Back to Journals » International Medical Case Reports Journal » Volume 13
Posterior Reversible Encephalopathy Syndrome Due to Acute Water Intoxication in a Patient with Schizophrenia
Authors Takaoka Y , Akaho R , Inada K , Muraoka H, Hokama C , Inoue A, Nishimura K
Received 5 November 2019
Accepted for publication 26 February 2020
Published 7 April 2020 Volume 2020:13 Pages 117—121
DOI https://doi.org/10.2147/IMCRJ.S237430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Yohei Takaoka,1,2 Rie Akaho,1 Ken Inada,1 Hiroyuki Muraoka,1 Choryo Hokama,1 Atsuko Inoue,1 Katsuji Nishimura1
1Department of Psychiatry, Tokyo Women’s Medical University, Tokyo, Japan; 2Department of Psychiatry, Kuki Suzunoki Hospital, Kuki, Saitama, Japan
Correspondence: Rie Akaho
Department of Psychiatry, Tokyo Women’s Medical University, Tokyo, Japan
Email [email protected]
Abstract: Posterior reversible encephalopathy syndrome (PRES) is a clinical syndrome that presents as transient cerebral edema (vasogenic edema), usually on a background of hypertensive encephalopathy, puerperal eclampsia, or immunosuppressant drug use. We describe a case of PRES that arose in the context of a psychiatric disorder. The patient was a 26-year-old woman with schizophrenia who was hospitalized upon falling into a catatonic stupor and then suffered acute anxiety leading to impulsive polydipsia and subsequent water intoxication. She lost consciousness, and brain magnetic resonance imaging revealed a high density area, primarily affecting the cortex and subcortical white matter in areas in the occipital and parietal lobes, leading to the diagnosis. We did not treat the hyponatremia by means of aggressive sodium supplementation but rather balanced the extracellular fluid by continuous infusion of isotonic electrolyte replacement fluid. The patient’s level of consciousness improved gradually, but a total 141 days passed before hospital discharge was appropriate. The prognosis for PRES is generally favorable, but irreversible neurological damage can occur. We believe, therefore, that brain magnetic resonance imaging should be performed promptly whenever PRES is suspected and that timely, appropriate treatment is of utmost importance. If PRES is observed in a psychiatric patient, it is important to investigate whether the condition might have been caused by water intoxication and to treat the condition accordingly.
Keywords: posterior reversible encephalopathy, schizophrenia, water intoxication
Introduction
Posterior reversible encephalopathy syndrome (PRES), first reported by Hinchey et al in 1996,1 is a clinical syndrome that presents as transient cerebral edema (vasogenic edema) on a background of hypertensive encephalopathy, puerperal eclampsia, or immunosuppressant drug use. There is often a subsequent increase in blood pressure resulting from damage to vascular endothelial cells. Symptoms include headache, seizures, visual impairment, and loss of consciousness. To date, there has been no report of PRES due to water intoxication. This report documents a case of PRES that we believe was caused by acute water intoxication resulting from polydipsia in a patient with schizophrenia. We obtained a written informed consent from the patient to have her case details and images published. We also obtained an approval from our department to publish the data.
Case Report
The patient was a 26-year-old woman with schizophrenia who was admitted to our hospital’s department of psychiatry in a catatonic state. The schizophrenia had been diagnosed 3 years before her presentation to us. Her medical history included a lumbar vertebral fracture that occurred at the time of schizophrenia onset; she had tried to jump during a state of confusion. After the diagnosis, she visited psychiatric departments at numerous hospitals, but she loathed the weight gain that occurred with the use of antipsychotic drugs, so she did not return for follow-up examinations or take her prescribed medications regularly. A few weeks before admission to our hospital, she began displaying stereotyped behaviors, such as suddenly lying down and getting up again repeatedly. Shortly thereafter, she became unresponsive when addressed and fell into a catatonic stupor.
