Back to Journals » International Medical Case Reports Journal » Volume 15
Postoperative Intracardiac Thrombus in a Child with Nephrotic Syndrome
Authors Maghrabi K , Aldhaheri R , Aljunaid N, Alzahrani AS, Alsayyad HM, Kalakattawi N, Safdar O
Received 27 November 2021
Accepted for publication 15 February 2022
Published 10 March 2022 Volume 2022:15 Pages 91—96
DOI https://doi.org/10.2147/IMCRJ.S349740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Supplementary video 1 of "Postoperative intracardiac thrombus in a child" [ID 349740].
Views: 180
Khadijah Maghrabi,1 Ruba Aldhaheri,2 Nora Aljunaid,2 Amal Saeed Alzahrani,2 Hanan Mohammad Alsayyad,2 Nada Kalakattawi,2 Osama Safdar2
1Abdullah Bakhsh Children’s Heart Center, Department of Pediatrics, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 2Department of Pediatrics, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence: Khadijah Maghrabi, Abdullah Bakhsh Children’s Heart Center, Department of Pediatrics, Faculty of Medicine, King Abdulaziz University, PO Box 80215, Jeddah, 21589, Saudi Arabia, Tel +996 2 6401000 Ext. 20208, Email [email protected]
Background: Thromboembolic events are a known complication of nephrotic syndrome in children. Intracardiac thrombi are a rare location for thrombus formation but can lead to severe clinical consequences.
Case Presentation: We report an intracardiac left atrial thrombus in a child with new onset steroid-resistant nephrotic syndrome and recently repaired atrial septal defect and cor triatriatum. The thrombus was successfully treated with surgical excision.
Conclusion: Intracardiac thrombus is a rare but serious complication of nephrotic syndrome in children, especially in children with risk factors for potentially increased intracardiac thrombogenicity such as history of recent intervention.
Keywords: nephrotic, pediatric, congenital heart disease, cardiac surgery, intracardiac thrombus
Introduction
Nephrotic syndrome is defined by nephrotic-range proteinuria (urine protein/creatinine ratio ≥200 mg/mg creatinine), hypoalbuminemia (<25 g/L), periorbital or pitting edema and hyperlipidemia.1,2 It is often associated with thromboembolism and increased risk of infection. The most common cause of nephrotic syndrome in children is minimal change glomerulonephritis.2 Most of the patients respond to corticosteroids, although some patients experience disease relapse. In those patients, immunosuppressive medications are used to achieve remission and prevent relapse in corticosteroid-resistant disease.3
Complications of nephrotic syndrome can be divided into two categories: disease-associated and drug-related complications. Disease-associated complications include infections, thromboembolism, hypovolemic crisis, cardiovascular problems, acute renal failure, anemia, and others.4 One of the most serious complications in nephrotic syndrome is thromboembolic events.5 The incidence of thromboembolic complications is about 3% among children.6
The pathophysiology of thromboembolic disease in patients with nephrotic syndrome is thrombophilic tendency such as loss of coagulation factor; (protein S, antithrombin III, plasminogen, and plasmin), treatment-related hazards, and disease-related hypercoagulopathy.7 The most common sites is renal-vein thrombosis which affects 20–30% of adult patients who have membranous glomerulonephritis.5 Deep vein thrombosis and pulmonary embolism are also common,8 although less so in children. The most concerning thromboembolic complication is intracardiac thrombus formation which has high morbidity and mortality, although it is considered to be significantly infrequent.9–11
We present a case of intracardiac thrombus in a child after surgical repair of congenital heart disease.
