Back to Journals » OncoTargets and Therapy » Volume 11
Postmastectomy radiotherapy reduces locoregional and disease recurrence in patients with stage II–III triple-negative breast cancer treated with neoadjuvant chemotherapy and mastectomy
Authors Chen XX, Xia F, Luo JR, Ma JL, Yang ZZ, Zhang L, Feng Y, Shao ZM, Yu XL, Guo XM
Received 30 November 2017
Accepted for publication 10 February 2018
Published 5 April 2018 Volume 2018:11 Pages 1973—1980
DOI https://doi.org/10.2147/OTT.S158482
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Carlos E Vigil
Xingxing Chen,1,2,* Fan Xia,1,2,* Jurui Luo,1,2,* Jinli Ma,1,2 Zhaozhi Yang,1,2 Li Zhang,1,2 Yan Feng,1,2 Zhimin Shao,2,3 Xiaoli Yu,1,2 Xiaomao Guo1,2
1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China; 3Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
*These authors contributed equally to this work
Background: This study investigated the effect of postmastectomy radiotherapy (PMRT) in patients with stage II–III triple-negative breast cancer (TNBC) after neoadjuvant chemotherapy (NAC) and modified radical mastectomy (MRM).
Patients and methods: A total of 104 women with stage II–III TNBC who received NAC and MRM at our institution between January 2000 and July 2007 were identified. Patients were divided into 2 groups (PMRT and non-PMRT) for statistical analysis.
Results: The median follow-up time was 64 months (range 12–123 months). The 5 year cumulative locoregional recurrence (LRR) and disease recurrence (DR) rates were 26.5% and 49.6%, respectively. Despite their more adverse prognostic features, patients with PMRT had lower 5 year cumulative LRR and DR rates than those without PMRT (LRR: 18.3% vs 52.2%, respectively, p=0.0005; DR: 45% vs 69.1%, p=0.0334, respectively). On multivariate analysis of the entire study cohort, forgoing PMRT was significantly associated with developing LRR and DR. Subset analysis revealed that PMRT significantly reduced the 5 year LRR rate in patients with pre-chemotherapy clinical stages IIA (8.3% vs 46.2%, p=0.019) and IIIA (16% vs 66.7%, p=0.003). PMRT also significantly reduced the 5 year DR rate in patients with pre-chemotherapy clinical stage IIA (24.5% vs 69.3%, p=0.0151) and ≥IIIB (70.8% vs 100%, p=0.0481).
Conclusion: In our cohort of patients with TNBC treated with NAC and MRM, PMRT significantly improved locoregional control and disease-free survival in the entire cohort as well as in patients with stage IIA disease. Our results may help in tailoring adjuvant treatment decisions for these particular patient populations.
Keywords: triple-negative breast carcinoma, neoadjuvant chemotherapy, surgery, adjuvant radiation therapy, locoregional recurrence, disease recurrence
Introduction
Patients with triple-negative breast cancer (TNBC), defined as a breast malignancy that lacks estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) expression, are documented to have higher risks of locoregional recurrence (LRR) and distant metastasis (DM), as well as shorter overall survival (OS), than those with other breast cancer subsets. The lack of effective targeted treatments and its aggressive course make TNBC a notable clinical challenge.1,2 Nevertheless, TNBCs have been shown to be chemosensitive in previous studies.3,4
Large randomized trials and meta-analyses have demonstrated the benefit of postmastectomy radiotherapy (PMRT) in terms of locoregional control (LRC) as well as OS in high-risk breast cancer patients.5–7 Based on these studies, PMRT administration criteria were largely determined according to traditional T and N stages, including initial surgery followed by adjuvant chemotherapy, without considering the molecular heterogeneity of breast cancers.8,9 However, the use of neoadjuvant therapy has become increasingly common, particularly for treating locally advanced disease. Notably, patients experience significant changes in the extent of their disease following neoadjuvant chemotherapy (NAC), which reduces the applicability of traditional pathological guidelines for PMRT. Therefore, patients are generally selected for PMRT according to their clinical stage before NAC administration. However, consensus guidelines have not been established regarding the use of PMRT after NAC. With the increasing popularity of NAC as a standard treatment for large subsets of patients with breast cancer, data concerning the risk of LRR and the efficacy of PMRT in the described setting are urgently needed.
