Back to Journals » Infection and Drug Resistance » Volume 12
Potential effects of microbial air quality on the number of new cases of diabetes type 1 in children in two regions of Poland: a pilot study
Authors Michalska M , Wąż P, Zorena K, Bartoszewicz M , Korzeniowska K , Krawczyk S, Beń-Skowronek I , Myśliwiec M
Received 28 February 2019
Accepted for publication 12 June 2019
Published 29 July 2019 Volume 2019:12 Pages 2323—2334
DOI https://doi.org/10.2147/IDR.S207138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Małgorzata Michalska,1,* Piotr Wąż,2,* Katarzyna Zorena,1 Maria Bartoszewicz,1 Katarzyna Korzeniowska,3 Sylwia Krawczyk,4 Iwona Beń-Skowronek,4 Małgorzata Myśliwiec3
1Department of Immunobiology and Environment Microbiology, Faculty of Health Sciences, Medical University of Gdańsk, Gdańsk, Poland; 2Department of Nuclear Medicine, Faculty of Health Sciences, Medical University of Gdańsk, Gdańsk, Poland; 3Clinic of Pediatrics, Diabetology and Endocrinology, Faculty of Medicine, Medical University of Gdańsk, Gdańsk, Poland; 4Department of Pediatric Endocrinology and Diabetology, Faculty of Medicine, Medical University of Lublin, Lublin, Poland
*These authors contributed equally to this work
Aim: The aim of the study was to investigate the relationship between the concentration of psychrophilic bacteria, mesophilic bacteria and mold fungi in bioaerosols, and the number of new cases of type 1 diabetes mellitus (T1DM) in children.
Methods: Air samples from the Lubelskie and Pomeranian voivodeships in Poland were collected from January 2015 to December 2016 in winter, spring, summer and autumn. Thirty-three samples were collected in the Pomeranian and 27 in the Lubelskie voivodeship. The air samples were collected on the first day of each month at 1:00 pm for 10 mins at a height of 1.5 m above the ground. The number of mesophilic bacteria was detected after 24–48 hrs incubation at 37°C on tryptone soya agar (TSA; Merck, Darmstadt, Germany). The number of psychrophilic bacteria was detected after 72 hrs incubation at 22°C on TSA. The number of fungi was detected by a 5-day long incubation at 28°C on chloramphenicol yeast glucose agar.
Results: In the Lubelskie voivodeship, the mean concentration of psychrophilic bacteria was significantly higher than in the Pomeranian voivodeship (2739 vs 608 CFU/m3, respectively), the mean concentration of mesophilic bacteria was significantly higher (2493 vs 778/m3, respectively) and the concentration of fungi was significantly higher (3840 vs 688 CFU/m3, respectively). We also showed a statistically significant relationship between the number of children with recently diagnosed T1DM and the mean concentration of psychrophilic and mesophilic bacteria in the Pomeranian and Lubelskie voivodeships (P<0.001). Moreover, we found a significant relationship between the number of new cases of T1DM in children and the mean concentration of fungi in bioaerosols in the Lubelskie voivodeship (P<0.001), but not in the Pomeranian voivodeship (P=NS).
Conclusion: The results of our research showed that there is a higher concentration of microbial particles in the Lublin voivodeship. Therefore, we recommend changes in climate for children (trips to the sea, mountains, etc) as often as possible.
Keywords: psychrophilic bacteria, mesophilic bacteria, mold fungi, children, incidence of type 1 diabetes mellitus
Introduction
Primary biological aerosols (PBA) are a subset of atmospheric particles released directly from the biosphere to the atmosphere. They consist of both living and non-living organisms including fungi, bacteria, viruses, pollens and their metabolic products (eg, endotoxins, mycotoxins). The term “bioaerosol” is commonly used synonymously with organic dust.1–3 Bioaerosols can be emitted from the ground, soil, forests, desert dust, farming and composting, rural areas, marshy areas, inshore areas and maritime areas.4,5 Modern industrial activities, including the storage and sorting of organic waste, composting, agricultural production, food processing, animal husbandry and wastewater treatment systems also emit large volumes of bioaerosols, which leads to a significant exposure to biological factors.6,7 The first studies devoted to the occurrence and spread of microorganisms and spores in the air date back to the beginning of the 19th century.8 Since that time, studies on bioaerosols have gone through a long way and air samples collected by planes, balloons and racquets showed that PBA released from the land and ocean can be transferred over long distances and to great heights. Some types of fungi, such as Aspergillus fumigatus, can be transported to more than 200 m from the composter and at concentrations higher than locally, near the composter.9 The elements contained in bioaerosols can differ in respect to size and do not necessarily have to occur in the air as independent particles. As early as 20 years ago, Shaffer and Lighthart showed that most bacteria living in inland areas bind with particles with an aerodynamic diameter of more than 3 μm.10 Bacteria can form cell agglomerations or can be dispersed in the air, on plants or animal skin, soil, pollen or on spores in the air. Anemophilous plant pollens are typically 17–58 μm in diameter, fungal spores 1–30 μm, bacteria 0.25–8 μm, and viruses usually not more than 0.3 μm.11,12
The extent to which biological particles suspended in the air are inhaled greatly depends on their ability to penetrate the respiratory system and their deposition within it. Particles larger than 10 μm in diameter can form deposits in the upper respiratory tract, nose and throat, causing hay fever. Particles smaller than 5 μm in diameter penetrate the alveoli, causing allergic alveolitis and asthma. Bound and non-bound fungal allergens, ultra-small particles measuring <0.1 μm can deeply penetrate the human respiratory system. Their size and shape also determine their removal from the airways.13,14
