Back to Journals » Cancer Management and Research » Volume 11
Potential Antiangiogenic Treatment Eligibility of Patients with Squamous Non-Small–Cell Lung Cancer: EPISQUAMAB Study (GFPC 2015-01)
Authors Vergnenègre A , Basse V, Le Garff G, Bylicki O , Dubos-Arvis C, Comet B, Marcq M, Le Treut J, Auliac JB , Madroszyk A, Fraboulet G , Crequit J , Thomas P, Paleiron N, Monnet I
Received 19 June 2019
Accepted for publication 5 November 2019
Published 27 December 2019 Volume 2019:11 Pages 10821—10826
DOI https://doi.org/10.2147/CMAR.S219984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Alain Vergnenègre,1 Victor Basse,2 Gwenaelle Le Garff,3 Olivier Bylicki,4 Catherine Dubos-Arvis,5 Bénédicte Comet,6 Marie Marcq,7 Jacques Le Treut,8 Jean-Bernard Auliac,9 Anne Madroszyk,10 Gislaine Fraboulet,11 Jacky Crequit,12 Pascal Thomas,13 Nicolas Paleiron,14 Isabelle Monnet15 On behalf of the French Lung Cancer Group
1Centre Hospitalier Universitaire (CHU) Dupuytren, Limoges, France; 2CHU Morvan, Brest, France; 3CH Yves-Le-Foll, Saint-Brieuc, France; 4Hôpital d’Instruction des Armées Percy, Clamart, France; 5Centre François-Baclesse, Caen, France; 6Centre Catalan d’Oncologie, Perpignan, France; 7CH Départemental Vendée, Les Oudairies, La Roche-Sur-Yon, France; 8CH du Pays d’Aix, Aix-En-Provence, France; 9Hôpital Quesnay, Mantes-La-Jolie, France; 10Institut Paoli-Calmettes, Marseille, France; 11CH René-Dubos, Cergy-Pontoise, France; 12CH Laennec, Creil, France; 13CH Intercommunal (CHI) Des Alpes-Du-Sud, Gap, France; 14Hôpital d’Instruction Des Armées Sainte-Anne, Toulon, France; 15CHI Créteil, Créteil, France
Correspondence: Alain Vergnenègre
Unité d’Oncologie Thoracique et Cutanée, CHU de Limoges Hôpital Dupuytren, 2, Avenue Martin-Luther-King, Limoges Cedex 87042, France
Tel +33 5 55 05 62 13
Fax +33 5 55 05 64 17
Email [email protected]
Background: Antiangiogenic agents have improved the prognosis of non-squamous non-small–cell lung cancers (NSCLCs), even though all the patients are not eligible to receive them because of counterindications linked to the tumor’s characteristics or comorbidities. Much less information is available about the eligibility of patients with squamous non-small–cell lung cancers (SQ-NSCLCs) to receive antivascular endothelial growth-factor (VEGF) treatments, even though such molecules are being developed for this histology. This study was undertaken to determine the percentage of advanced SQ-NSCLC patients who would be eligible to receive an antiVEGF agent as second-line systemic therapy.
Methods: This observational, multicenter, prospective study evaluated advanced SQ-NSCLC patients’ criteria for ineligibility to receive an antiVEGF during a multidisciplinary meeting to choose their standard second-line systemic therapy.
Results: Among the 317 patients included, 53.6% had at least one ineligibility criterion, and ∼20% had at least two, with disease extension to large vessels (39.8%), tumor cavitation (20.5%), cardiovascular disease (11%) and/or hemoptysis (7.2%) being the most frequent. Patients with an ECOG performance score of 1/2 had more cardiovascular contraindications that those with scores of 0.
Conclusion: Almost half of the SQ-NSCLC patients included in this study would have been eligible to receive an antiVEGF agent. The development of these molecules for these indications should be encouraged.
