Back to Journals » Open Access Journal of Sports Medicine » Volume 5
Post-traumatic knee osteoarthritis in the young patient: therapeutic dilemmas and emerging technologies
Authors Stiebel M, Miller L, Block J
Received 4 February 2014
Accepted for publication 26 February 2014
Published 8 April 2014 Volume 2014:5 Pages 73—79
DOI https://doi.org/10.2147/OAJSM.S61865
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Matthew Stiebel,1 Larry E Miller,2,3 Jon E Block3
1Palm Beach Sports Medicine, West Palm Beach, FL, USA; 2Miller Scientific Consulting, Inc, Asheville, NC, USA; 3The Jon Block Group, San Francisco, CA, USA
Abstract: Traumatic knee injury is common in young adults and strongly contributes to premature development of knee osteoarthritis (OA). Post-traumatic knee OA poses a therapeutic dilemma to the physician, since no known therapy has an acceptable safety profile, effectively relieves joint pain, and enjoys reasonable patient acceptance. Consequently, these young patients will ultimately be faced with the decision to either undergo surgical intervention, despite prosthesis durability concerns, or to continue with ineffective nonsurgical treatment. Emerging therapies, such as biologics, disease-modifying drugs, partial joint resurfacings, and minimally invasive joint-unloading implants are currently being studied to fill this therapeutic void in the young patient with post-traumatic knee OA.
Keywords: injury, knee, osteoarthritis, post-traumatic, implant, joint unloading, KineSpring, minimally invasive
Introduction
Knee osteoarthritis (OA) is a progressive joint disease that represents the leading cause of disability in adults.1,2 This disease involves not only articular cartilage, but all tissue components of the joint, including periarticular muscles, ligaments, subchondral bone, and synovial membrane.3 Although knee OA is generally believed to be a disease of the elderly, the median age at initial diagnosis is only 55 years.4 Considering the continued aging of the population, the absence of proven disease-modifying treatments, and the general unwillingness of patients to undergo early arthroplasty,5–7 most patients must tolerate the burden of knee OA for decades. While the etiology of knee OA remains controversial and is likely multifactorial,8 it is clear that an unfavorable biomechanical environment at the joint is a primary contributing factor to disease development.9 When chronic excessive loads are applied to the knee joint, especially in a joint with altered kinematics, the mechanical demand eventually exceeds the ability of the joint to repair itself, setting the stage for OA development.
Traumatic knee injury strongly contributes to knee OA development in the young adult. The incidence of acute knee injury is approximately 900,000 cases per year in the US.10 Joint injury triggers a lengthy remodeling process in the cartilage and surrounding tissues that has adverse biomechanical and biochemical implications that encourage joint degeneration. Numerous types of joint injury, including meniscal, ligament, and joint capsule tears, joint dislocations, and intra-articular fractures are known to markedly increase knee OA risk.11–15 OA secondary to joint injury is typically diagnosed earlier in life and progresses much quicker, resulting in a lengthier period of joint-related morbidity compared to patients with primary OA.16,17
In a prospective study of over 1,300 adults with 36-year follow-up, the relative risk for developing knee OA was five-fold higher in patients who suffered a knee injury during follow-up compared to those with no injury.14 Evidence of rapid post-injury disease onset includes a reported 30% incidence of knee OA by 5 years and 50% incidence by 10–20 years following major knee injury, anterior cruciate ligament (ACL) injury, and total meniscectomy.18–22 Since most knee injuries occur in young active adults, onset of post-traumatic knee OA in the 30s and 40s is a distinct possibility. In clinical practice, post-traumatic knee OA accounts for 12% of all knee OA cases.10 Patient factors, such as longer life expectancy and desire to continue physical activity later in life, combined with treatment factors, including lack of efficacy with nonsurgical care and durability concerns with surgical treatments, pose a challenging therapeutic dilemma to the physician. Identification of knee injury prevention strategies and ideal post-traumatic OA treatments targeted to the younger patient are topic areas that clearly demand concerted research efforts.
Why does knee injury increase OA susceptibility?
