychloroquine for COVID-19”, “Chincona Bark for COVID-19” and “traditional herbal medicine for COVID-19”. The inclusion criteria for the articles were that they had to be in English; they had to include activity against SARS-Cov-2 and Angiotensin Converting Enzyme 2 (ACE2) receptor; and they had to be clinical trials, meta-analysis, randomized controlled trials, reviews or systematic reviews. Articles were not used if the language of publication was not English, or if the article did not contain the desired keywords. The search results from the total database yielded 104 articles. Eighty-four of these articles were excluded because they did not meet the inclusion criteria or met at least one of the exclusion criteria. Finally, 20 articles were available for review discussing the potential of QS for COVID-19 treatment and its safety profile.
Quinine Sulfate
Quinine is an alkaloid compound contained in Cinchona bark.4 The Cinchona plant belongs to the Plantae kingdom, the Magnoliophyta division, the Magnoliopsida class, the Gentianales order, the Rubiaceae family, and the Cinchonoideae subfamily.10 The most common compounds from the Cinchona plant are quinine, quinidine, cinchonine and cinchonidine.
Over the years, the active ingredient in Cinchona bark has been used to treat fevers. Quinine was isolated and identified as the oldest effective drug for the treatment of malaria to date. In 1934, a chemist from Germany, Hans Andersag, synthesized the CQ molecule from quinine for the first time.11 HCQ was synthesized in 1946 and proposed as a safer alternative to CQ in 1955.12 Until now, the quinine compound, which is the main component of secondary metabolites in the Cinchona plant, is still used as a potent malaria drug.4 Several studies have revealed that the alkaloids in quinine have other potential activities, such as antiobesity, anticancer, antioxidant, anti-inflammatory, antimicrobial, and antiviral.13,14
Potential of QS for COVID-19 Treatment
CQ, HCQ and QS are antimalarial drugs derived from the alkaloid quinoline.15 Quinoline is a heterocyclic aromatic organic compound with the molecular formula C9H7N and has a double ring structure containing a benzene ring that fuses with pyridine on two adjacent carbon atoms.16 CQ, HCQ and QS have a similar structure with a specific benzene ring (Figure 1).17,18
 |
Figure 1 Structure of Chloroquine, Hydroxychloroquine, Quinine. |
CQ, HCQ and QS inhibit quinone reductase-2 (hQR2) in human red blood cells.19 The inhibitory activity of quinoline on hQR2 has also been demonstrated through other in-vitro studies.20 Inhibition of the hQR2 enzyme involved in the biosynthesis of sialic acid (a cell transmembrane protein acid monosaccharide required for ligand recognition) makes CQ a broad antiviral agent. It should be noted that human coronavirus HCoV-O43 and orthomyxoviruses use sialic acid groups as receptors.21,22 Since HCQ and CQ are quinine derivatives and similar chemical structures, it is thought that they can be used as a therapeutic agent in the treatment or prevention of COVID-19.23 Thus, QS, like CQ, has potential antiviral activity.