Data specific to TNBCs treated with NAC are more limited. Although previous studies have shown that TNBCs are more likely to respond to NAC, they are associated with inferior LRR as well as other adverse prognoses.10,11 On the other hand, including PMRT in the treatment of high-risk breast cancer, particularly stage III disease, has been shown to produce superior outcomes, including in pathological complete response (pCR) rates.12 However, there are few published studies on the risk of LRR following PMRT in patients with TNBCs treated with NAC.
The purpose of this study was to evaluate variables associated with LRR and disease recurrence (DR) in patients with TNBC, and to examine the effect of PMRT in such patients with stage II–III disease after they have already undergone NAC and mastectomy.
Patients and methods
Patient population
Between January 1, 2000 and July 31, 2007, a total of 104 women with stage II–III TNBC who underwent NAC and modified radical mastectomy (MRM) were identified at our institution. This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center, and written informed consent was obtained from each patient. The definition of TNBC was based on immunohistochemistry (IHC) staining of ER, PR, and HER2, which was routinely performed in the pathology department of our hospital as previously described.13 Patients were categorized as negative for ER and PR if nuclear staining for these receptors was ≤1%, whereas patients were considered HER2 negative if their IHC scores for this receptor were 0–2+ and no HER2 amplification was observed by fluorescent in situ hybridization. The clinical evaluation of tumor response to NAC was determined clinically according to the Response Evaluation Criteria in Solid Tumors.14 pCR was defined as no evidence of residual invasive disease within either the breast or axillary lymph nodes (LNs). All patients’ clinicopathological data were recorded in a computerized database at accrual; data included age, menopausal status, tumor characteristics, treatment, and outcome. Patients with DM at diagnosis or those with less than 1 month of follow-up were excluded.
Treatment
All patients were clinically staged at diagnosis according to the 2003 American Joint Committee on Cancer (AJCC) staging guidelines before and after undergoing NAC. NAC regimens were chosen at the discretion of the oncologist; 93 patients (89.4%) received an anthracycline-based regimen. All patients underwent MRM including level I–II axillary dissection. The median number of excised axillary LNs was 14 (range 1–38). The decision to administer PMRT was taken by the patients and their physicians. The radiotherapy protocol for breast cancer was developed circa the year 2000 based on our experiences in conjunction with evidence-based international guidelines; the institutional review board approved our protocol for the individual treatment of patients with breast cancer. At the discretion of the radiation oncologist, PMRT was delivered to 79 patients (76%), using external-beam irradiation with 46–50 Gy in 1.8–2.0 Gy fractions to the chest wall and/or regional nodal areas that generally included the internal mammary and supraclavicular regions. The axillary nodes were not routinely included in the PMRT field. The chest wall was treated with opposed tangential beams using cobalt-60 or 4–6 MeV photons. The regional nodal area was treated with an anterior photon field with a design matched to the tangential fields.
Endpoints
Regular follow-up was performed, including clinical and/or telephone follow-up by surgeons, radiation oncologists, or nurses. Patients were followed every 3 months during the first 2 years, and every 6 months thereafter. All patients’ information was recorded in a computerized database at accrual and included clinicopathological details. The primary endpoint of this study was LRR, defined as any clinical and/or biopsy-proven tumor recurrence involving the ipsilateral chest wall and/or the axillary, supraclavicular, intraclavicular, or internal mammary nodes. All LRR events were recorded regardless of their concurrence with DM. The secondary endpoints were DR, defined as either locoregional and/or distant recurrence, or death during follow-up. Both locoregional-recurrence free survival (LRFS) and disease-free survival (DFS) were calculated from the date of surgery to the date of event occurrence. In the absence of such events, patients were censored as of the final follow-up date.