The pathologic cascade of joint damage following traumatic injury varies with the severity of mechanical impact and the extent and involvement of tissue damage. Lower energy injuries such as ligamentous and meniscal injuries often cause articular cartilage damage with possible microfracturing of subchondral bone, while higher energy injuries frequently result in intra-articular fracture. In the acute post-injury phase, hemarthrosis, chondrocyte death, and dilution of synovial fluid occur.23 Collagen and proteoglycan synthesis is suppressed and matrix-degrading enzymes and inflammatory mediators are overexpressed.24,25 Cell necrosis occurs beyond the initial area of injury due to apoptotic mechanisms. After a prolonged period (up to 1 year), these biochemical processes slowly revert back to pre-injury conditions, although their adverse impact on joint structures are permanent.26
With the joint in a vulnerable state, injury-induced biomechanical alterations further degrade the articular cartilage, and ultimately, lead to subchondral lesions and osteophyte formation. Meniscal lesions and ACL injuries represent the greatest proportion of traumatic knee injuries, each resulting in distinct chronically pathologic biomechanical alterations that greatly increase OA susceptibility. Meniscus injury increases OA risk concomitant with the amount of damaged tissue by increasing load transmission at articular surfaces.27,28 Injury to the cruciate ligaments can influence the location where joint surfaces make contact, resulting in localized areas of cartilage degeneration not typically loaded before injury.29 Loss of ACL stability causes increased medial femoral condyle translation on the tibial plateau and altered wear patterns. Additionally, posterior cruciate ligament (PCL) injury also leads to increased translation and rotation, with eventual increased patellofemoral cartilage wear after progressive posterior tibial subluxation. Unfortunately, neither meniscectomy13,30 nor surgical ACL or PCL reconstruction18,19,31,32 mitigate the risk for OA, since kinematic aberrations related to biomechanical alterations inside the reconstructed joint and muscle wasting remain unaddressed.33 Ultimately, pre-injury multi-dimensional joint stability cannot be perfectly replicated with surgery.34 Following knee injury, deconditioning of the musculoskeletal system, including quadriceps weakness,35 proprioceptive deficiencies,36–38 and postural control limitations,39,40 is common, all of which subtly, yet adversely, impact articular loading patterns.41,42 In summary, traumatic knee injury contributes to OA risk via the combination of acute and chronic degradative biochemical processes, alterations in joint loading patterns, and chronic loss of muscle strength and control. Detailed discussions of the etiology of disease in the post-traumatic knee OA patient have been published elsewhere.43–46
Current treatments for the young post-traumatic knee OA patient
The clinical presentation, diagnostic methods, and treatment considerations in the patient with post-traumatic knee OA are similar to that of the primary OA patient, with the major exception of a considerably earlier age at initial diagnosis. Knee OA treatment in these younger patients represents a therapeutic dilemma where the physician seeks a compromise between clinically meaningful pain relief with functional improvement and treatment safety and durability. The traditional first-line therapeutic approach to the post-traumatic knee OA patient of any age consists of nonsurgical treatments such as weight loss, lateral wedge insoles, bracing, and physical therapy. Pharmacological treatments include analgesics, nonsteroidal anti-inflammatory drugs, opioids, hyaluronic acid, or corticosteroid injections, and various disease-modifying osteoarthritis drugs. Despite the fact that all of the twelve existing guidelines for knee OA management dictate that optimal management of OA requires a combination of non-pharmacological and pharmacological modalities,47 these conservative therapies have major limitations, most notably a failure to successfully correct the underlying pathology – namely, abnormal joint loading resulting in continued disease progression. Despite marginal symptom relief with some nonsurgical therapies, none have been shown to alter disease progression.48
As a result, many younger OA patients will eventually be faced with the decision to either undergo surgical intervention or to continue with ineffective conservative therapies. In older adults, only one in three candidates are willing to consider arthroplasty;7 presumably, this number is even lower in younger adults. The decision to undergo arthroplasty in the younger patient is further complicated by the higher physical demand placed on the prosthesis components and concerns regarding implant survival.
The incidence of joint replacement surgery has increased in older and younger patients over the last decade.49 In the US, approximately 70,000 total knee arthroplasties (TKAs) are performed annually in patients younger than 55 years, with the incidence expected to increase to 1 million annually by 2030.50 Despite the rapidly increasing utilization, TKA outcomes are suboptimal in the younger population. Unlike older patients who place lower mechanical demands on prosthetic components following TKA, younger patients expect to remain physically active after surgery, with the resulting high likelihood of outliving the implant. Consequently, surgical outcomes with TKA in the young patient are less reliable than for primary knee OA. Patients under 40 years of age have only a 40% chance of good or excellent Knee Society function scores postsurgery, a one in eight chance of revision over the next 8 years, and will almost undoubtedly outlive the implant, necessitating riskier revision surgery.51 In the younger patient with single compartmental disease, unicompartmental knee arthroplasty (UKA) suffers from the same limitations as TKA. In fact, the durability of UKA is inferior to TKA, and consequently, this surgery is also an imperfect option given the increased risk of polyethylene wear and lower survival rates due to increased activity demands.52
Given the durability concerns with arthroplasty, high tibial osteotomy (HTO) is widely held to be a suitable surgical option for the younger, physically active patient with either large uncontained grade IV chondral defects or entire medial compartment wear with tibiofemoral “kissing lesions”.53,54 Despite the overall decline in tibial osteotomies over the last two decades, the incidence has slightly increased in patients under 50 years of age due to a lack of other acceptable surgical options.55 Significant safety concerns limit the utility of HTO, which include infection (2%–55%), deep vein thrombosis (1%–10%), delayed or non-union (0%–14%), and peroneal nerve injury (0%–20%).56 Although early patient outcomes with HTO are generally favorable, the benefits of surgery gradually deteriorate over time due to disease progression.56 Additionally, many patients find the change to an overcorrected valgus position, which is necessary for full medial joint offloading, to be esthetically unacceptable. Approximately 10 years after HTO, only 60% of patients report a good or excellent result and 20%–50% will undergo TKA.57,58 There is a clear need for novel treatments to fill the therapeutic void between ineffective nonsurgical treatment and invasive surgical options with limited durability and safety concerns in the younger patient with post-traumatic knee OA.