Bacteria, fungal spores, pollens and other bioparticles are transported by the air, and on the one hand, they are essential for the reproduction and spread of organisms in various ecosystems, but on the other hand, they may contribute to and/or aggravate many diseases in plants, animals and humans.15,16 Healthy persons are usually adapted to the presence of bioaerosols in the natural environment. However, for people in high-risk groups, a high concentration of organic dust can pose an additional health threat, causing cardiovascular diseases, chronic obstructive pulmonary disease neoplasms and diabetes.17,18
Until 1998, the Polish population was one among nations with low incidence of type 1 diabetes mellitus (T1DM). Within the last 30 years, there has been a dynamic increase in the number of new cases of T1DM in Poland – a trend moving this country up to the medium level19,20 in the classification of countries according to incidence of this disease. The prevalence of T1DM is constantly increasing worldwide and it is not always the genetic predispositions that contribute to the initiation and/or propagation of abnormal immune response leading to the loss of beta cells. T1DM is more and more often diagnosed in people who do not present high-risk genetic alleles.21 This indicates that it is the environment that may be the main factor contributing to T1DM.22,23 Because so many environmental factors have changed in the last 3 decades, it would be helpful to narrow down the change for environmental exposures. Studies carried out so far showed the negative effect of bacteria and mold fungi in the course of diabetes.16,24,25 Dayal et al described mucormycosis in children with T1DM aged 3–12 years.24 The acute onset of T1DM has been reported in a child with toxic shock syndrome due to Staphylococcus aureus.25 Thus, we made an attempt to check whether there was any relationship between the concentration of psychrophilic bacteria, mesophilic bacteria and mold fungi in the bioaerosol, and the number of new cases of T1DM in children in the Pomeranian and Lubelskie voivodeships.
Selection of sampling sites
The geographical situation of the Lubelskie and Pomeranian voivodeships, Poland
The Pomeranian voivodeship is located in the north of Poland, by the Baltic Sea. It borders Russia through the Gulf of Gdansk. The total area of the Pomeranian voivodeship is 18,310 km2 and is inhabited by more than 2.4 million people. It consists of 16 districts, including the Tricity an agglomeration of Gdansk, Gdynia and Sopot. In the Tricity, there are industrial plants producing fuels and petroleum-derived products, factories producing chemical fertilizers, feed manufacturing companies, heat and power stations and thermal power stations and shipyards. There are also two ports (in Gdansk and Gdynia) which are the most important transport chain link connecting Scandinavia with the countries of Southern Europe. The natural resources in the region include a vast green area. Over 46% of the surface of the Pomeranian voivodeship is covered by the forest (Figure 1). The area of the Lubelskie voivodeship is 25,000 km2 and is inhabited by more than 2.1 million people. It consists of 20 districts. Due to the presence of fertile soils, the Lubelskie voivodeship is an important agricultural region in Poland, including the cultivation of wheat, sugar beet, fruits, vegetables, hops and tobacco. Agricultural land covers 56% of the voivodeship area (the average for Poland: 48%). Forests in the Lubelskie voivodeship show considerable diversity in terms of distribution and size of forest complexes and most of all in terms of habitats and phytosociology. According to official statistics by Zgłobicki, forests cover only 23% of the area (the average for Poland: 30%)26 (Figure 1).
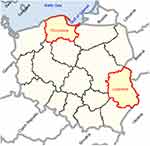 |
Figure 1 A map showing the Pomeranian and Lubelskie voivodeships in Poland. |
Sampling methods
Air samples from the Lubelskie and Pomeranian voivodeships were collected from January 2015 to December 2016 in winter, spring, summer and autumn. Thirty-three samples were collected in the Pomeranian voivodeship and 27 in the Lubelskie voivodeship. The air samples were collected by impaction with a SAS Super ISO 100 (Milan, Italy) sampler. The samples automatically collected 100 L of air. The samples automatically collected 100 L of air. The maximum efficiency of collection is for particulate matter with a d50=2–4 μm. The flow rate is 90 lpm. All removable parts of the air sampler were sterilized by autoclaving before sampling and sterilized sampler head between samples with 70% ethanol. Sucked air was then transported through small holes to a head with a Petri dish containing an agar medium appropriate for each type of microorganisms. The air samples were collected on the first day of each month at 1:00 pm for 10 mins at a height of 1.5 m above the ground. If there was any rainfall or snowfall approx. 2–3 hrs before scheduled we collected them on the following day at 1:00 pm. The air temperature, humidity, wind speed and direction were recorded using a Beurer Termohygrometer HM55 (Ulm, Germany). The air temperatures in the Lubelskie voivodeship during the tested seasons ranged from 2ºC to 13ºC (winter), from 12ºC to 19ºC (spring), from 11ºC to 27 (summer), and from 4ºC to 15ºC (autumn). Relative humidity in the winter ranged from 40% to 72%, from 55% to 61% in the spring, from 47% to 93% in the summer, and from 67% to 77% in the autumn. The wind speed was 15–16 km/hr in the winter, 11–15 km/hr in the spring, 2–22 km/hr in the summer and 6–8 km/hr in the autumn. The air temperatures in Pomeranian voivodeship during the tested season ranged from 1ºC to 14ºC (winter), from 8ºC to 24ºC (spring), from 15ºC to 24ºC (summer), and from 2ºC to 9.6ºC (autumn). Relative humidity in the winter season ranged from 44% to 86%, from 40% to 88% in the spring, from 41% to 80% in the summer, and from 81% to 99% in the autumn. The wind speed was 15–24 km/hr in the winter, 9–35 km/hr in the spring, 2–22 km/hr in the summer and 4–11 km/hr in the autumn.