Statistical analysis
Patients were divided into 2 groups for statistical analysis based on whether or not they received PMRT. Comparisons of tumor and treatment characteristics between the PMRT and non-PMRT groups were performed using chi-square and Fisher’s exact tests. The Fisher’s exact test was used when the cell counts were too low to perform chi-square tests. The 5 year cumulative LRR and DR rates were calculated using the Kaplan–Meier method, and comparisons between the 2 groups were performed using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model, testing only those factors that were found to be significant on univariate analysis. All statistical analyses were performed using STATA version 11.0 (Stata Corporation, College Station, TX, USA). All p-values were 2-sided, and a value of p≤0.05 was considered statistically significant.
Results
Patient characteristics
The median follow-up times for the entire cohort and for the irradiated-only and non-irradiated patients were 64 (range 12–123), 65 (range 12–112), and 60 (range 12–123) months, respectively. Comparisons of the key characteristics between the PMRT and non-PMRT groups are shown in Table 1. Patients in the PMRT group had significantly more advanced pre-chemotherapy clinical T stage, pathological N stage, and AJCC clinical stage than those in the non-PMRT group (all p<0.05) (Table 1). There were no differences between the 2 groups with respect to age, clinical N stage, pathological tumor size, lymphovascular invasion (LVI) status, or grade histology (all p>0.05) (Table 1). Among the 79 patients (76%) who received PMRT, 1 (1.27%) received treatment directed to the chest wall only, 64 (81.01%) to the chest wall as well as the regional nodal areas, and 14 (17.72%) to the regional nodal areas only.
LRR
At the last follow-up visit, 25 patients (24%) had developed LRR with a 5 year cumulative rate of 26.5%. Seven patients (6.7%) with pre-chemotherapy clinical stage IIA, IIB, or IIIB achieved pCR after NAC; none developed LRR. Despite exhibiting more adverse prognostic features, patients who underwent PMRT had a lower 5 year cumulative LRR rate than those who did not undergo PMRT (18.3% vs 52.2%, respectively, p=0.0005) (Figure 1A). Table 2 shows the effect of PMRT on the LRR rates according to various patient parameters. Patients with clinical T2–T3 tumors and those with clinical N-positive disease who underwent PMRT had significantly lower LRR than same-category patients who did not (all p<0.05). With respect to pathological features, PMRT reduced LRR in patients with T2 stage, as well as those with N0 or N2 (all p<0.05). Patients with clinical stage IIA or IIIA had a significantly lower 5 year LRR rate if their treatment included PMRT than if it did not (IIA: 8.3% vs 46.2%, respectively, p=0.019; IIIA: 16% vs 66.7%, respectively, p=0.003).
  | Figure 1 Kaplan–Meier probability curves of (A) locoregional recurrence and (B) disease recurrence according to the use of postmastectomy radiation therapy (RT) in the entire cohort. |
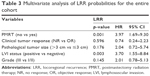
Univariate analysis of LRR showed that lack of PMRT, poorer clinical tumor response to NAC, pathological tumor size >3 cm, LVI positivity, and grade III disease were associated with a higher risk of LRR (all p<0.05). On multivariate analysis of LRR, forgoing PMRT was the most powerful significant predictor of LRR, with an HR of 3.97 (95% CI 1.7–9.3; p=0.001) (Table 3). LVI positivity was another significant factor predicting LRR development (95% CI 1.55–8.84; p=0.003) (Table 3).