Emerging technologies for the young post-traumatic knee OA patient
It is appealing to envision a therapy that could prevent, delay, or effectively manage knee OA in the young patient with post-traumatic knee injury. Indeed, several effective therapies are currently available for post-traumatic OA patients with isolated cartilage lesions such as autologous chondrocyte implantation, osteochondral allografts, and lesion resurfacing techniques. Unfortunately, numerous studies of biologic therapies (eg, platelet-rich plasma, mesenchymal stem cells) and disease-modifying drugs (eg, anabolics, anti-catabolics) intended to slow joint narrowing, prevent chondrocyte death, inhibit post-injury inflammatory mediators, or preserve and strengthen subchondral bone have reported disappointing results in larger tibiofemoral lesions.59 To date, no known therapy has been shown to alter the natural history of post-traumatic tibiofemoral knee OA. It is generally held that the failure of biologics and disease-modifying drugs to impact joint-space narrowing and control disease-related symptoms is due to their inability to overcome the destructive effects of aberrant joint biomechanics.59 Correction of excessive and abnormal joint biomechanics appears to be a prerequisite for any concomitant therapy to exert therapeutic benefit in post-traumatic knee OA.
There has been recent interest in using joint-sparing implants to achieve an optimal biomechanical environment at the knee joint in patients with mild-to-moderate OA. The first studies on such devices utilized interpositional spacers that replicated the meniscal shape. The theoretical advantages of this implant included no bone resection or implant fixation. However, clinical outcomes were disappointing with revision rates of 21%–44%.60–65
Minimally invasive implants that partially unload the diseased medial compartment in patients with unicompartmental knee OA via an extracapsular absorber unit affixed to femoral and tibial bases have been extensively studied (Figure 1).66–72 The genesis for such technology developed from the observation that lower extremity OA onset and progression are strongly related to excessive and/or abnormal loading across the joint.73,74 Consequently, technologies that transfer loads away from the affected compartment have potential in the treatment of this disabling condition.75 A notable advantage of these implants is the minimally invasive nature of the implant procedure, and if needed, the procedure is completely reversible. The implant/explant procedure does not violate the joint capsule, avoids soft tissue or osseous trauma, and does not interfere with future surgical procedures.76 The implant absorbs a maximum load of 13 kg during full knee extension, comparable to the magnitude of knee adduction moment reduction that improves function and reduces knee pain in OA patients.77 The device accommodates the natural motions of the knee joint by using two ball-and-socket joints with the capability of >60° internal–external rotation, 50° of varus–valgus angulation, and 155° of flexion–extension.
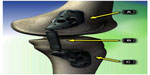  | Figure 1 Components of the KineSpring System. (A) Femoral base, (B) absorber, and (C) tibial base. |
An in vitro study determined that all test specimens survived 10 million cycles of fatigue loading with no evidence of cracking or plastic deformation as a result of the cyclic fatigue loading. Examination of the components with special consideration for the interfaces between the screws and bases, screws and bones, and bases and bones showed no evidence of wear or abrasion. Static loading to failure established the construct strength as 4,050±209 N and the failure mode was uniformly seen to be fracture of the bone analog, with no breaking or cracking of any socket, base, or screw components. Simulated-use testing of the implant showed that all implants survived 15 million cycles of flexion–extension motion and loading. Visual examination revealed no evidence of cracking or plastic deformation. Soft tissue response to the articulating subcutaneous implant was studied in a chronic ovine model.66 Macroscopically, early evidence of an acute inflammatory response was observed at 4 weeks, but subsequently resolved. Skin incisions were completely healed with no evidence of irritation or ulceration by 26 weeks in all animals. Histological evidence at 4 weeks showed that the device was covered with a soft tissue membrane that was edematous, slightly inflamed, and had surface fibrin deposition, although this inflammatory response resolved by 12 weeks. To determine the effect of the implant on intra-articular loads, a gait simulation study was performed on six cadaver knees.70 Femorotibial forces in the medial compartment of the knee throughout stance phase were reduced by 31±11 lb (P=0.002) when the device was implanted. The reductions in peak medial forces were greatest around heel strike (29±18 lb, P=0.01) and around toe-off (44±20 lb, P=0.008). In addition, the total joint load was also significantly reduced in the treated knees. Reductions in total joint load during stance averaged 22±9 lb (P=0.002). Larger reductions in total joint load were observed at foot flat (mid-stance) (24±18 lb, P=0.019) and around toe-off (31±13 lb, P=0.005). These reductions in medial and total intra-articular loads were within the clinically effective ranges of other joint unloading therapies.77 The treated knees showed no substantial difference in lateral compartment load compared to the untreated knees.