Keywords: lung cancer, squamous non-small cell, antiangiogenic treatments
Introduction
Lung cancer is the first cause of cancer deaths of men and women in the United States,1 with a 5-year survival rate of ~16%.2,3 Lung cancers are separated into two major categories based on histology, clinical management and prognosis: non-small–cell lung cancer (NSCLC) and small-cell lung cancer (SCLC).3 NSCLCs represent more than 85% of these tumors.4 Its two major histologies are non-squamous and squamous (SQ) carcinomas, with the latter representing 30% of NSCLCs.4 NSCLC outcomes changed remarkably during the early 2000s, particularly for advanced lung adenocarcinomas.4 Those changes reflect the development of new agents devoted to specific oncological drivers: inhibitors of epidermal growth factor-receptor (EGFR), anaplastic lymphoma kinase (ALK) and vascular endothelial growth factor (VEGF), and finally immunotherapy.5,6 However, median survival time was not prolonged for SQ-NSCLCs.7 The difference between the two subtypes may be due to a modest effect against SQ-NSCLCs of the agents used to treat adenocarcinomas.8,9 Therefore, immune-checkpoint inhibitors (ICIs) for SQ-NSCLCs, developed after those for non-squamous NSCLCs, could modify their prognoses.10
Because angiogenesis is a pejorative factor for several tumors, inhibiting proangiogenic factors represents a potential avenue for therapeutic development.9 While the role of VEGF in angiogenesis is well established,9,11,12 studies on SQ-NSCLCs have been limited9,11–13 by concerns about life-threatening pulmonary hemorrhage14,15 and guidelines excluded these patients from the indication.16
Bevacizumab (BVZ) was the first agent targeting VEGF to prolong survival when combined with chemotherapy for selected NSCLC patients.6,14 Despite BVZ’s demonstrated efficacy in phase II and III trials on NSCLC patients,5,9 adverse events like significant bleeding, including major hemoptysis, delayed its development for SQ-NSCLC patients.15,16
Tolerability of BVZ in combination with chemotherapy was established in a phase I trial on all NSCLC subtypes.17 In an early phase II trial of BVZ for NSCLC patients,18 among six patients experiencing life-threatening pulmonary hemorrhages, four had SQ-NSCLCs; four of the six patients died. Pertinently, all six patients had centrally located tumors close to major blood vessels and five had cavitation or necrosis. Results of observational studies confirmed BVZ safety11,12 and excluded certain initial contraindications, like brain metastases. Multiple trials have evaluated BVZ as second-line therapy. In the phase III ULTIMATE trial,19 166 patients with advanced NSCLCs progressing after first- or second-line therapy were randomized to receive weekly the paclitaxel–BVZ combination compared to docetaxel; progression-free survival (PFS) was significantly longer for the former group but overall survival (OS) was comparable for the two groups.
New agents with antiVEGF activity have been developed for SQ-NSCLCs.20 A phase III trial that included 1253 randomized patients (all NSCLC histology, 25% SQ-NSCLCs) compared docetaxel (75 mg/m2) in combination with ramucirumab (10 mg/kg) or placebo.21 Ramucirumab adjunction to docetaxel was associated with significantly prolonged PFS and OS. That OS benefit was also retained for the SQ-NSCLC subgroup (respective median OS, 9.5 vs 8.2 months).22 Those results led to the US Food and Drug Administration and European Medicines Agency approvals of ramucirumab for both NSCLC histologies.
Nintedanib, a multitarget antiangiogenic agent, was evaluated in combination with docetaxel in a large phase III randomized trial,9,23 comparing docetaxel to placebo for all NSCLC histological subtypes. Significantly improved OS rates were obtained for patients randomized to receive docetaxel and nintedanib vs placebo: those whose adenocarcinomas progressed within medians of 10.9 vs 7.9 months, respectively, and for the entire adenocarcinoma subset (12.6 vs 10.3 months). However, the entire study population did not benefit from OS prolongation. The European Medicines Agency—but neither the US Food and Drug Administration nor Health Canada—approved nintedanib to treat non-squamous NSCLCs.
However, few real-life data from SQ-NSCLC patients are available.
This study was undertaken to assess prospectively the clinical and radiological characteristics of advanced SQ-NSCLC patients about to receive second-line therapy to determine the percentage of them who would have been eligible to receive antiVEGF therapy.