QS, CQ and HCQ are weak bases.24 This property is known to increase the pH of acidic intracellular organelles, such as endosome and lysosomes. This is essential for membrane fusion so that it interferes with the fusion process of SARS-CoV-2 in cells, thus SARS-CoV-2 cannot reproduce itself.22,25
SARS-Cov-2 uses the ACE-2 receptor to infect human cells.26 A recent in-silico study by Lestari et al aimed to compare the affinity of CQ, HCQ and QS bonds to ACE-2 receptors using molecular docking studies. The results showed that all compounds (CQ, HCQ and QS) can interact with amino acid residues in the ACE-2 receptor peptidase domain. Of the three compounds, quinine showed the strongest affinity for the ACE-2 receptor with the value of free energy bonding (ΔG = −4.89 kcal/mol) followed by HCQ (ΔG = −3.87 kcal/mol), and CQ (ΔG = −3.17 kcal/mol).5 The more negative the ΔG value, the stronger the ligand-receptor complex bonds.27
In addition, these three compounds also form hydrogen bonds with Lys353. From this study, it can be concluded that QS has a higher binding affinity than 2 other drugs currently used as COVID-19 therapy so that it has the potential to be used as a therapeutic agent for COVID-19 by binding to the ACE-2 receptor.5
Another study analyzed 9 antimalarial phytochemical compounds in-silico using Chimera software, the ligands that have the most affinity with the ACE2 receptor are CQ, quinine, artemisinin and febrifugine. These four compounds form hydrogen bonds with Thr371, Glu406, Arg518, Asp36828. An in-silico study has also been carried out to see if the interaction between quinine and doxycycline was targeted against non-structural protein (nsp 12), which plays a vital role in replication and transcription of the corona viral genome. The compounds doxycycline and quinine were found to have good binding affinity with the corona viral non-structural protein.29 An in-silico study of 13 compounds with the best binding affinity towards SARS-CoV-2 protease was carried out. The ligands were subjected to molecular docking using Autodock Vina. Of the 13 traditional herbal compounds, including quinine, all had good binding affinity for the SARS-CoV-2 protease, Epicatechin and apoquinine showed the highest binding affinity.30
Roza et al, 2020, determine the interaction between SARS-CoV-2 and quinine derivative compounds. The results showed that from the 10 tested compounds against SARS-CoV-2 virus cells, all of them have the ability to act as an antivirus, including quinine.31 Trina et al, 2020, conducted a molecular docking study of 13 plant bioactive compounds against SARS-CoV-2 Main Protease (MPro) and Spike (S) Glycoprotein Inhibitors, including quinine. Quinine has a better binding affinity than CQ and HCQ (as standard).32 A summary of several in silico studies of Quinine Sulfate against SARS-CoV-2 is shown in Table 1.
 |
Table 1 In-Silico Study of QS Against SARS-CoV-2 |
A study conducted by Grobe et al, 2020, investigated the effect of QS, CQ and HCQ on the inhibition of SARS-CoV-2 cell replication in Vero B4 cells. Three days post infection, cell culture supernatants were harvested and virus production analyzed by Western blot. The results showed that 10 μM QS could reduce the replication of the strong SARS-CoV-2 virus where virion progeny production was almost completely blocked. The inhibition of SARS-CoV-2 virus replication by QS was better than that by CQ and HCQ, with 10 μM QS, virus replication was reduced by 90%, whereas, with HCQ, it was only reduced by 50%. It can be concluded that QS has a stronger activity than CQ and HCQ.6 The study also measured the spread of the virus in living cells using epithelial cells derived from human Caco-2 colon carcinoma. To facilitate detection and analysis of infected cells, as well as to measure the spread of the virus in living cells, an infectious clone of SARS-CoV-2 expressing mNeonGreen reporter gene was used.33
Caco-2 cells were infected on 96-well plates with a relatively high multiplicity of infection (MOI) of 3, for strong fluorescence readings. At 48 hpi (hour post infection) the cells were fixed, and the nucleus was stained with Hoechst to determine the level of relative infection (mNeonGreen +/Hoechst + cells) and potential toxic effects of treatment and infection. This analysis confirmed the results obtained from Vero cells and showed that QS concentrations up to 100 μM were non-toxic to Caco-2 cells. Furthermore, the 50 μM and 100 μM QS treatments could almost completely inhibit SARS-CoV-2 infection at high MOI. Therefore, QS has the potential as a treatment option that can be tolerated and widely used for SARS-CoV-2 infection, with a predictable toxicological profile and is significantly better when compared with CQ or HCQ.6
In Vero cells, quinine inhibited SARS-CoV-2 infection more effectively than CQ, and HCQ and was less toxic. In human Caco-2 colon epithelial cells, as well as the lung cell-line A549 stably expressing ACE2 and TMPRSS2, quinine also showed antiviral activity. In consistency with Vero cells, quinine was less toxic in A549 as compared to CQ and HCQ. This study also confirms that in Calu-3 lung cells, expressing ACE2 and TMPRSS2 endogenously. In Calu-3, infections with high titers of SARS-CoV-2 were completely blocked by quinine, CQ, and HCQ in concentrations above 50 μM. The estimated IC50 were ~25 μM in Calu-3, while overall, the inhibitors exhibit IC50 values between ~3.7 to ~50 μM, dependent on the cell line and multiplicity of infection (MOI). Conclusively, these data indicate that quinine could have the potential as a treatment option for SARS-CoV-2, as the toxicological and pharmacological profile seems more favorable when compared to its progeny drugs HCQ or CQ.34
Another in-vitro study using Vero B6 cells infected with the SARS-CoV-2 strain (IHUMI-3). Quinine showed medium antiviral in-vitro activity with EC50 of 10.7 ± 2.0 uM and EC50 of 38.8 ± 34 uM.35 A 600 mg single dose of QS led to blood Cmax around 3.5 mg/L (around 8.5 uM).36 In rat, after intravenous dose of 10 mg/kg of quinine, the observed concentration lung/blood ratio was 246.37 An in-vitro effective concentration in the lungs to cure SARS-CoV-2 is achievable in human. If its clinical efficacy in human is confirmed, quinine could be administered intravenously in patients before the cytokine storm.35 A summary of several in vitro studies of Quinine Sulfate against SARS-CoV-2 is shown in Table 2.