The microbiological assessment of air in the Lubelskie and Pomeranian voivodeships
The mesophilic bacteria were counted after a 24–48 hrs incubation at 37°C on tryptone soya agar (TSA; Merck, Darmstadt, Germany). In order to culture psychrophilic bacteria, we used TSA. Incubation was carried out at 22°C for 72 hrs. To culture fungi, we used the chloramphenicol yeast glucose agar medium by Merck. The plates with growth medium were incubated at 28°C for 5 days.
Chapmann agar (Merck) was used for culturing Staphylococcus sp. Staphylococcus sp. was differentiated from Micrococcus sp. species in terms of the oxidase and coagulase activity. Mannitol egg yolk polymyxin agar (Merck) was used for culturing and enumeration of Bacillus cereus. Agar plates were incubated at 30°C for 24–28 hrs and examined for typical B. cereus colonies. Trypticase soy 5% sheep blood agar (Graso Biotech, Starogard Gdański, Poland) was used for the determination of hemolytic reactions.
Isolation of the Enterobacteriaceae Gram-negative bacteria was performed on the McConkey agar (Merck) and on TSA (Merck) by 24–48 hrs incubation at 37°C. All Enterobacteriaceae bacteria were biochemically identified using the API 20E system for (BioMerieux, Paris, France) according to the manufacturer’s instructions. Bacteria of the genus Pseudomonas were determined using Cetrimide Agar (Merck), incubated at 37°C for 24–48 hrs. The production of pyocyanin and fluorescein pigments was observed on Pseudomonas agar P and Pseudomonas agar F.
Bacterial identification
The isolated bacteria were divided into Gram-positive cocci, Gram-positive aerobic bacilli and Gram-negative aerobic bacteria. The bacteria were identified based on their morphological features in a microscopic preparation stained with Gram’s staining method.27,28 Cells from the stain were viewed under an oil immersion at 1000×, using a Nikon Eclipse E2000 microscope (Tokyo, Japan). We used the properties from the Bergey’s manual of systematic bacteriology and the textbooks on microbiological diagnostics.29
Mold fungi identification
Mold fungi were identified based on their macro- and microscopic features, with use of a Nikon Eclipse E2000 microscope at 400×, 600×, 1000× magnification and a key for the identification of fungi.30,31 Mold colonies were identified on the basis of color, texture, topography of the culture surface, smell of the colony, color of the reverse of the colony and the presence of the diffuse pigment. Microscopic features of the fungal colonies were identified based on their microscopic features, ie the presence of macroconidia and microconidia, their shape and appearance.30,31
Sample analysis
The number of colonies of bacteria and fungi were expressed as a colony-forming unit (CFU) per 1 m3 of the air (CFU/m3). When applying the impact method, we used the Feller table attached to the manual of the air sampler.32 The colonies collected should be revised by the equation:
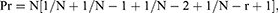
where Pr is the revised colony in stage, N is the number of sieve pores, and r is the number of viable particles counted on the agar plate.
The number of colonies of bacteria and fungi (CFU/m3) was calculated using the following equation:
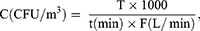
where C – airborne bacterial concentration; CFU – colony-forming unit; T – total colonies after application of the Pr statistical correction; t – sampling time and F – airflow rate.
Incidence of type 1 diabetes mellitus in children and adolescents in the Pomeranian and Lubelskie voivodeships
The number of new cases incidence of T1DM was obtained from the Clinic of Pediatric Endocrinology and Diabetology of the Medical University of Gdansk and the Clinic of Endocrinology and Pediatric Diabetology of the Medical University in Lublin. The number of children of 0–18 years rate in 2015 and 2016 in the Pomeranian and Lubelskie voivodeships was acquired from the statistical yearbooks by the Polish Central Statistical Office (GUS).33,34 The study was approved by the Ethics Committee of the Medical University of Gdańsk (no. NKBBN/314/2016) and the investigation was carried out in accordance with the principles of the Declaration of Helsinki as revised in 1996.
Statistical analysis
The results were generated using R statistics language (a language and environment for statistical computing) 2018 version.35 The main procedure in the calculation is a generalized linear model – glm. Due to the high flexibility of the glm function, we have applied it to the creation of a model basedon the Poisson's distribution of errors and the natural logarithm as the link function. For the data collected in the work, the Poisson error distribution and the natural logarithm as a link function have been determined. The model chosen in this way is suitable for data counting. Moreover, Chi-square test for independence was used for qualitative variables. The assumed significance level is α=0.05.
Results
Assessment of microbiological bioaerosol in the Pomeranian and the Lubelskie voivodeships
In the Lubelskie voivodeship, the mean concentration of psychrophilic bacteria was higher (2739 vs 608 CFU/m3) than in the Pomeranian voivodeship (P<0.0001), the mean number of mesophilic bacteria was higher (2493 vs 778 CFU/m3) than in the Pomeranian voivodeship (P<0.0001), and the mean concentration of fungi was higher (3840 vs 688 CFU/m3) than in the Pomeranian voivodeship (P<0.0001) (Figure 2).
 |
Figure 2 The concentration of bacteria and mold fungi in outdoor air in the Lubelskie and Pomeranian voivodeships.Abbreviation: CFU/m3, colony-forming unit per cubic meter of air. |
Qualitative assessment of bacteria in the air in the Lubelskie voivodeship
From the samples of the air collected in the Lubelskie voivodeship, we isolated Gram-positive cocci (20.97%), including Micrococcus luteus 8.97%, Micrococcus lylar 0.96%, Sarcina lutea 2.29%, Kocuria rosea 1.17%, Staphylococcus aureus 0.93%, Staphylococcus epidermidis 3.42% and Staphylococcus saprophyticus 3.23%. We also isolated Gram-positive Bacillus (78.51%), including Bacillus subtilis 16.76%, B. cereus 5.27%, Bacillus mycoides 18.94%, Bacillus macerans 37.55% and Gram-negative bacilli (0.52%), including Pseudomonas aeruginosa 0.32%, Citrobacter freundi 0.07% and Enterobacter aerogenes 0.13%.