Methods
This observational, multicenter, prospective study included consecutive advanced SQ-NSCLC patients >18 years old, whose disease progressed after first-line chemotherapy, and evaluated their criteria rendering them ineligible to receive an antiangiogenic treatment. That evaluation was carried out in each center, at the time of multidisciplinary meetings to choose their standard second-line systemic regimen.
The following information was collected: Eastern Cooperative Oncology Group performance status (ECOG PS); lung cancer characteristics: histology, TNM grade, stage, number and type of metastases; and first- and second-line treatments.
The criteria retained for ineligibility to receive an antiVEGF were: tumor with central cavitation; tumor extension to a large vessel, with a 180° branching angle; hemoptysis >3 mL during the previous 3 months; prior thromboembolic events, myocardial infarction, unstable angina, stroke, transient ischemic attack during the preceding 6 months; uncontrolled hypertension >150/90 mm Hg; grade-3/4 hemorrhagic disorder, vasculitis or gastrointestinal bleeding; gastrointestinal perforation or digestive tract fistula during the previous 6 months; inflammatory bowel disease, digestive tract obstructions or intestinal resection, Crohn’s disease, ulcerative colitis or chronic diarrhea.
Data were analyzed with Excel software (version 16.16.10, Microsoft 2018).
The Ethics Committee of Limoges University Hospital approved the protocol for this observational study on December 12, 2015. All participants provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Results
From July 2016 to July 2017, 40 centers included 317 patients: 256 (80.8%) men; most in good general condition (ECOG PS 0/1: 82.0%); only 3.5% were non-smokers; and 65.3% had metastatic SQ-NSCLCs at diagnosis (Table 1). All patients had received first-line chemotherapy. As second-line therapy, 82% received chemotherapy, 7% were given a tyrosine-kinase inhibitor and radiotherapy was prescribed for 11%.
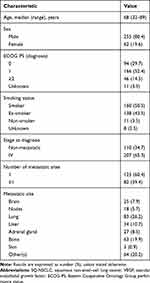 |
Table 1 Characteristics of the 317 Advanced SQ-NSCLC Patients Evaluated for Eligibility to Receive Anti-VEGF Therapy |
The VEGF-eligibility evaluation found that 53.6% of the patients had an ineligibility criterion and 20% had at least two (Figure 1, Table 2).
 |
Figure 1 Flow chart of the study. Patients could have more than 1 ineligibility criterion. |
 |
Table 2 Reasons for Ineligibility of Advanced SQ-NSCLC Patients to Receive Antiangiogenics* |
Table 3 reports the distributions of certain characteristics of these SQ-NSCLC patients, according to the main antiVEGF ineligibility criteria: tumor cavitation, extension to large vessels, hemoptysis, cardiovascular diseases.
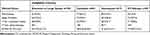 |
Table 3 Percentages of Clinical Factors as a Function of Ineligibility Criteria |
Discussion
The results of this novel, observational evaluation of previously unassessed eligibility of advanced SQ-NSCLC patients to receive a second-line antiangiogenic agent showed that slightly less than half were eligible. Based on the more abundant data available on non-squamous NSCLC patients, about 20–30% of them were ineligible.4,6 No study on such patients has yet been published. Our results and conclusions have to be confirmed by prospective studies.