DR
Fifty-two patients in the cohort (50%) developed DR, and the 5 year accumulative DR rate was 49.6%. Despite their more adverse prognostic features, patients with PMRT had a lower 5 year cumulative DR rate than those who did not undergo PMRT (45% vs 69.1%, respectively, p=0.0334) (Figure 1B). Analysis of various patient parameters revealed that PMRT significantly reduced DR in patients with pre-chemotherapy clinical T3–T4 stage and clinical N-negative disease. As for pathological features, PMRT reduced DR for patients with pathological T2–T3, and pN2 (all p<0.05). For patients with clinical stage IIA or ≥IIIB, the 5 year DR rate was significantly lower for patients treated with PMRT than for those who were not (IIA: 24.5% vs 69.3%, respectively, p=0.0151; ≥IIIB: 70.8% vs 100%, respectively, p=0.0481) (Table 2).
On univariate analysis for DR, no PMRT, clinical non-response, pathological T >5 cm, LVI positivity, grade 3 histology, pathological lymph nodes (LN) ≥4, and ≥25% positive LNs were associated with increased DR (all p<0.05). Multivariate analysis revealed that no PMRT, LVI positivity, and ≥25% positive LNs were independent significant prognostic factors for DR (PMRT: 95% CI 1.94–10.36; p=0.000; LVI; 95% CI 1.10–5.90; p=0.028; ≥25% positive LN; 95% CI 2.43–135.31; p=0.005) (Table 4).
Discussion
Ours is the first large-scale single-institution study to investigate the efficacy of PMRT in patients with locally advanced TNBC who were treated with NAC in terms of LRR and DR. Our data indicate that adding PMRT to NAC and MRM produces a significant reduction of LRR and DR in patients with stage II–III TNBC. When patients with pre-NAC clinical stage IIA disease were analyzed separately, the improvement in LRFS and DFS owing to PMRT inclusion remained statistically significant. Our findings support recommending PMRT in this group of patients, and should be considered regardless of their response to initial chemotherapy.
Triple-negative status has previously been reported as a predictor of worse outcome in patients with breast cancer.15–17 Although several studies revealed that the pCR rates in patients with TNBC were significantly higher than those in patients with non-TNBC, TNBC patients were reported to have significantly poorer LRFS, DFS, and OS rates than those with luminal subtypes in the setting of NAC; this was attributed to a higher likelihood of relapse in patients who did not achieve pCR.18
Randomized controlled trials and meta-analyses of patients with breast cancer encompassing all molecular subtypes have demonstrated that substantial improvements in LRC could translate into longer OS by including PMRT for patients at high risk of LRR.5–7 Accordingly, current guidelines recommend the use of PMRT in patients with 4 or more positive LNs or T3/T4 disease.8,9 However, the indications for PMRT remain unclear and controversial in patients with less advanced disease, including those with smaller primary tumors and 1–3 positive LNs, for whom PMRT is thought to be of little benefit. Notably, the large trials that enrolled patients with all molecular subtypes were performed in the setting of initial surgery followed by systemic therapy; therefore, there is insufficient evidence to recommend PMRT for patients with potentially high-risk features such as TNBC, and recommendations regarding patients who receive NAC are also not concretely established.
Our results suggest that TNBC patients with stage II–III disease have a significantly improved LRC and DFS when PMRT is included in their treatment plans. The 5 year LRR rate was reduced by two-thirds (18.3% vs 52.2% with and without PMRT, respectively) and the 5 year DR rate by almost 25% (45.0% vs 69.1% with and without PMRT, respectively). When analyzed separately, those with clinical stage IIA disease continued to derive a significant benefit in both LRFS and DFS from the use of PMRT. Our data are in relative agreement with an analysis by Abdulkarim et al,19 who stratified their patients according to locoregional management (breast-conserving therapy, MRM without PMRT, and MRM with PMRT) and found that the highest LRR rates were in patients who underwent MRM without PMRT. Furthermore, MRM without PMRT was found to be the only independent adverse prognostic factor associated with increased LRR in patients with T1–2N0 TNBC compared to breast-conserving therapy. On the other hand, we also found that PMRT improved LRFS and DFS in TNBC patients with pre-chemotherapy clinical stage IIA disease; this was not observed in a study of similarly treated patients with all molecular breast cancer subtypes.20 Our results, taken together with those of Abdulkarim et al, indicate that T and N stage may be insufficient for predicting LRR risk in all patients with TNBC (compared to other molecular subtypes) when considering adjuvant radiation therapy, and suggest that TNBC might be a powerful prognostic factor when considering the benefit of PMRT in patients with less advanced disease treated with NAC and MRM. However, our findings should be interpreted with caution owing to the study’s small sample size. Prospective studies are required to validate our findings.