Qualitative assessment of bacteria in the air in the Pomeranian voivodeship
From the samples of air collected in the Pomeranian voivodeship, we isolated Gram-positive cocci (33.19%), including Micrococcus luteus 16.17%, Micrococcus lylar 2.20%, Sarcina lutea 6.48%, K. rosea 1.21%, Staphylococcus aureus 3.69%, Staphylococcus epidermidis 1.53% and Staphylococcus saprophyticus 1.90%. We also isolated Gram-positive Bacillus (65.33%), including B. subtilis 17.17%, B. cereus 3.25%, B. mycoides 13.48%, B. macerans 31.44% and Gram-negative bacilli (1.48%), including Pseudomonas aeruginosa 1.17%, C. freundi 0.11%, Enterobacter aerogenes 0.10%, Escherichia coli 0.05% and Aeromonas hydrophila 0.06%.
Qualitative assessment of mold fungi in the air in the Lubelskie voivodeship
From the air samples collected in the Lubelskie voivodeship, we isolated 9 types of mold fungi and 1 yeast-like fungus (Rhodotorula mucilaginosa) (14.8%). The most commonly isolated types of mold fungi included Penicillium chrysogenum (31.7%), Aspergillus niger (20.7%) and Penicillium viridicatum (7.7%). Less common were Alternaria alternata (6.3%), Paecilomyces sp. (6.3%), Aspergillus flavus (6.0%), Chrysosporium sp. (4.9%) and Mucor mucedo (1.5%). The least common was Rhizopus nigricans (0.2%).
Qualitative assessment of mold fungi in the air in the Pomeranian voivodeship
From the air samples collected in the Pomeranian voivodeship, we isolated 8 types of fungi and 1 yeast-like fungus (Rhodotorula mucilaginosa) (0.12%). The most commonly isolated types of fungi included Penicillium chrysogenum (44.2%), Aspergillus niger (32.2%) and Mucor mucedo (12.4%). Rhizopus nigricans (6.7%), Geotrychum sp. (2.5%), Alternaria alternata (1.2%), Chrysosporium sp.(0.8%) were less common. The least common was Biopolaris sp., isolated only once (0.01%).
The number of children aged 0–18 years in the Pomeranian voivodeship and the Lubelskie voivodeship vs the number of new cases of T1DM
In the years 2015–2016, the number of children of 0–18 years old amounted to 947,362 in the Pomeranian voivodeship, and the number of new cases of T1DM was 219. In the Lubelskie voivodeship, there were 809,235 children at that time and the number of new cases of T1DM amounted to 152. T1DM incidence in the Pomeranian and Lubelskie voivodeships in years 2015 and 2016 is presented in Figure 3.
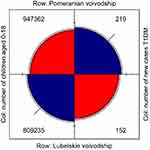 |
Figure 3 The number of new cases of T1DM in the Pomeranian and Lubelskie voivodeships.Abbreviations: Col, column; T1DM, type 1 diabetes mellitus. |
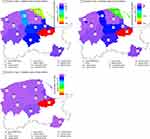
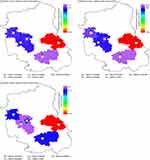
Concentration of psychrophilic bacteria, mesophilic bacteria and mold fungi (CFU/m3) in the air vs the number of new cases of T1DM in the Pomeranian and Lubelskie voivodeships
Map of Pomorskie voivodeship (Figure 4) and Lubelskie voivodeship (Figure 5) presents districts in which air outdoor samples were taken and marked with red color – highest mean concentrations of psychrophilic, mesophilic bacteria, mold fungi (CFU/m3) to the lowest concentration of psychrophilic bacteria, mesophilic bacteria, mold fungi (CFU/m3) with violet color.
The relationship between the number of new cases of T1DM and the concentration of psychrophilic bacteria, mesophilic bacteria and mold fungi in the air in the Lubelskie and Pomeranian voivodeships
In the Lubelskie voivodeship, there was a relationship observed between the number of new cases of T1DM and the number of psychrophilic bacteria (β=2.860; P<0.001), mesophilic bacteria (β=2.824; P<0.001) and mold fungi (β=2.923; P<0.001). In the Pomeranian voivodeship, there was a relationship observed between the number of new cases of T1DM and the mean number of psychrophilic bacteria (β=2.960; P<0.001) and mesophilic bacteria (β=2.898; P<0.001), but not with mold fungi (β=2.756, P=NS).