 |
Table 2 In-Vitro Study of QS Against SARS-CoV-2 |
Research on the effect of quinine as an antiviral was first reported by Seeler et al, 1946. This study concluded that quinine has an antiviral effect against influenza viruses by consistently slowing the course of infection with the influenza virus.38 Quinine’s antiviral activity has also been demonstrated in several other viruses, such as H1N139, Influenza A virus (IAV), Human Immunodeficiency Virus (HIV), Zika virus, Ebola and dengue.40,41 In addition, the antiviral effect of quinine was described by Baroni at al, who evaluated QS in HaCat HSV-1 infected cells. The results of these studies indicate that quinine has the effect of inhibiting viral infection through indirect pathways, such as activating the protein heat shock response, interfering with several viral replication pathways, and inhibiting Nuclear Factor kB (NF-kB) by blocking gene expression.42
Antiviral mechanisms of action of quinine include the inhibition of cytokine production (management of cytokine storm), and T cell release of IL-1,2,6,18, TNFα and INFγ, reduce levels of chemokines CCL2 and CXCL10, inhibition of micro-RNA expression, decreased TH17-related cytokines, decreased DNA, RNA and protein synthesis in thymocytes.43,44 Besides the direct antiviral activity, it is also possible to act by suppressing the synthesis of cytokines and especially pro-inflammatory factors with its anti-inflammatory effect. In-vitro data show that quinine, CQ and HCQ inhibit SARS-CoV-2 replication.23 Quinine is a good candidate for the development of an effective drug to treat SARS-CoV-2 because of its DNA-intercalating properties.45,46 As fever is one of the most common side effects of COVID-19 and quinine has prominent antipyretic effects, these alkaloids could be introduced as a treatment for handling this complication of COVID-19.35,47
A recent study demonstrated that the antiviral mechanism of quinine is indirect killing of the virus. The study was conducted to investigate the effects of QS on dengue virus-infected cells. Due to the relative similarity of the structure of the dengue virus and SARS-CoV-2, it is possible for SARS-CoV-2 to use several relatively similar methods to infect cells and trigger cytokines in fighting viruses.48 Host cells infected with the virus can initiate viral RNA release and interfere with normal protein synthesis. However, the expression of the Pathogen Recognition Receptor (PRR) known as Retinoic acid-Inducible Gene I (RIG-I) in infected host cells increases slowly to promote the Interferon-I (IFN-I) signaling pathway and increases the expression of IFN-stimulated genes (RNase L, PKR), which can inhibit protein synthesis, thereby inhibiting viral replication.49 The RNase L pathway can remove ssRNA in virus-infected cells, meanwhile PKR blocks translation, and affects signal transduction.50 The targets of quinine action are inhibition of genomic replication and translation of infected host cells and increased expression of RIG-I and IFN-α (Figure 2).51 It has been shown that IFN-α is a cytokine secreted by host cells to fight viruses.48
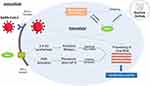 |
Figure 2 Mechanism of Quinine Sulfate as an antiviral agent. Reproduced from Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid Based Complement Altern Med. 2020;2020:1–12. doi:10.1155/2020/2560645.51 Abbreviations: ACE-2, Angiotensin Converting Enzyme-2; INF-α, Interferon-α; PRR, Pattern Recognition Receptor; RIG-I, Retinoic acid-Inducible Gene I; PKA, Protein Kinase A. |
The cytokine storm that is currently being discussed may not appear after quinine administration for COVID-19 because available data indicate that the release of TNF-α, the most important cytokine in determining the severity of COVID-19 symptoms, is inhibited by quinine.52,53 Because quinine blocks TNF-α expression at the transcription level of mRNA, analyzed by northern blot, it will reduce the inflammatory reaction in infected individuals rather than promote the inflammatory process.52,54,55 For example, research on IBD patients relative to SARS-CoV-2 shows possible protective effects of anti-TNFα antibodies in Crohn's patients.56 A summary of several potentials of Quinine Sulfate against SARS-CoV-2 is shown in Table 3.