According to the rates of central lesions and large-vessel extensions, it is not surprising that a higher percentage of these patients were ineligible. In the REVEL trial,22 219 (26.6%) of the 825 screened patients did not meet inclusion criteria, knowing that patients with ECOG PS=2 and those with brain metastases were excluded; only 13% had large-vessel involvement and 10% had comorbidities. To help guide physicians’ use of BVZ to treat NSCLC patients, an expert panel reviewed the available data to identify factors predictive of pulmonary hemoptysis and concluded that large blood-vessel infiltration might do so; however, no consensus has been established to define radiological infiltration. Eligibility for BVZ use is not affected by patient age, ECOG PS, or anticoagulation or antiplatelet therapy. All NSCLC patients for whom antiVEGF therapy is being considered should undergo individualized risk-benefit assessments.24
This study had several limitations. First, it was observational. It also relied on multidisciplinary meetings in each center and the usual practices of the radiologists and investigators in each center, which could have engendered some heterogeneity and modified the final results. However, the findings confirmed that almost one out of two advanced SQ-NSCLC patients was, indeed, eligible for antiVEGF therapy. Notably, eligibility evaluation is often difficult and observer-dependent, especially for the radiological criteria of vessel invasion of tumors. Those criteria were assessed by chest computed-tomography scans for eligibility to receive BVZ in an analysis of NSCLC patients with centrally located tumors;25 discordance for eligibility was found for 55% patients. While interobserver strength of agreement was fair-to-moderate (mean kappa: 0.40), intraobserver strength of agreement was good-to-very-good (mean kappa: 0.74). Multivariate analysis retained the risk of discrepancy as essentially reflecting the assessment of contact between the tumor and vessels. Second, we did not collect the outcomes, particularly survival, as it was not the objective of the study.
Literature data are very scarce. The large majority of papers are limited to non-squamous NSCLCs.26 Two recent studies reported real-life information about patients treated with angiogenesis inhibitors. For Nadler et al,27 13% received first-line BVZ, but none for the second line. For Armochalam et al,28 in a large study (n=2899), BVZ was used only as second-line therapy for non-squamous NSCLC patients: BVZ alone for 2.7%, combined with pemetrexed or docetaxel for 4.8%, a platin doublet for 6.2%, or carboplatin–paclitaxel–atezolizumab for 6.9%.
The risk of bleeding did not stop further investigation of an antiangiogenic effect in SQ-NCLC patients.29–32 A phase II trial on SQ-NSCLC patients evaluated targeting the VEGF pathway with axitinib, a novel pan-VEGF-receptor (R) inhibitor (e.g., inhibitor of all three: VEGFR-1, −2 and −3) and compared it to standard first-line cisplatin–gemcitabine regimen.24 Experimental arm patients reached a median PFS of 6.2 months and a median OS of 14.2 months. The most frequent grade-≥3 toxicities were neutropenia (13.2%) and hypertension (13.2%), with only three (7.9%) patients experiencing hemoptysis, which was fatal for one (2.6%).30
More recently, several studies used a strategy to limit and closely monitor toxicity, and reexamined the potential of BVZ to treat SQ-NSCLCs. In the BRIDGE trial,33 a new, sequential administration regimen of chemotherapy and BVZ was applied in an attempt to minimize BVZ toxicity in SQ-NSCLC patients. In that study, patients received two carboplatin–paclitaxel cycles, followed by that combination and BVZ for cycles 3–6, then BVZ maintenance alone, until progression or toxicity. Grade-3 pulmonary hemorrhage occurred in 1/31 (3.2% [95% confidence interval, 0.3–13.5%]) patients; PFS was 6.2 months. While the pulmonary hemorrhage rate was lower than that reported in the phase II study described above,18 using BVZ to treat SQ-NSCLCs remains investigational.34,35
That positive outcome encourages continuing studies on SQ-NSCLC patients and their combination in the near future with immune-checkpoint inhibitors ICIs.36 ICIs will modify the use of these molecules. Hakozaki et al37 showed that BVZ could increase the eligibility rate by 20% (paclitaxel–carboplatin–atezomizumab–BVZ combination) for EGFR-mutated NSCLCs that had received first-line tyrosine-kinase inhibitors. Angiogenesis inhibitors could have an immunosuppressive action;38 in a small number of patients (16 adenocarcinomas and 3 SQ-NSCLCs), the disease-control rate was 90% with the ramucirumab–docetaxel combination after nivolumab. BVZ and other antiangiogenics could be included in the global therapeutic strategy for metastatic SQ-NSCLCs.
Abbreviations
NSCLC, non-small–cell lung cancer; SQ-NSCLC, squamous NSCLC; BVZ, bevacizumab; VEGF, vascular endothelial growth factor; EGFR, endothelial growth-factor receptor; ECOG PS, Eastern Cooperative Oncology Group performance status.