Discussion
The microbiological study of bioaerosol in the Lubelskie voivodeship revealed a higher mean concentration of psychrophilic bacteria, mesophilic bacteria and mold fungi (CFU/m3) compared to the concentrations revealed in the bioaerosol in the Pomeranian voivodeship. The higher concentration of bacteria and mold fungi in the Lubelskie voivodeship may result from unfavorable meteorological conditions, including temperature inversion and lack of wind. Horticulture, fruit farming, hops and tobacco cultivation are widely practiced in the Lubelskie voivodeship. Poland has a long-standing tradition of tobacco cultivation and is one of its largest producers in the European Union.36 What is specific to tobacco cultivation is its high labor intensity, low level of mechanization and significant amount of manual work, which leads to a significant exposure to biological aerosols.26,37,38 Another factor increasing the concentration of bacteria and mold fungi in the air in the Lubelskie voivodeship may not be anthropogenic bioaerosol exclusively from farming but also naturally occurring from forests, parks and meadows. Our results are consistent with the ones of the study by Lymperopoulou et al. The authors concluded that in the air collected directly above vegetation the concentration of microorganisms was 2–10-fold higher than that in the air collected simultaneously in an adjacent non-vegetated area.39
In the next stage of the study, we made an attempt to identify the types of bacteria present in the bioaerosol. The most common genus found in the air samples detected in the Lubelskie and Pomeranian voivodeships was Gram-positive bacteria, including Micrococcus luteus and B. macerans. Micrococcus luteus is believed to be an opportunistic pathogen. It tends to be responsible for recurrent bacteremia, septic shock, arthritis, meningitis, suppuration and pneumonia.40,41 In the literature, there are cases described of infective endocarditis caused by Micrococcus luteus and of leukocytosis caused by Micrococcus luteus and Streptococcus intermedium.42
The results obtained in our study indicate that Gram-positive Bacillus sp. is dominant in the air in both voivodeships. Similar results were obtained in studies conducted by other authors, where the dominating bacteria in the air turned out to be the bacteria of the Bacillus genus.7,11,43 Bacillussp. are characterized by their ability to adapt to changes in environmental conditions. They are typical saprophytes living mainly in soil and water.44,45 Many of them can be opportunistic pathogens, able to cause an illness in people with compromised immunity, especially small children.44 Less common were Gram-positive cocci, mannitol-negative of the Staphylococcus sp. genus. In most studies Staphylococcus saprophyticus found in animals and Staphylococcus epidermidis being part of the human flora. However, there are reports stating that they may be the cause of peritoneal dialysis in immunocompromised patients.46,47 The types of bacteria Pseudomonas sp. that were found in the investigated samples are mainly bacteria living in the natural environment and are an opportunistic pathogen. It often causes chronic lung infections associated with respiratory failure, clinic deterioration or burn patients.48,49 However, it should be pointed out that they are a source of endotoxins too. Endotoxins, being an element of the cell wall of the Gram-negative mesophilic bacteria, are biologically active lipopolysaccharides causing inflammatory reactions. When released into the environment they take on a form of spherical particles of 30–50 nm (respirable fraction). Inhalation of endotoxins contained in the polluted air can lead to toxic pneumonisis and consequently to adult respiratory distress syndrome, as well as to increased incidence of neoplasms or diabetic neuropathy.50,51 Pathogenic mold fungi found in the air samples collected in the Pomeranian and Lubelskie voivodeships included Penicillium chrysogenum, Aspergillus niger, Mucor mucedo, and Rhizopus nigricans. Studies carried out so far showed the negative effect of fungi, including Aspergillus flavus, Aspergillus niger and Alternaria alternata in the course of diabetes.24,52 Mucormycosis affecting the maxilla is rare because of rich blood vessel supply to maxillofacial areas although more virulent fungi such as Mucor can overcome this difficulty.
A common form of this infection is seen in the rhinomaxillary region and in patients with immunocompromised state such as diabetes.24,52 In studies, the authors reported a case of a 62-year-old man with diabetes, who was admitted to hospital due to painful ophthalmoplegia caused by Aspergillus niger.53 Additionally, there was a case of a 56-year-old gardener with type 2 diabetes mellitus and an infection with Penicillium capsulatum.54 Exposure to fungi can result in allergies, most commonly manifested as rhinitis and asthma, as well as allergic alveolitis (AZPP).55 Infection with mold spores is possible through inhalation, wound contamination or damage of the mucous membrane.55,56 In several studies, it was observed that inhalation of toxins can even have a tenfold stronger toxic effect than exposure through the skin and digestive tract, where most of defensive reactions of the human body take place. It is associated with the ease with which mycotoxins penetrate through the barrier of capillaries in the alveoli directly into the bloodstream. It is believed that for intoxication through the respiratory tract much lower dose of mycotoxin is needed and the symptoms appear when the number of spores in the inhaled air amounts to 1000 (CFU/m3).37,57,58 The mechanism by means of which air pollutants (biological aerosols and particulate matter) contribute to the occurrence of diseases and premature death is not fully known. The in vivo and in vitro studies conducted so far showed that even exposing healthy volunteers for short periods to pollutant particles in the air triggers an inflammatory reaction on several different levels.59–61 The immune system recognizes the antigens via toll-like receptors that are stimulated directly or indirectly. Microbial infections have long been considered as an important modulator of T1DM risk. One of the mechanisms leading to the disease development is the direct damage and destruction of the pancreatic β-cells by the viruses and other microbes.62,63 However, in most of the cases, a diabetogenic influence of the infection is of non-direct character, aimed rather at the induction of an autoimmune process directed against pancreatic β-cells and therefore leading to their destruction.61,62 The persistent destruction of the β-cells by active cytotoxic T lymphocytes, natural killer and K cells, as well as some of the cytokines (IL1, tumor necrosis factor-α, interferon-γ) probably releases new autoantigens that induce new cellular reactions and formation of new antibodies, thus augmenting the process of destruction β-cells.64 The increase in T1DM incidence in children and adolescents is worrying and not yet fully explored. Biological aerosols, including viruses, molds fungi, bacteria and their metabolic products, eg endotoxins, mycotoxins may be one of the main factors contributing to the autoimmunity.62,64
In the current research, the relationship between the number of psychrophilic bacteria, mesophilic bacteria and mold fungi in Lubelskie voivodeship have been demonstrated. In the Pomeranian voivodeship, we detected a relationship between the number of new cases of T1DM and the concentration of psychrophilic bacteria and mesophilic bacteria but not mold fungi. The lower number of microorganisms in the air in the Pomeranian voivodeship compared to the Lubelskie voivodeship may result from the geomorphological diversity of the Pomeranian voivodeship. The close proximity to the Baltic Sea and location within the influence of atmospheric centers both contribute to the distinct diversity of climatic conditions in the Pomeranian voivodeship. In the coastal region, the most significant factor affecting the quality of the air is the force and direction of wind, which can decrease the number of bacteria and mold fungi in the bioaerosol.65,66
In summary, we conclude that in the Pomeranian and Lubelskie voivodeships the most commonly occurring bacteria in the air were Gram-positive ones, including Micrococcus luteus and B. macerans. However in the Lubelskie voivodeship, we found a relationship between the number of new cases of T1DM and the concentration of psychrophilic bacteria, mesophilic bacteria and mold fungi. We suggest that children living in the Lubelskie voivodeship should spend holidays by the sea or in the mountains as frequently as possible. In this way, they could avoid significant exposure to bacteria and molds occurring in the air in areas where they live.