 |
Table 3 Summary of Journal Reviews on the Potential of QS Against SARS-CoV-2 |
Currently, clinical trials of QS to determine its efficacy and safety are being carried out in Indonesia. In Indonesia QS has been used to treat patients with malaria. Currently, there is no clinical research data to suggest a dose of QS for COVID-19. The dosage form currently available in Indonesia is QS 200 mg. Therefore, based on the recommendations of the COVID-19 Treatment Protocol 2nd Edition regarding the dose of HCQ and available doses from QS, with the concept of re-purposing drug, this study uses the same dose as HCQ, so it is still in its current therapeutic dose has been used. In addition, the HCQ dose and QS dose used for malaria are the same, so the dose QS used for COVID-19 refers to the HCQ dose previously used for COVID-19. Nevertheless, in vitro studies showed that the toxicity profile of QS was better than that of HCQ in Vero B6 cells with CC50 > 100 M, while HCQ was 20.4± 1.4 M.35 The plasma concentration of QS that can cause toxicity ranges from 5 to 17.8 mg/L, meanwhile the plasma concentration in 100 mg QS is 0.5 mg/L so that the dose of QS used for COVID-19 is still within safe limits.57
It can be concluded that QS has immunostimulating and immunosuppressant activity in fighting viral infections. When quinine effectively intensifies the production of IFN-α cytokines, it functions as an immunostimulator to inhibit viruses. In contrast, quinine inhibits TNF-α release and has an immunosuppressant effect. These two different activities may have beneficial effects on people who are infected with COVID-19.
Safety Profile of QS
Some investigators consider quinine to be substantially safe at therapeutic doses, but above the therapeutic dose may have serious side effects. Accidental or intentional overdoses have been linked to serious and fatal cardiac arrhythmias.58,59
The most common side effect is a symptom called “cinchonism syndrome”. Mild cinchonism syndrome includes headache, vasodilation and sweating, nausea, tinnitus, hearing loss, vertigo or dizziness, blurred vision, and color perception disorders. More severe cinchonism syndromes include vomiting, diarrhea, abdominal pain, deafness, blindness, and disturbances in the heart rhythm or conduction. Most of the cinchonism syndrome can be cured and treated with quinine discontinuation (reversible).60,61
Apart from these side effects, quinine has been used for more than 70 years and shows a good safety profile as long as it is used as prescribed and the therapeutic dose is not exceeded. Besides being used as a malaria drug, several studies have revealed that quinine contains alkaloids with have other potential activities, such as antiobesity, anticancer, antioxidant, anti-inflammatory, antimicrobial, and antiviral. The side effects that occur due to the use of quinine are also reversible, can be cured and are overcome by discontinuing quinine.60,61
Conclusion
QS is an alkaloid compound contained in Cinchona bark. The potential of QS for COVID-19 treatment, among others, has the same basic structure with CQ and HCQ, namely Quinoline, which can inhibit viral fusion; is weakly alkaline so that it can increase the pH of cell organelles; has higher binding affinity to SARS-CoV-2 compared with CQ and HCQ; has antiviral activity against SARS-CoV-2 in-vitro; has other antiviral activity and acts as an immunomodulator. It is undeniable that QS also has some side effects, but the side effects caused are reversible and in long-term use and large doses. The in-vitro study also stated that the toxicity profile of QS is better than both CQ and HCQ. We conclude that QS has the potential to be developed as a COVID-19 treatment with a better safety profile than that of CQ and HCQ.