Case Presentation
A 2-year-old boy was diagnosed with secundum atrial septal defect (ASD) and cor-triatriatum upon evaluation of an incidental murmur. He underwent surgical repair shortly after diagnosis (on 17/12/2019) and had a smooth postoperative course. 4 days after discharge, the patient presented to emergency department with generalized body swelling and facial puffiness, he had gained 2Kg since discharge, it was associated with hematuria and oliguria. On examination, the patient looked edematous with bilateral pitting edema, his weight was 9.67 kg, height 73 cm, head circumference 41cm, all growth parameters were below 3rd centile. His vital signs were as follows: blood pressure 115/67mmHg, heart rate 131 beats/minute, respiratory rate 35 breaths/minute, oxygen saturation 100% on room air, temperature 37C. Cardiovascular examination showed midline sternotomy scar, no visible pulsation, apex beat on the 4th intercostal space, normal heart sounds with no added sound and no murmur. Abdominal examination showed distended abdomen with abdominal girth 50 cm (2 cm above umbilicus), positive shifting dullness, no hepatosplenomegaly with bilateral ballotable kidneys. Other systemic examinations were unremarkable. The results of laboratory investigations are detailed in Tables 1–3.
 |
Table 1 Laboratory Investigations at the Time of Admission |
 |
Table 2 Accumulative Results of Platelet Count (Pre- and Post-Operative) |
 |
Table 3 Accumulative Results of Albumin (Preoperative) |
Abdomen and pelvis ultrasound were done and showed increased renal echogenicity and mild ascites. The patient was started on Prednisolone 60mg/m2/day and Hydralazine as needed. The patient continued to have worsening edema and respiratory distress, and he was started on regular Albumin infusion. During his hospital stay he was treated for Acinetobacter sepsis. 10 days from admission the patient continued to show progressive generalized edema and started to develop respiratory distress requiring non-invasive respiratory support. Transthoracic echocardiography showed intact atrial septum with no residual shunt. However, there was an atrial mass measuring 17 mm x 7 mm attached to the left atrial side of the interatrial septum, normal left ventricular (LV) systolic function (Figure 1, Supplementary Videos 1 and 2). Electrocardiogram showed normal sinus rhythm with sinus arrhythmia, and incomplete right bundle branch block.
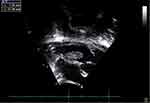 |
Figure 1 Echocardiographic measures of the intracardiac thrombus. |
The patient was started on heparin infusion and 3 days later he underwent surgical resection of the atrial mass. Histopathology revealed fibrocollagenous tissue with myxoid changes and foci of ulceration, acute and chronic cells’ infiltration with foreign body giant cell reaction. The patient had a smooth postoperative course with no complications. Given the positive blood culture on admission, he was treated with IV antibiotics for a total of 6 weeks as is recommended for infective endocarditis. He was also maintained on low molecular weight heparin injections for 6 months. As noted from Table 2, his platelet counts gradually decreased to normal levels with no intervention. No anti-platelet treatment was given.
From a renal perspective, he was started on cyclosporine and pulse prednisolone. He achieved complete remission within 72 hours and was discharged on cyclosporine and prednisone. He was admitted several times with relapses and renal biopsy showed focal segmental glomerulosclerosis.
Discussion
The hypercoagulable state in NS is multifactorial, attributed mainly to profound abnormalities of the coagulation inhibitors eg, antithrombin III (ATIII) deficiency, the increase in protein C and protein S, fibrinolytic system, platelet hyper aggregation, altered endothelial-cell function and hyper viscosity of blood.5,12–16 Other factors include associated infections and hypovolemia due to inappropriate use of diuretics therapy, and immobilization.15–18 Other studies showed that corticosteroids play a major role in this type of hypercoagulability.5,12
On the molecular level, several changes in NS lead to enhanced thrombogenicity. The loss of protein in the urine and low albumin level increase hepatic fibrinogen synthesis.19 Fibrinogen in turn enhances platelet activity and aggregation of admission. Hypoalbuminemia increases thromboxane A2 synthesis, which stimulates platelet red blood cells, increased in patients with nephrotic syndrome. These patients also have higher levels of adhesiveness.19–21 Moreover, factor V Leiden deficiency plays an important part in the thrombogenic nature of the disease, due to the leakage of high-molecular-weight proteins such as albumin through the polymorphisms associated with the prothrombotic state or other unknown genetic predispositions, also the primary defect in the glomeruli causes massive proteinuria.19–21 Furthermore, platelet hyperactivity is enhanced by hypercholesterolemia and factor V, factor VII, and alpha-2 macroglobulin, which promote thrombus formation.19 Increased levels of Von Willebrand factor promotes platelet adhesion. Fibrinolysis is also compromised by urinary loss of plasmin.20 A combination of these factors increases the levels of Von Willebrand factor, which promotes platelet adhesion, as well as hypovolemia resulting in increased incidence of thromboembolic events in patients with nephrotic syndrome.22
In the case we presented here, several factors effected the development of an intracardiac thrombus. Heavy proteinuria in the context of nephrotic syndrome is the main factor. However, the presence of high platelet count upon admission, steroid use also contributed to a hypercoagulable state. Moreover, the endothelial injury of surgical intervention at the interatrial septum no doubt acted as nidus for intracardiac thrombus formation. The combination of these factors resulted in this rare presentation in our patient.