Once admitted to our department, the patient was prescribed oral quetiapine at 400 mg per day, lorazepam at 3 mg per day, and eszopiclone at 1 mg per day. Blood tests performed soon after admission revealed no electrolyte abnormalities (Na 139 mEq/L, K3.4 mEq/L, and Cl 100 mEq/L), and brain computed tomography (CT) showed no organic abnormality despite a slight difference in size between the left and right cerebral ventricles. The catatonic stupor improved after admission, but the patient became delusional, expressing a belief that “war is about to break out.” This resulted in extreme anxiety, and from the evening of hospital day 3 to the morning of hospital day 4, the patient impulsively drank an excessive amount of water, attested to by the empty PET bottles, which together would have contained 4500 mL, and by the fact that the patient readily obtained water from other sources. She complained of a headache and vomited on the morning of hospital day 4. Over the next several hours, her level of consciousness decreased to Glasgow Coma Scale (GCS) 3 (E1, V1, M1), and she became unresponsive to speech. Her vital signs remained stable, with blood pressure of 128/89 mmHg and body temperature of 36.4°C. Both pupils measured 5 mm, and both were sluggishly responsive to light. No seizure was observed. Her blood counts were normal, and blood tests revealed the following: Na 116 mEq/L, K 3.6 mEq/L, and Cl 82 mEq/L. Her blood gas levels were normal. Brain CT was once again performed, this time revealing narrowing of the cerebral ventricles and globally indistinct cerebral sulci, findings suggestive of cerebral edema (Figure 1A). A brain surgeon suspected subarachnoid hemorrhage on the basis of the CT images, but 2 days later, the part that appeared to be bleeding had disappeared, and no aneurysm was evident on the 3D image, so subarachnoid hemorrhage was ruled out. CSF examination revealed no abnormality. T2-weighted, fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted brain magnetic resonance (MR) imaging were also performed, revealing areas of high signal intensity, primarily in the cortex and subcortical white matter of the occipital and parietal lobes. In addition, an apparent diffusion coefficient (ADC) map of these areas, obtained on hospital day 7, showed decreased signal intensity (Figure 1B). PRES was suspected on the basis of the imaging findings.
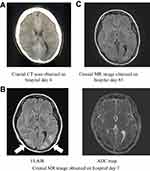 |
Figure 1 Images obtained over the clinical course. |
Cerebrospinal fluid (CSF) analysis was performed, but no abnormalities were detected. Autoimmune encephalitis was ruled out on the basis of the CSF test results and results of blood tests for antinuclear antibodies, rheumatoid factors, and complement proteins. We did not treat the hyponatremia by means of aggressive sodium supplementation but rather balanced the extracellular fluid by continuous infusion of isotonic electrolyte replacement fluid. The serum sodium concentration increased to 138 mEq/L within 2 days. Despite rapid correction of the hyponatremia, none of the magnetic resonance (MR) images obtained over the next 5 days suggested central pontine myelinolysis. The patient opened her eyes in response to speech on hospital day 6, with her level of consciousness improving to GCS 14 (E4, V4, M6) by day 8. However, she scored 20 points on the Hasegawa Dementia Scale-Revised (HDS-R) on day 23, a score indicative of decreased cognitive function. (HDS-R scores are known to be correlated with MMSE scores.2) Her level of consciousness continued to improve, but she suffered from a persistent mild attention disorder and decreased visual memory. Brain T2-weighted FLAIR MR imaging performed on hospital day 85 showed the high signal intensity regions in the occipital subcortical white matter to be gradually disappearing, and we believe this was part of the healing process (Figure 1C). She was discharged on day 141 after admission. Upon brain MR imaging performed 1 year after her presentation to us, we noted that the high density area had disappeared. We also noted absence of residual executive function deficits.