Abbreviations list
CFU, colony-forming unit; CFU/m3, colony-forming unit per cubic meter of air; Col, column; GUS, Polish Central Statistical Office; T1DM, type 1 diabetes mellitus; TSA, tryptone soya agar; PBA, primary biological aerosols.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Medical University of Gdańsk (number NKBBN/314/2016) and the investigation was carried out in accordance with the principles of the Declaration of Helsinki as revised in 1996.
No informed consent from the participants was required for the present analysis.
Acknowledgment
The study was financed by the Medical University of Gdańsk (Grants: ST-02-0108/07/780 and ST-02-0120/07/156).
Disclosure
The authors declare that they have no competing interests in regard to this work.
References
1. Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg. 2003;47(3):187–200. doi:10.1093/annhyg/meg032
2. Peccia J, Hernandez M. Incorporating polymerase chain reaction-based identification, population characterization, and quantification of microorganisms into aerosol science: a review. Atmos Environ. 2006;40(21):3941–3961. doi:10.1016/j.atmosenv.2006.02.029
3. Walser SM, Gerstner DG, Brenner B, et al. Evaluation of exposure-response relationships for health effects of microbial bioaerosols – a systematic review. Int J Hyg Environ Health. 2015;218(7):577–589. doi:10.1016/j.ijheh.2015.07.004
4. Yoo K, Lee TK, Choi EJ, et al. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: a review. J Environ Sci. 2017;51(234–247):2418. doi:10.1016/j.jes.2016.07.002
5. Gandolfi I, Bertolini V, Ambrosini R, Bestetti G, Franzetti A. Unravelling the bacterial diversity in the atmosphere. Appl Microbiol Biotechnol. 2013;97(11):4727–4736. doi:10.1007/s00253-013-4901-2
6. Kłapeć T, Cholewa G, Cholewa A, Dutkiewicz J, Wójcik-Fatla A. Fungal diversity of root vegetables and soil rhizosphere collected from organic and conventional farms in Eastern Poland. Ann Agric Environ Med. 2018;25(2):374–381. doi:10.26444/aaem/92143
7. Brodie EL, DeSantis TZ, Parker JPM, et al. Urban aerosols harbor diverse and dynamic bacterial populations. Proc Natl Acad Sci. 2007;104(1):299–304. doi:10.1073/pnas.0608255104
8. Berche P. Louis Pasteur, from crystals of life to vaccination. Clin Microbiol Infect. 2012;18:1–6. doi:10.1111/j.1469-0691.2012.03945.x
9. Herr CEW, Zur Nieden A, Jankofsky M, Stilianakis NI, Boedeker R-H, Eikmann TF. Effects of bioaerosol polluted outdoor air on airways of residents: a cross sectional study. Occup Environ Med. 2003;60(5):336–342. doi:10.1136/oem.60.5.336
10. Shaffer BT, Lighthart B. Survey of culturable airborne bacteria at four diverse locations in Oregon: urban, rural, forest, and coastal. Microb Ecol. 1997;34(3):167–177.
11. Fang Z, Ouyang Z, Zheng H, Wang X, Hu L. Culturable Airborne bacteria in outdoor environments in Beijing, China. Microb Ecol. 2007;54(3):487–496. doi:10.1007/s00248-007-9216-3
12. Fahlgren C, Bratbak G, Sandaa R-A, Thyrhaug R, Zweifel UL. Diversity of airborne bacteria in samples collected using different devices for aerosol collection. Aerobiologia (Bologna). 2011;27(2):107–120. doi:10.1007/s10453-010-9181-z
13. Hargreaves M, Parappukkaran S, Morawska L, Hitchins J, He C, Gilbert D. A pilot investigation into associations between indoor airborne fungal and non-biological particle concentrations in residential houses in Brisbane, Australia. Sci Total Environ. 2003;312(1–3):89–101. doi:10.1016/S0048-9697(03)00169-4
14. Sturm R. Bioaerosols in the lungs of subjects with different ages – Part 2: clearance modeling. Ann Transl Med. 2017;5(5):95. doi:10.21037/atm.2017.03.05
15. Joung YS, Ge Z, Buie CR. Bioaerosol generation by raindrops on soil. Nat Commun. 2017;8:1–10. doi:10.1038/s41467-016-0009-6
16. Fröhlich-Nowoisky J, Kampf CJ, Weber B, et al. Bioaerosols in the earth system: climate, health, and ecosystem interactions. Atmos Res. 2016;182:346–376. doi:10.1016/j.atmosres.2016.07.018
17. Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187(7):721–727. doi:10.1164/rccm.201211-2004OC
18. Howard SG. Exposure to environmental chemicals and type 1 diabetes: an update. J Epidemiol Community Health. 2019;73(6):483–488. doi:10.1136/jech-2018-210627
19. Mysliwiec M, Balcerska A, Zorena K, Jedrzejczyk A, Malinowska E, Mysliwska J. Increasing incidence of diabetes mellitus type 1 in children – the role of environmental factors. Polish J Environ Stud. 2007;16(1):109–112.