In a review of literature, the incidence of nephrotic syndrome-associated thromboembolism shows a significant difference in NS-associated thromboembolism between children and adults, the incidence of thromboembolic complications is much more common in adults with NS by 26.7% compared to children, 2.8%.22 In another study conducted in 1986, it was revealed that children with steroid-resistant nephrotic syndrome have a higher incidence of thromboembolic complications (by 3.8%) than those with steroid-sensitive nephrotic syndrome (1.5%).15 One of the most serious forms of venous thromboembolism is acute pulmonary embolism; however, there are few reports or studies of pulmonary embolism affecting young adults and children.23
Intracardiac thrombus has been infrequently identified in children.24,25 The majority of thromboembolic incidents in patients with nephrotic syndrome were treated with anticoagulants including heparin or warfarin and were treated conservatively.12,25,26 In other studies, patients with nephrotic syndrome associated with intracardiac thrombus were treated with surgical removal of the thrombus along with anticoagulation due to the characteristic high risk of the thrombus.11,24 These case reports in our literature are summarized in Table 4. Our patient had a large freely mobile thrombus, attached to the left side of the interatrial septum. Due to high risk of detachment and embolization leading to stroke or sudden death, it was decided that surgical resection was needed.
 |
Table 4 Reported Cases of Intracardiac Thrombi in Children with NS |
Compliance with Ethical Standards
Institutional approval: is not required for publishing case reports.
Informed Consent
Written informed consent was obtained from the patient’s father (legal guardian) for publication of this case report and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Downie ML, Gallibois C, Parekh RS, Noone DG. Nephrotic syndrome in infants and children: pathophysiology and management. Paediatr Int Child Health. 2017;37(4):248–258. doi:10.1080/20469047.2017.1374003
2. McCloskey O, Maxwell AP. Diagnosis and management of nephrotic syndrome. Practitioner. 2017;261(1801):11–15.
3. Wang CS, Greenbaum LA. Nephrotic syndrome. Pediatr Clin North Am. 2019;66(1):73–85. doi:10.1016/j.pcl.2018.08.006
4. Park SJ, Shin JI. Complications of nephrotic syndrome. Korean J Pediatr. 2011;54(8):322–328. doi:10.3345/kjp.2011.54.8.322
5. Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338(17):1202–1211. doi:10.1056/NEJM199804233381707
6. Suri D, Ahluwalia J, Saxena AK, et al. Thromboembolic complications in childhood nephrotic syndrome: a clinical profile. Clin Exp Nephrol. 2014;18(5):803–813. doi:10.1007/s10157-013-0917-2
7. Kerlin BA, Haworth K, Smoyer WE. Venous thromboembolism in pediatric nephrotic syndrome. Pediatr Nephrol. 2013;29(6):989–997. doi:10.1007/s00467-013-2525-5
8. Bellomo R, Atkins RC. Membranous nephropathy and thromboembolism: is prophylactic anticoagulation warranted? Nephron. 1993;63(3):249–254. doi:10.1159/000187205
9. Weisz W, Kemper MJ, Weil J, Müller-Wiefel DE. Asymptomatic intracardiac thrombus in steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2002;17(4):287–289. doi:10.1007/s00467-002-0831-4
10. Skalova S, Lukes A, Vanicek H, et al. Intracardiac thrombus–a rare complication of the steroid resistant nephrotic syndrome. Bratisl Lek Listy. 2008;109(12):573–575.