Discussion
Hyponatremia occurs when the serum sodium concentration falls below 135 mEq/L. It is a common electrolyte abnormality, but acute severe hyponatremia can be life-threatening. It is most prevalent in patients with a psychiatric disorder,3,4 and when it occurs in such patients, it is important for clinicians to consider the likelihood of water intoxication due to polydipsia. Polydipsia is defined as water intake behavior that is sufficient to cause a marked increase in body weight. The causes of polydipsia in patients with schizophrenia vary. The patients themselves give various reasons, such as “because my mouth was dry,” “because voices told me to,” and “I was trying to relieve my stress.” Sometimes patients do not give a particular reason but simply say “I had to drink,” and some patients appear to be engaging in polydipsia as a form of addictive behavior. Vieweg et al noted that polydipsia in patients with schizophrenia can be attributed to (1) psychological stress, (2) hallucination, delusion, or stereotypy, (3) enhanced impulsiveness, (4) addictive behavior, (5) excessive release of antidiuretic hormone, i.e., of vasopressin (syndrome of inappropriate antidiuretic hormone secretion [SIADH]), (6) organic brain abnormality, (7) genetic polymorphism, or (8) the effect of certain antipsychotic drugs.5
The circulating blood becomes diluted as a result of polydipsia, and this causes hyponatremia and sudden fluctuations in osmotic pressure, so water migrates into the cells. When hyponatremia progresses rapidly, it causes increased intracranial pressure and cerebral edema and may cause headaches, fatigue, lethargy, irritability, seizures, and consciousness disorders such as coma. Various neuropsychiatric symptoms can also result. In the field of psychiatry, this condition is known as water intoxication.
As noted above, PRES was first reported by Hinchey et al in 1996.1 Patients with this clinical syndrome presented with transient vasogenic edema that occurred in the subcortical areas in the occipital and parietal lobes. At first, PRES was referred to as reversible posterior leukoencephalopathy syndrome (RPLS), but in reality, the condition involves considerable injury to both the cerebral white matter and cortex. Therefore, in recent years, the term PRES has been widely used because it refers to an encephalopathy that includes lesions in both the white matter and cortex. Factors leading to the onset of PRES include sudden blood pressure fluctuations, renal failure, autoimmune disorders, eclampsia, septicemia, and use of drugs such as immunosuppressants administered after organ transplantation or anti-cancer drugs.6,7
Background factors for PRES are hypertension, immunosuppressant drug use, anti-cancer drug use, eclampsia, collagen disorders, and electrolyte abnormalities. In this disease state, sudden increases in blood pressure or the effects of cytokines cause vascular endothelial injury, resulting in transient cerebral edema (vasogenic edema). We believe this is why the disorder tends to predominantly affect regions supplied by the posterior circulation, which have little sympathetic innervation.8–10 Infarction or hemorrhage occurs due to vasospasm in some cases.11 MR imaging is useful for diagnosing PRES because the areas of high signal intensity that appear on T2-weighted and FLAIR images, the areas of low or normal signal intensity that appear on diffusion-weighted images, and the ADC elevation. If the condition is determined to be drug-induced, the causative agent can be withdrawn, or the therapeutic dose can be reduced. Treatment of symptoms might include the administration of anticonvulsants or antihypertensive drugs.
Our patient experienced psychological stress during the acute phase of catatonic schizophrenia, and this stress led to impulsive polydipsia, which resulted in acute water intoxication. On the day following her excessive fluid intake, she suffered headache, vomiting, and loss of consciousness. Her state upon presentation was that of profound hyponatremia, and MR imaging performed at that time showed lesions that were distributed in areas that were not consistent with areas supplied by the vessels for the cortex and subcortical white matter in the occipital and parietal lobes. The clinical course, especially the transient headache and loss of consciousness, led us to suspect PRES. There is a report of PRES associated with hyponatremia, as in our case.12
Once the hyponatremia improved, the high density area began to recede. No further neurological symptoms occurred over the subsequent clinical course, and the high density area disappeared spontaneously, which we believe further supports the diagnosis of PRES. Blood and CSF tests ruled out the presence of infectious or autoimmune encephalitis and demyelination disorders. Decreased signal intensity was observed on the ADC map during MR imaging, and this was associated with infarction, so the PRES presented in a nonclassical manner. However, as in our case, high signal intensity on diffusion-weighted MR images and low signal intensity on ADC maps have been documented in cases of PRES.11 Restricted diffusion has been reported in 15–30% of 151 PRES patients.13 As noted above, PRES can be caused by hypertension, immunosuppressant drug use, anti-cancer drug use, eclampsia, collagen disorders, and electrolyte abnormalities. However, causes other than electrolyte abnormalities were ruled out in our case, and thus we believe that our patient’s PRES was caused by hyponatremia resulting from water intoxication.