20. Szalecki M, Wysocka-Mincewicz M, Ramotowska A, et al. Epidemiology of type 1 diabetes in Polish children: a multicentre cohort study. Diabetes Metab Res Rev. 2018;34:e2962. doi:10.1002/dmrr.2962
21. Steck AK, Armstrong TK, Babu SR, Eisenbarth GS. Type 1 diabetes genetics consortium the T 1 DG. Stepwise or linear decrease in penetrance of type 1 diabetes with lower-risk HLA genotypes over the past 40 years. Diabetes. 2011;60(3):1045–1049. doi:10.2337/db10-1419
22. Hathout EH, Beeson WL, Nahab F, Rabadi A, Thomas W, Mace JW. Role of exposure to air pollutants in the development of type 1 diabetes before and after 5 yr of age. Pediatr Diabetes. 2002;3(4):184–188. doi:10.1034/j.1399-5448.2002.30403.x
23. Beyerlein A, Krasmann M, Thiering E, et al. Ambient air pollution and early manifestation of type 1 diabetes. Epidemiology. 2015;26(3):e31–e2. doi:10.1097/EDE.0000000000000254
24. Dayal D, Bakshi J, Jain P, Shivaprakash MR, Singhi S. Outcome of rhino-sinus mucormycosis in children with type 1 diabetes. Indian J Pediatr. 2015;82(7):651–652. doi:10.1007/s12098-014-1680-4
25. Couper JJ, Kallincos N, Pollard A, et al. Toxic shock syndrome associated with newly diagnosed type I diabetes. J Paediatr Child Health. 2000;36(3):279–282.
26. Zgłobicki W, Gawrysiak L, Baran-Zgłobicka B, Telecka M. Long-term forest cover changes, within an agricultural region, in relation to environmental variables, Lubelskie province, Eastern Poland. Environ Earth Sci. 2016;75(20):1373. doi:10.1007/s12665-016-6195-z
27. Washington JA. Principles of Diagnosis. In: Baron S, editor. Medical Microbiology. 4th ed. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
28. Sneath PHA, Mair NS, Sharpe ME, Holt JG. eds. Bergey’s Manual of Systematic Bacteriology. Vol.2. Baltimore-London-Los Angeles-Sydney: Williams & Wilkins; 1986.
29. Boone DR, Castenholz RW, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York: Springer; 2001.
30. Käärik A, Keller J, Kiffer E, et al. Atlas of Airborne Fungal Spores in Europe. Berlin: Springer; 1983.
31. Campbell CK, Johnson EM, Warnock DW, editors. Identification of Pathogenic Fungi. 2nd ed. Chichester: Wiley-Blackwell; 2013.
32. Feller W. An Introduction to Probability Theory and Its Applications – Vol. II. New York: John Wiley and Sous Inc.; 1950.
33. Statistical Yearbook. Pomorskie Voivodship 2017. Available from: https://gdansk.stat.gov.pl/publikacje-i-foldery/roczniki-statystyczne/rocznik-statystyczny-wojewodztwa-pomorskiego-2017,4,18.html.
34. Statistical yearbook. Lubelskie Voivodship 2017. Available from: https://lublin.stat.gov.pl/publikacje-i-foldery/roczniki-statystyczne/rocznik-statystyczny-wojewodztwa-lubelskiego-2017,2,18.html.
35. R: the R project for statistical computing. Available from: https://www.r-project.org/. Accessed June 21, 2018.
36. Jassem J, Przewoźniak K, Zatoński W. Tobacco control in Poland-successes and challenges. Transl Lung Cancer Res. 2014;3(5):280–285. doi:10.3978/j.issn.2218-6751.2014.09.12
37. Góra A, Mackiewicz B, Krawczyk P, et al. Occupational exposure to organic dust, microorganisms, endotoxin and peptidoglycan among plants processing workers in Poland. Ann Agric Environ Med. 2009;16(1):143–150.
38. Golec M, Skórska C, Mackiewicz B, et al. Relationship between COPD and lower socioeconomic status in farmers from South-Eastern Poland (Lublin region). Rural Remote Health. 2014;14:2531.
39. Lymperopoulou DS, Adams RI, Lindow SE. Contribution of vegetation to the microbial composition of nearby outdoor air. Appl Environ Microbiol. 2016;82(13):3822–3833. doi:10.1128/AEM.00610-16
40. Yang S, Sugawara S, Monodane T, et al. Micrococcus luteus teichuronic acids activate human and murine monocytic cells in a CD14- and toll-like receptor 4-dependent manner. Infect Immun. 2001;69(4):2025–2030. doi:10.1128/IAI.69.4.2025-2030.2001
41. Dhyani A, Gupta V, Chauhan A, Seshendra Kumar RN, Chakravarty S. Meningitis caused by Micrococcus luteus: case report and review of literature. IP Int J Med Microbiol Trop Dis. 2019;5(1):63–64. doi:10.18231/2581-4761.2019.0015
42. Ioannou A, Xenophontos E, Karatsi A, Petrides C, Kleridou M, Zintilis C. Insidious manifestation of pyogenic liver abscess caused by Streptococcus intermedius and Micrococcus luteus: a case report. Oxford Med Case Rep. 2016;2016(1):1–3. doi:10.1093/omcr/omv071
43. Aydogdu H, Asan A, Tatman Otkun M. Indoor and outdoor airborne bacteria in child day-care centers in Edirne City (Turkey), seasonal distribution and influence of meteorological factors. Environ Monit Assess. 2010;164(1–4):53–66. doi:10.1007/s10661-009-0874-0
44. Brągoszewska E, Pastuszka JS. Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia (Bologna). 2018;34(2):241–255. doi:10.1007/s10453-018-9510-1
45. Soto T, Lozano M, Vicente-soler J, et al. Microbiological survey of the aerial contamination in urban areas of the city of Murcia, Spain. An Biol. 2009;31:7–13.