Acknowledgments
This paper was presented in part at Toronto WCLC 2018 and the abstract was published: A Vergnenègre et al J Thor Oncol. 2018;13:S828-S829. DOI: https://doi.org/10.1016/j.jtho.2018.08.1471.
Funding
Supported by an academic grant from Lilly Pharmaceuticals.
Disclosure
A. Vergnenègre has received honoraria for consultancies and fees for medical conferences from MSD, Hoffman–La Roche, BMS, Pierre-Fabre Oncology, AstraZeneca, and Boehringer Ingelheim. Dr Olivier Bylicki reports personal fees from MSD, personal fees from ROCHE, personal fees from ASTRA-ZENECA, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. American Cancer Society. Cancer Facts & Figures. Vol. 1; 2019:219
2. Ferrara R, Mezquita L, Besse B. Progress in the management of advanced thoracic malignancies in 2017. J Thorac Oncol. 2018;13(3):301–322. doi:10.1016/j.jtho.2018.01.002
3. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi:10.1001/jama.2014.3741
4. Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1416–1423. doi:10.1097/JTO.0b013e3181da36f4
5. Zhang H, Huang Z, Zou X, et al. Bevacizumab and wound-healing complications: a systematic review and meta-analysis of randomized controlled trials. Oncotarget. 2016;7(50):82473–82481. doi:10.18632/oncotarget.12666
6. Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. doi:10.1200/JCO.2007.14.5466
7. Tsironis G, Ziogas DC, Kyriazoglou A, et al. Breakthroughs in the treatment of advanced squamous-cell NSCLC: not the neglected sibling anymore? Ann Transl Med. 2018;6(8):143. doi:10.21037/atm2018.02.18
8. Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25(5):1044–1052. doi:10.1093/annonc/mdu098
9. Alshangiti A, Chandhoke G, Ellis PM. Antiangiogenic therapies in non-small-cell lung cancer. Curr Oncol. 2018;25(Suppl 1):S45–S58. doi:10.3747/co.25.3747
10. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous Non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
11. Crino L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol. 2010;11(8):733–740. doi:10.1016/S1470-2045(10)70151-0
12. Lynch TJ
13. Piperdi B, Merla A, Perez-Soler R. Targeting angiogenesis in squamous non-small cell lung cancer. Drugs. 2014;74(4):403–413. doi:10.1007/s40265-014-0182-z
14. Sheng J, Yang Y, Ma Y, et al. The efficacy of combining antiangiogenic agents with chemotherapy for patients with advanced non-small cell lung cancer who failed first-line chemotherapy: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0127306. doi:10.1371/journal.pone.0127306
15. Clement-Duchene C, Godbert B, Martinet Y. Anti-angiogenic agents in the treatment of lung cancer: indications and toxicities. Rev Mal Respir. 2012;29(2):161–177. doi:10.1016/j.rmr.2011.06.017
16. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(S4):iv192–iv237.