We encountered PRES resulting from water intoxication in a patient with schizophrenia who engaged in polydipsia, perhaps as a result of the schizophrenia. SIADH due to water intoxication is not uncommon in patients with schizophrenia, and such cases are often encountered in clinical psychiatric practice. In addition, it is widely known that cases of hyponatremia due to water intoxication can be complicated by central pontine myelinolysis. However, there are no reports of water intoxication being complicated by PRES, as occurred in our case.
Patients with schizophrenia are prone to suffer from water intoxication, and it is important for clinicians to realize that PRES can result from water intoxication. The prognosis for PRES is generally favorable, but there have been reports of irreversible neurological damage.5 We believe, therefore, that brain MR imaging should be performed promptly whenever PRES is suspected and that timely, appropriate treatment is of utmost importance. PRES must also be differentiated from stroke. If PRES is observed in a psychiatric patient within the setting of neurosurgery, neurology, or emergency medicine, it is important to investigate whether the condition may have been caused by water intoxication, to treat the condition appropriately, and to prevent recurrence.
Disclosure
Dr Ken Inada reports personal fees from Eisai, Eli Lilly, Janssen, Meiji-Seika Pharma, Mitsubishi Tanabe Pharma, Mochida, MSD, Novartis, Otsuka, Shionogi, Sumitomo Dainippon Pharma, and Yoshitomiyakuhin, outside the submitted work. Prof. Dr. Katsuji Nishimura reports personal fees from Meiji Seika Pharma, Astellas, Yoshitomiyakuhin, Pfizer, Shionogi, Janssen, Daiichi Sankyo, Chugai, Nipro, and Kissei; grants, personal fees from Eli Lilly, MSD, Mochida, Takeda, Eisai, Otsuka, Tsumura, Novartis, and Mitsubishi Tanabe; grants from Dainippon Sumitomo, GlaxoSmithKline, and Mebix, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. doi:10.1056/NEJM199602223340803
2. Kim KW, Lee DY, Jhoo JH, et al. Diagnostic accuracy of mini-mental status examination and revised hasegawa dementia scale for alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(5–6):324–330. doi:10.1159/000084558
3. Siegel AJ. Hyponatremia in psychiatric patients: update on evaluation and management. Harv Rev Psychiatry. 2008;16(1):13–24. doi:10.1080/10673220801924308
4. Manu P, Ray K, Rein JL, De Hert M, Kane JM, Correll CU. Medical outcome of psychiatric inpatients with admission hyponatremia. Psychiatry Res. 2012;198(1):24–27. doi:10.1016/j.psychres.2012.01.022
5. Vieweg V, Pandurangi A, Levenson J, Silverman J. The consulting psychiatrist and the polydipsia hyponatremia syndrome in schizophrenia. Int J Psychiatry Med. 1994;24(4):275–303. doi:10.2190/5WG5-VV1V-BXAD-805K
6. Thompson RJ, Sharp B, Pothof J, Hamedani A. Posterior reversible encephalopathy syndrome in the emergency department: case series and literature review. West J Emerg Med. 2015;16(1):5–10. doi:10.5811/westjem.2014.12.24126
7. Iwafuchi Y, Okamoto K, Oyama Y, Narita I. Posterior reversible encephalopathy syndrome in a patient with severe uremia without hypertension. Intern Med. 2016;55(1):63–68. doi:10.2169/internalmedicine.55.5563
8. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036–1042. doi:10.3174/ajnr.A0928
9. Granata G, Greco A, Iannella G, et al. Posterior reversible encephalopathy syndrome: insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev. 2015;14(9):830–836. doi:10.1016/j.autrev.2015.05.006
10. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–925. doi:10.1016/S1474-4422(15)00111-8
11. Rowley C, Onslow J. Posterior reversible encephalopathy syndrome (PRES). Int J Obstet Anesth. 2008;17(2):195–196. doi:10.1016/j.ijoa.2007.11.003
12. Aulakh P, Fatakhov E, Koch CF
13. Siebert E, Bohner G, Liebig T, Endres M, Liman TG. Factors associated with fatal outcome in posterior reversible encephalopathy syndrome: a retrospective analysis of the Berlin PRES study. J Neurol. 2017;264(2):237–242. doi:10.1007/s00415-016-8328-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