11. Ueno K, Nagasako H, Ueno M, et al. Large intracardiac thrombus in a child with refractory nephrotic syndrome. Pediatr Int. 2010;52(1):e51–e53. doi:10.1111/j.1442-200X.2009.02991.x
12. Citak A, Emre S, Sâirin A, Bilge I, Nayir A. Hemostatic problems and thromboembolic complications in nephrotic children. Pediatr Nephrol. 2000;14(2):138–142. doi:10.1007/s004670050029
13. Lilova MI, Velkovski IG, Topalov IB. Thromboembolic complications in children with nephrotic syndrome in Bulgaria (1974–1996). Pediatr Nephrol. 2000;15(1–2):74–78. doi:10.1007/s004679900253
14. Sagripanti A, Barsotti G. Hypercoagulability, intraglomerular coagulation, and thromboembolism in nephrotic syndrome. Nephron. 1995;70(3):271–281. doi:10.1159/000188604
15. Hoyer PF, Gonda S, Barthels M, Krohn HP, Brodehl J. Thromboembolic complications in children with nephrotic syndrome. Risk and incidence. Acta Paediatr Scand. 1986;75(5):804–810. doi:10.1111/j.1651-2227.1986.tb10294.x
16. Fabri D, Belangero VM, Annichino-Bizzacchi JM, Arruda VR. Inherited risk factors for thrombophilia in children with nephrotic syndrome. Eur J Pediatr. 1998;157(11):939–942. doi:10.1007/s004310050972
17. Martínez Ara J, Gómez Rioja R, Riñón C, García Muñoz MS, Ruiz Caravaca ML, Miguel JL. Prevalence of genetic prothrombotic factors (factor V Leiden and II20210 prothrombin mutation) in glomerular nephropathies with or without thrombosis. Nefrologia. 2000;20(2):139–144.
18. Schöning M, Klein R, Krägeloh-Mann I, et al. Antiphospholipid antibodies in cerebrovascular ischemia and stroke in childhood. Neuropediatrics. 1994;25(1):8–14. doi:10.1055/s-2008-1071574
19. Mirrakhimov AE, Ali AM, Barbaryan A, Prueksaritanond S, Hussain N. Primary nephrotic syndrome in adults as a risk factor for pulmonary embolism: an up-to-date review of the literature. Int J Nephrol. 2014;2014:916760. doi:10.1155/2014/916760
20. Gigante A, Barbano B, Sardo L, et al. Hypercoagulability and nephrotic syndrome. Curr Vasc Pharmacol. 2014;12(3):512–517. doi:10.2174/157016111203140518172048
21. Fahal IH, McClelland P, Hay CR, Bell GM. Arterial thrombosis in the nephrotic syndrome. Postgrad Med J. 1994;70(830):905–909. doi:10.1136/pgmj.70.830.905
22. Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome–associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7(3):513–520. doi:10.2215/CJN.10131011
23. Song Z, Wu H, Cao H, Tang M, Yang S, Qin L. Nephrotic syndrome with acute pulmonary embolism in young adults: two case reports. Medicine. 2018;97(29):e11495. doi:10.1097/MD.0000000000011495
24. Mortazavi F, Samadi M. Asymptomatic intracardiac thrombus in a child with nephrotic syndrome. Arch Iran Med. 2006;9(4):426–428.
25. Lin CC, Lui CC, Tain YL. Thalamic stroke secondary to straight sinus thrombosis in a nephrotic child. Pediatr Nephrol. 2002;17(3):184–186. doi:10.1007/s00467-001-0785-y
26. Divekar AA, Ali US, Ronghe MD, Singh AR, Dalvi RB. Superior sagittal sinus thrombosis in a child with nephrotic syndrome. Pediatr Nephrol. 1996;10(2):206–207. doi:10.1007/BF00862081
27. Ekici F, Çakar N. A large intracardiac thrombus in a child with steroid-resistant nephrotic syndrome. Cardiol Young. 2013;23(3):440–442. doi:10.1017/S1047951112000923
28. Tavil B, Kara F, Topaloglu R, et al. Case series of thromboembolic complications in childhood nephrotic syndrome: Hacettepe experience. Clin Exp Nephrol. 2015;19(3):506–513. doi:10.1007/s10157-014-1005-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