17. Margolin K, Gordon MS, Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol. 2001;19(3):851–856. doi:10.1200/JCO.2001.19.3.851
18. Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–2191. doi:10.1200/JCO.2004.11.022
19. Cortot A, Audigier-Valette C, Molinier O, et al. Weekly paclitaxel plus bevacizumab versus docetaxel as second or third-line treatment in advanced non-squamous non-small cell lung cancer (NSCLC): results from the phase III study IFCT-1103 ULTIMATE. J Clin Oncol. 2016;34(15):9005. doi:10.1200/JCO.2016.34.15_suppl.9005
20. Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763–774. doi:10.1016/S1470-2045(15)00021-2
21. Reck M, Paz-Ares L, Bidoli P, et al. Outcomes in patients with aggressive or refractory disease from REVEL: a randomized phase III study of docetaxel with ramucirumab or placebo for second-line treatment of stage IV non-small-cell lung cancer. Lung Cancer. 2017;112(11):181–187. doi:10.1016/j.lungcan.2017.07.038
22. Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi:10.1016/S0140-6736(14)60845-X
23. Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–155. doi:10.1016/S1470-2045(13)70586-2
24. Reck M, Barlesi F, Crino L, et al. Predicting and managing the risk of pulmonary haemorrhage in patients with NSCLC treated with bevacizumab: a consensus report from a panel of experts. Ann Oncol. 2012;23(5):1111–1120. doi:10.1093/annonc/mdr463
25. Barlesi F, Balleyguier C, Besse B, et al. Inter- and intraobserver consistency in assessing eligibility for bevacizumab (BVZ) in non-small-cell lung cancer (NSCLC) patients with centrally located tumors. Ann Oncol. 2010;21(8):1682–1686. doi:10.1093/annonc/mdp590
26. Lunacsek OE, Ravelo A, Coutinho AD, et al. First line treatment with bevacizumab and platinum doublet combination in non squamous non small cell lung cancer: a retrospective cohort study in US oncology community practices. Real World Outcomes. 2016;3(3):333–343. doi:10.1007/s40801-016-0090-5
27. Nadler E, Forsyth M, Satram-Hoang S, Reye C. Costs and clinical outcomes among patients with second line non small cell lung cancer in the outpatient community setting. J Thorac Oncol. 2012;7(1):212–218. doi:10.1097/JTO.0b013e3182307f33
28. Arunachalam A, Li H, Bittoni MA, et al. Real world treatment patterns, overall survival and occurrence and costs of adverse events associated with second line therapies for Medicare patients with advanced non small cell lung cancer. Clin Lung Cancer. 2018;19(5):e783–e799. doi:10.1016/j.cllc.2018.05.016
29. Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol. 2012;30(25):3084–3092. doi:10.1200/JCO.2011.39.7646
30. Bondarenko IM, Ingrosso A, Bycott P, Kim S, Cebotaru CL. Phase II study of axitinib with doublet chemotherapy in patients with advanced squamous non-small-cell lung cancer. BMC Cancer. 2015;15:339. doi:10.1186/s12885-015-1350-6
31. Heist RS, Wang X, Hodgson L, et al. CALGB 30704 (Alliance): a randomized phase II study to assess the efficacy of pemetrexed or sunitinib or pemetrexed plus sunitinib in the second-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9(2):214–221. doi:10.1097/JTO.0000000000000071
32. Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619–626. doi:10.1016/S1470-2045(10)70132-7
33. Hainsworth JD, Fang L, Huang JE, et al. BRIDGE: an open-label phase II trial evaluating the safety of bevacizumab + carboplatin/paclitaxel as first-line treatment for patients with advanced, previously untreated, squamous non-small cell lung cancer. J Thorac Oncol. 2011;6(1):109–114. doi:10.1097/JTO.0b013e3181f94ad4
34. Assoun S, Brosseau S, Steinmetz C, Gounant V, Zalcman G. Bevacizumab in advanced lung cancer: state of the art. Future Oncol. 2017;13(28):2515–2535. doi:10.2217/fon-2017-0302
35. Russo AE, Priolo D, Antonelli G, et al. Bevacizumab in the treatment of NSCLC: patient selection and perspectives. Lung Cancer (Auckl). 2017;8(12):259–269. doi:10.2147/LCTT.S110306
36. Das M, Wakelee H. Targeting VEGF in lung cancer. Expert Opin Ther Targets. 2012;16(4):395–406. doi:10.1517/14728222.2012.669752
37. Hakozaki T, Okuma Y, Hashimoto K, Hosomi Y. Correlation between the qualification for bevacizumab use and the survival of patients with non-small cell lung cancer harboring the epidermal growth factor receptor mutation: a retrospective analysis. J Cancer Res Clin Oncol. 2019;145(10):2555–2564. doi:10.1007/s00432-019-02985-1.
38. Malapelle U, Rossi A. Emerging angiogenesis inhibitors for non-small cell lung cancer. Expert Opin Emerg Drugs. 2019;24(2):71–81. doi:10.1080/14728214.2019.1619696.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