A previous study demonstrated that PMRT confers LRC and a survival benefit in patients presenting with stage III–IV disease following NAC and MRM,20 a finding consistent with our own observations in terms of improved LRR in the setting of PMRT after MRM and NAC, although we did not find that PMRT provides any DFS benefit. This may be explained as follows: First, the small number of patients and their imbalance in the 2 groups limited the statistical significance and power of the data obtained. Second, it is generally assumed that PMRT can improve LRC and reduce the risk of secondary dissemination from residual locoregional tumor deposits.21 However, patients who would potentially achieve additional survival benefits are those without micrometastatic disease at presentation or with micrometastatic disease effectively treated by systemic therapy.22 Therefore, it can be posited that TNBC patients with stage III disease are more likely to harbor a systemic subclinical disease burden, making any improvements in LRC following PMRT less likely to translate into survival benefits. Finally, the TNBC-specific characteristics themselves may play a role, as previous studies revealed that TNBC has a predilection for DM,17,23 even at relatively early stages of the disease. The competing risk of DM very probably limits the translatability of improved LRC following PMRT into a survival benefit. Clearly, more data from clinical trials are needed to guide treatment recommendations regarding this group of patients.
Our study also found that the 7 patients with pre-chemotherapy clinical stage II–IIIA breast cancer who achieved pCR had excellence prognoses; only 1 had DM but none experienced LRR. This result corroborates previous findings that patients who achieve a pCR have remarkably good prognoses compared to those who have residual disease after NAC.4,18,24 Although the sample size of this subgroup was small and did not provide sufficient statistical power to test the impact of PMRT, it is notable that none of the 3 patients who received PMRT relapsed. This result may in part reflect the positive benefit of PMRT in this subset of patients with TNBC, a finding also reported in a previous study of patients with various molecular subtypes of breast cancer, demonstrating that PMRT provides a significant clinical benefit for patients who achieve a pCR after NAC.12 Nevertheless, additional studies are required to clarify the role of PMRT restricted to TNBC patients who achieve pCR after NAC and MRM.
On our multivariate analysis, LVI-positive status was found to be significantly associated with an increased risk of both LRR and DR. As a precursor to nodal involvement, LVI status has been reported to have an independent prognostic value for survival in patients with invasive breast cancer.25,26 Our study confirms this relationship after separately analyzing TNBC patients in the NAC setting. The other variable found to be associated with higher DR was pathological ≥25% N-positive status; otherwise, other factors that were previously reported to be prognostic (such as young age, increased tumor size, and high nuclear grade) did not significantly influence LRR or DR according to our multivariate analysis. This may be attributable to the changes in disease extent after NAC administration as well as the TNBC-specific phenotypes observed in the present study.
Limitations
There were certain limitations in this study. First, it was prone to selective bias toward particular patients and treatments owing to its retrospective design. Although we endeavored to mitigate several risk variables by controlling them in the multivariate model, it is possible that other unmeasured confounders that we did not consider may have influenced our results in part. Second, since the majority of patients with HER2 IHC scores of 2+ (n=21) could not be reevaluated with fluorescent in situ hybridization, some patients may potentially have been misclassified as triple-negative, which could skew our findings. Finally, a larger sample size and longer follow-up period may validate our findings and better define the subgroups of patients most likely to benefit from PMRT. Nevertheless, our study evaluated the LRR and DR risks exclusively in TNBC patients treated with NAC and MRM, a population in which data regarding outcomes following PMRT are scarce, and provides evidence confirming the benefit of PMRT in selected patients within this specific population.