Acknowledgments
This research was funded by the Ministry of Research, Technology and Higher Education, the Republic of Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cereda D, Tirani M, Rovida F, et al. The early phase of the COVID-19 outbreak in Lombardy, Italy; 2020. Available from: http://arxiv.org/abs/2003.09320.
2. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382(24):2302–2315. doi:10.1056/nejmoa2006100
3. Mehra MR, Desai SS, Ruschitzka F, Patel AN. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;6736(20):31180–6. doi:10.1016/S0140-6736(20)31180-6
4. Gachelin G, Garner P, Ferroni E, Tröhler U, Chalmers I. Evaluating Cinchona bark and quinine for treating and preventing malaria. J R Soc Med. 2017;110(1):31–40. doi:10.1177/0141076816681421
5. Lestari K, Sitorus T, Instiaty MS, Levita J. Molecular docking of quinine, chloroquine and hydroxychloroquine to angiotensin converting enzyme 2 (ACE2) receptor for discovering new potential COVID-19 antidote. J Adv Pharm Educ Res. 2020;10(2):1–4.
6. Große M, Ruetalo N, Businger R, et al. Evidence that quinine exhibits antiviral activity against SARS-CoV-2 infection in vitro. Preprints. 2020;19:2020070102.
7. Taylor WRJ, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27(1):25–61. doi:10.2165/00002018-200427010-00003
8. Lim AKH, Ho L, Levidiotis V. Quinine-induced renal failure as a result of rhabdomyolysis, haemolytic uraemic syndrome and disseminated intravascular coagulation. Intern Med J. 2006;36(7):465–467. doi:10.1111/j.1445-5994.2006.01104.x
9. Robinson J. Goodman; 2013. doi:10.4324/9780203813034
10. Phillipson JD, O’Neill MJ. Novel antimalarial drugs from plants? Parasitol Today. 1986;2(12):355–359. doi:10.1016/0169-4758(86)90058-X
11. Krafts K, Hempelmann E, Skórska-Stania A. From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy. Parasitol Res. 2012;111(1):1–6. doi:10.1007/s00436-012-2886-x
12. Tzekov R. Ocular toxicity due to chloroquine and hydroxychloroquine: electrophysiological and visual function correlates. Doc Ophthalmol. 2005;110(1):111–120. doi:10.1007/s10633-005-7349-6
13. Marella A, Tanwar OP, Saha R, et al. Quinoline: a versatile heterocyclic. Saudi Pharm J. 2013;21(1):1–12. doi:10.1016/j.jsps.2012.03.002
14. Chen YL, Fang KC, Sheu JY, Hsu SL, Tzeng CC. Synthesis and antibacterial evaluation of certain quinolone derivatives. J Med Chem. 2001;44(14):2374–2377. doi:10.1021/jm0100335
15. Nqoro X, Tobeka N, Aderibigbe BA. Quinoline-based hybrid compounds with antimalarial activity. Molecules. 2017;22(12):2268. doi:10.3390/molecules22122268
16. Ferlin MG, Chiarelotto G, Gasparotto V, et al. Synthesis and in vitro and in vivo antitumor activity of 2-phenylpyrroloquinolin-4-ones. J Med Chem. 2005;48(9):3417–3427. doi:10.1021/jm049387x
17. Jomsky M. Could low-dose quinine prevent or treat coronavirus infection? Ec Pharmacol Toxicol. 2020:6–9. doi:10.31080/ecpt.2020.08.00455