46. Krajewski JA, Szarapińska-Kwaszewska J, Dudkiewicz B, Cyprowski M. Alive microorganism in the workplace ambient air in plants disposing communal waste. Med Pr. 2001;52(5):343–349.
47. Kao C-C, Chiang C-K, Huang J-W. Micrococcus species-related peritonitis in patients receiving peritoneal dialysis. Int Urol Nephrol. 2014;46(1):261–264. doi:10.1007/s11255-012-0302-1
48. Khosravi AD, Taee S, Asarehzadegan Dezfuli A, Meghdadi H, Shafie F. Investigation of the prevalence of genes conferring resistance to carbapenems in Pseudomonas aeruginosa isolates from burn patients. Infect Drug Resist. 2019;12:1153–1159. doi:10.2147/IDR.S197752
49. Sener Okur D, Yuruyen C, Gungor O, et al. Genotypic characterization of Pseudomonas aeruginosa isolates from Turkish children with cystic fibrosis. Infect Drug Resist. 2019;12:675–685. doi:10.2147/IDR.S183151
50. El-Nahas M, Gawish H, Tarshoby M, State O. The impact of topical phenytoin on recalcitrant neuropathic diabetic foot ulceration. J Wound Care. 2009;18(1):33–37. doi:10.12968/jowc.2009.18.1.32146
51. Noor S, Raghav A, Parwez I, Ozair M, Ahmad J. Molecular and culture based assessment of bacterial pathogens in subjects with diabetic foot ulcer. Diabetes Metab Syndr. 2018;12(3):417–421. doi:10.1016/j.dsx.2018.03.001
52. Afroze SN, Korlepara R, Rao GV, Madala J. Mucormycosis in a diabetic patient: a case report with an insight into its pathophysiology. Contemp Clin Dent. 2017;8(4):662–666. doi:10.4103/ccd.ccd_558_17
53. Siraj CA, Krishnan J, Nair RR, Girija AS. Invasive aspergillosis producing painful ophthalmoplegia. J Assoc Physicians India. 2005;53:901–902.
54. Chen M, Houbraken J, Pan W, et al. Pulmonary fungus ball caused by Penicillium capsulatum in a patient with type 2 diabetes: a case report. BMC Infect Dis. 2013;13(1):496. doi:10.1186/1471-2334-13-496
55. Schlosser O, Robert S, Debeaupuis C. Aspergillus fumigatus and mesophilic moulds in air in the surrounding environment downwind of non-hazardous waste landfill sites. Int J Hyg Environ Health. 2016;219(3):239–251. doi:10.1016/j.ijheh.2016.02.003
56. Tashiro T, Izumikawa K, Tashiro M, et al. A case series of chronic necrotizing pulmonary aspergillosis and a new proposal. Jpn J Infect Dis. 2013;66(4):312–316.
57. Ammann HM. Inhalation Exposure and Toxic Effects of Mycotoxins. Cham: Springer; 2016:495–523.
58. Ratnaseelan AM, Tsilioni I, Theoharides TC. Effects of mycotoxins on neuropsychiatric symptoms and immune processes. Clin Ther. 2018;40(6):903–917. doi:10.1016/j.clinthera.2018.05.004
59. Salvi S, Blomberg A, Rudell B. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159(3):702–709. doi:10.1164/ajrccm.159.3.9709083
60. Baldacci S, Maio S, Cerrai S, et al. Allergy and asthma: effects of the exposure to particulate matter and biological allergens. Respir Med. 2015;109(9):1089–1104. doi:10.1016/j.rmed.2015.05.017
61. Korsgren S, Molin Y, Salmela K, et al. On the etiology of type 1 diabetes. Am J Pathol. 2012;181(5):1735–1748. doi:10.1016/j.ajpath.2012.07.022
62. Kondrashova A, Hyöty H. Role of viruses and other microbes in the pathogenesis of type 1 diabetes. Int Rev Immunol. 2014;33(4):284–295. doi:10.3109/08830185.2014.889130
63. Kowalewska B, Zorena K, Szmigiero-Kawko M, Wąż P, Myśliwiec M. M. Higher diversity in fungal species discriminates children with type 1 diabetes mellitus from healthy control. Patient Prefer Adherence. 2016;10:591–599. doi:10.2147/PPA.S97852
64. Li M, Song L-J, Qin X-Y. Advances in the cellular immunological pathogenesis of type 1 diabetes. J Cell Mol Med. 2014;18(5):749–758. doi:10.1111/jcmm.12270
65. Marks R, Jankowska K, Michalska M, et al. The sea to air bacteria transfer from the coastal waters. Bull Inst Marit Trop Med Gdynia. 1996;47:1–4.
66. Michalska M, Bartoszewicz M, Cieszyńska M, et al. Bioaerosols on tri-city (Gdańsk-Sopot-Gdynia) beaches. Int Marit Health. 2010;61:1.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.