Conclusion
In this population-based study of patients with stage II–III TNBC treated with NAC and MRM, patients who underwent PMRT were found to have significantly lower LRR and DR rates despite possessing significantly worse prognostic factors. The addition of PMRT to the treatment protocol was associated with decreased LRR and DR rates in the entire cohort as well as in pre-chemotherapy clinical stage IIA patients. Our results may help in tailoring adjuvant treatment decisions in these specific patient populations. Our study was limited by its small sample size, and our findings should therefore be validated in a larger, multi-institutional study.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81602668) and the Shanghai Sailing Program (grant number 16YF1401700). The funders had no role in any of the stages from study design to submission of the paper for publication.
The abstract of this paper was presented at the 57th Annual Meeting of the American Society for Radiation Oncology (October 18–21, 2015, San Antonio, TX, USA) as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the International Journal of Radiation Oncology Biology Physics (http://www.redjournal.org/article/S0360-3016(15)01366-8/fulltext).
English writing support was provided by the professional editing service “Editage.”
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690. | ||
Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247–269. | ||
Kim H, Park W, Huh SJ, et al. Clinical outcomes according to molecular subtypes in stage II-III breast cancer patients treated with neoadjuvant chemotherapy followed by surgery and radiotherapy. Asia Pac J Clin Oncol. 2017;13(4):329–336. | ||
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. | ||
Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337(14):949–955. | ||
Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116–126. | ||
Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. | ||
Carlson RW, Anderson BO, Burstein HJ, et al. Breast cancer. J Natl Compr Canc Netw. 2005;3(3):238–289. | ||
Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1539–1569. | ||
Jwa E, Shin KH, Kim JY, et al. Locoregional recurrence by tumor biology in breast cancer patients after preoperative chemotherapy and breast conservation treatment. Cancer Res Treat. 2016;48(4):1363–1372. | ||
Zhang C, Wang S, Israel HP, et al. Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. Springerplus. 2015;4:386. | ||
McGuire SE, Gonzalez-Angulo AM, Huang EH, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68(4):1004–1009. | ||
Chen X, Yu X, Chen J, et al. Analysis in early stage triple-negative breast cancer treated with mastectomy without adjuvant radiotherapy: patterns of failure and prognostic factors. Cancer. 2013;119(13):2366–2374. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. | ||
Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(9):1419–1426. | ||
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. | ||
Millar EK, Graham PH, O’Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27(28):4701–4708. | ||
Tian M, Zhong Y, Zhou F, Xie C, Zhou Y, Liao Z. Effect of neoadjuvant chemotherapy in patients with triple-negative breast cancer: a meta-analysis. Oncol Lett. 2015;9(6):2825–2832. | ||
Abdulkarim BS, Cuartero J, Hanson J, Deschênes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29(21):2852–2858. | ||
Huang EH, Tucker SL, Strom EA, et al. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol. 2004;22(23):4691–4699. | ||
Marks LB, Zeng J, Prosnitz LR. One to three versus four or more positive nodes and postmastectomy radiotherapy: time to end the debate. J Clin Oncol. 2008;26(13):2075–2077. | ||
Pierce LJ. The use of radiotherapy after mastectomy: a review of the literature. J Clin Oncol. 2005;23(8):1706–1717. | ||
Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. | ||
Yang TJ, Morrow M, Modi S, et al. The effect of molecular subtype and residual disease on locoregional recurrence in breast cancer patients treated with neoadjuvant chemotherapy and postmastectomy radiation. Ann Surg Oncol. 2015;22(Suppl)3:S495–S501. | ||
Huang EH, Tucker SL, Strom EA, et al. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62(2):351–357. | ||
Chang DT, Feigenberg SJ, Indelicato DJ, et al. Long-term outcomes in breast cancer patients with ten or more positive axillary nodes treated with combined-modality therapy: the importance of radiation field selection. Int J Radiat Oncol Biol Phys. 2007;67(4):1043–1051. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.