18. Quinine _ C20H24N2O2 - PubChem.
19. Graves PR, Kwiek JJ, Fadden P, et al. Discovery of novel targets of quinoline drugs in the human purine binding proteome. Mol Pharmacol. 2002;62(6):1364–1372. doi:10.1124/mol.62.6.1364
20. Kwiek JJ, Haystead TAJ, Rudolph J. Kinetic mechanism of quinone oxidoreductase 2 and its inhibition by the antimalarial quinolines. Biochemistry. 2004;43(15):4538–4547. doi:10.1021/bi035923w
21. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi:10.1038/s41422-020-0282-0
22. Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):105932. doi:10.1016/j.ijantimicag.2020.105932
23. Gürü M, Gürü S, Yılmaz Aydın D. Effect of alkaloids on SARS-CoV-2. NATURENGS MTU J Eng Nat Sci Mal Turgut Ozal Univ. 2020;10–18. doi:10.46572/nat.2020.7
24. Bray PG, Mungthin M, Hastings IM, et al. PfCRT and the trans-vacuolar proton electrochemical gradient: regulating the access of chloroquine to ferriprotoporphyrin IX. Mol Microbiol. 2006;62(1):238–251. doi:10.1111/j.1365-2958.2006.05368.x
25. Biot C, Daher W, Chavain N, et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49(9):2845–2849. doi:10.1021/jm0601856
26. Wenhui L, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi:10.1038/nature02145
27. Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3(11):935–949. doi:10.1038/nrd1549
28. Benslama O, Mansouri N, Arhab R. Antimalarial phytochemicals as inhibitors against COVID-19 ACE2 receptor: computational screening. Not Sci Biol. 2021;13(2):10835. doi:10.15835/nsb13210835
29. Sumitha A, Devi PB, Hari S, Dhanasekaran R. COVID-19 - In silico structure prediction and molecular docking studies with doxycycline and quinine. Biomed Pharmacol J. 2020;13(3):1185–1193. doi:10.13005/bpj/1986
30. Srimathi R, Mohan Maruga Raja MK, Kathiravan MK. In silico screening of traditional herbal medicine derived chemical constituents for possible potential inhibition against sars-cov-2. J Nat Remedies. 2020;20(2):79–88. doi:10.18311/jnr/2020/25278
31. Roza D, Selly R, Munsirwan R, Fadhilah G. Molecular docking of quinine derivative as inhibitor in Sars-Cov-2. J Phys Conf Ser. 2021;1819(1):012053. doi:10.1088/1742-6596/1819/1/012053
32. Tallei TE, Tumilaar SG, Niode NJ, et al. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and Spike (S) glycoprotein inhibitors: a molecular docking study. Scientifica. 2020;2020. doi:10.1155/2020/6307457
33. Xie X, Muruato A, Lokugamage KG, et al. An infectious cDNA clone of SARS-CoV-2. Cell Host Microbe. 2020;27(5):841–848.e3. doi:10.1016/j.chom.2020.04.004
34. Große M, Ruetalo N, Layer M, et al. Quinine inhibits infection of human cell lines with sars-cov-2. Viruses. 2021;13(4):1–17. doi:10.3390/v13040647
35. Gendrot M, Andreani J, Boxberger M, et al. Antimalarial drugs inhibit the replication of SARS-CoV-2: an in vitro evaluation. Travel Med Infect Dis. 2020;37:1–6. doi:10.1016/j.tmaid.2020.101873
36. Adehin A, Igbinoba SI, Soyinka JO, Onyeji CO, Babalola CP, Bolaji OO. Pharmacokinetic parameters of quinine in healthy subjects and in patients with uncomplicated malaria in Nigeria: analysis of data using a population approach. Curr Ther Res Clin Exp. 2019;91:33–38. doi:10.1016/j.curtheres.2019.100567
37. Minchin RF, Ilett KF. Comparative uptake of quinine and quinidine in rat lung. J Pharm Pharmacol. 1981;33(1):464–466. doi:10.1111/j.2042-7158.1981.tb13835.x
38. Seeler AO, Graessle O, Ott WH. Effect of quinine on influenza virus infections in mice. J Infect Dis. 1946;79(2):156–158. doi:10.1093/infdis/79.2.156
39. Bazotte RB, Hirabara SM, Serdan TAD, et al. 4-aminoquinoline compounds from the Spanish flu to COVID-19. Biomed Pharmacother. 2021;135:111138. doi:10.1016/j.biopha.2020.111138
40. Khan SA, Al-Balushi K. Combating COVID-19: the role of drug repurposing and medicinal plants. J Infect Public Health. 2021;14(4):495–503. doi:10.1016/j.jiph.2020.10.012
41. Kashif M, Aamir M, Minhas S, Tahir R, Jahan S, Afzal N. Myths and facts: chloroquine May be a potential supportive/therapeutic drug in COVID-19 treatment; 2020. doi:10.20944/preprints202004.0341.v1
42. Baroni A, Paoletti I, Ruocco E, et al. Antiviral effects of quinine sulfate on HSV-1 HaCat cells infected: analysis of the molecular mechanisms involved. J Dermatol Sci. 2007;47(3):253–255. doi:10.1016/j.jdermsci.2007.05.009
43. Al-Bari AA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 2014;70(6):1608–1621. doi:10.1093/jac/dkv018
44. Inklebarger JJIJ, Gyer MG, Galanis N, Michael MJ, Adel DG. Cinchona bark for the treatment of covid-19 pneumonia: a modern review of the potential anti-viral therapeutic applications of an old treatment. Int J Med Sci Clin Invent. 2020;7(5):4795–4801. doi:10.18535/ijmsci/v7i05.02
45. Jahan I, Onay A. Potentials of plant-based substance to inhabit and probable cure for the covid-19. Turkish J Biol. 2020;44(3):228–241. doi:10.3906/biy-2005-114
46. Shika Tiwari NKD. Traditional medicinal plants as promising source of immunomodulator against Covid-19. J Exp Biol Agric Sci. 2020;8:128–138.
47. Majnooni MB, Fakhri S, Bahrami G, Naseri M, Farzaei MH, Echeverría J. Alkaloids as potential phytochemicals against SARS-CoV-2: approaches to the associated pivotal mechanisms. Evid Based Complement Altern Med. 2021;2021:1–21. doi:10.1155/2021/6632623
48. Malakar S, Sreelatha L, Dechtawewat T, et al. Drug repurposing of quinine as antiviral against dengue virus infection. Virus Res. 2018;255:171–178. doi:10.1016/j.virusres.2018.07.018
49. Noisakran JA, Punt J, Stranford SA. WHF. In: Kuby Immunology.
50. Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8(7):559–568. doi:10.1038/nri2314
51. Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid Based Complement Altern Med. 2020;2020:1–12. doi:10.1155/2020/2560645
52. Maruyama N, Kakuta Y, Yamauchi K, et al. Quinine inhibits production of tumor necrosis factor-alpha from human alveolar macrophages. Am J Respir Cell Mol Biol. 1994;10(5):514–520. doi:10.1165/ajrcmb.10.5.8179913
53. Ittarat W, Udomsangpetch R, Chotivanich KT, Looareesuwan S. The effects of quinine and artesunate treatment on plasma tumor necrosis factor levels in malaria-infected patients. Southeast Asian J Trop Med Public Health. 1999;30(1):7–10.
54. Margolin L, Luchins J, Margolin D, Margolin M, Lefkowitz S. 20-week study of clinical outcomes of over-the-counter COVID-19 prophylaxis and treatment. J Evid Based Integr Med. 2021;26:1–13. doi:10.1177/2515690X211026193
55. Liu W, Qi Y, Liu L, Tang Y, Wei J, Zhou L. Suppression of tumor cell proliferation by quinine via the inhibition of the tumor necrosis factor receptor-associated factor 6-AKT interaction. Mol Med Rep. 2016;14(3):2171–2179. doi:10.3892/mmr.2016.5492
56. Higgins PDR, Ng S, Danese S, Rao K. The risk of SARS-CoV-2 in immunosuppressed IBD patients. Crohn’s Colitis 360. 2020;2(2):1–4. doi:10.1093/crocol/otaa026
57. Brent J, Burkhart K, Dargan P, et al. Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient. Springer, Cham. 2017:1–3058. doi:10.1007/978-3-319-17900-1.
58. Achan J, Talisuna AO, Erhart A, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10(1):1–12. doi:10.1186/1475-2875-10-144
59. Bar-Oz B, Levichek Z, Koren G. Medications that can be fatal for a toddler with one tablet or teaspoonful: a 2004 update. Pediatr Drugs. 2004;6(2):123–126. doi:10.2165/00148581-200406020-00005
60. Jm V. Chemotherapy of malaria. In: The Pharmacological Basis of Therapeutics.
61. Adam Bykowski TDL. Chinconism. StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559319/#_article-19560_s15_.
62. Manzano-Santana PI, Tivillin JPP, Chóez-Guaranda IA, Lucas ADB, Orellana-Manzano AK, Rastrelli L. Potential bioactive compounds of medicinal plants against new Coronavirus (SARS-CoV-2): a review. Rev Bionatura. 2021;6(1):1653–1658. doi:10.21931/RB/2021.06.01.30
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