The initial clinical experience with this implant is promising.78 A recent study of 99 patients with 17 months mean follow-up reported excellent safety and effectiveness outcomes.78 All devices were successfully implanted and activated with no intraoperative complications. Statistically significant mean improvements of 56%, 50%, and 38% were observed for Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain, Function, and Stiffness scores, respectively (all P<0.001). WOMAC clinical success rates were 77.8% for pain, 77.8% for function, and 68.7% for stiffness. The worldwide experience with the current generation KineSpring System yielded excellent safety and durability outcomes, with only 8% of patients undergoing device removal during follow-up.
If joint unloading implants are ultimately proven to durably ameliorate symptoms in a cost effective manner, patient willingness to accept treatment may increase and younger patients with post-traumatic knee OA may receive treatment sooner, thereby lowering disease-related morbidity, improving quality of life, and reducing medical costs related to invasive surgery and subsequent revisions. To date, no joint unloading device has received marketing clearance through the Food and Drug Administration in the US although Phase III trials are currently underway. Future studies with these implants should include randomized controlled trials with sham or surgical (eg, HTO) controls as well as large-scale registries to better characterize the long-term safety and durability of these implants.
Conclusion
Knee OA is a common diagnosis in the patient with previous traumatic knee injury. Following joint injury, OA develops and progresses rapidly, with no known therapies that can prevent or alter the course of disease. In an effort to prevent or delay invasive joint arthroplasty in the post-traumatic knee OA patient, biologics and disease-modifying drugs are emerging technologies intended to address the biochemical environment at the joint, whereas minimally invasive unloading implants address abnormal joint biomechanics.
Disclosure
LM and JB received financial support from Moximed, Inc., (Hayward, CA, USA). The authors report no other conflicts of interest in this work.
References
Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. | |
Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. | |
Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis – an untreatable disease? Nat Rev Drug Discov. 2005;4(4):331–344. | |
Losina E, Weinstein AM, Reichmann WM, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res (Hoboken). 2013;65(5):703–711. | |
Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212–3220. | |
Hawker GA, Wright JG, Badley EM, Coyte PC. Perceptions of, and willingness to consider, total joint arthroplasty in a population-based cohort of individuals with disabling hip and knee arthritis. Arthritis Rheum. 2004;51(4):635–641. | |
Hawker GA, Wright JG, Coyte PC, et al. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients’ preferences. Med Care. 2001;39(3):206–216. | |
Aspden RM. Osteoarthritis: a problem of growth not decay? Rheumatology (Oxford). 2008;47(10):1452–1460. | |
Waller C, Hayes D, Block JE, London NJ. Unload it: the key to the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1823–1829. | |
Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–744. | |
Buckwalter JA. Osteoarthritis and articular cartilage use, disuse, and abuse: experimental studies. J Rheumatol Suppl. 1995;43:13–15. | |
Buckwalter JA, Lane NE. Athletics and osteoarthritis. Am J Sports Med. 1997;25(6):873–881. | |
Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48(8):2178–2187. | |
Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133(5):321–328. | |
Sahin V, Karakas ES, Aksu S, Atlihan D, Turk CY, Halici M. Traumatic dislocation and fracture-dislocation of the hip: a long-term follow-up study. J Trauma. 2003;54(3):520–529. | |
Thambyah A. A hypothesis matrix for studying biomechanical factors associated with the initiation and progression of posttraumatic osteoarthritis. Med Hypotheses. 2005;64(6):1157–1161. | |
Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192–202. | |
Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. | |
Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. | |
Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687–693. | |
Lohmander LS, Roos H. Knee ligament injury, surgery and osteoarthrosis. Truth or consequences? Acta Orthop Scand. 1994;65(6):605–609. | |
Frobell RB, Roos HP, Roos EM, Roemer FW, Ranstam J, Lohmander LS. Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ. 2013;346:f232. | |
Szczodry M, Coyle CH, Kramer SJ, Smolinski P, Chu CR. Progressive chondrocyte death after impact injury indicates a need for chondroprotective therapy. Am J Sports Med. 2009;37(12):2318–2322. | |
DiMicco MA, Patwari P, Siparsky PN, et al. Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum. 2004;50(3):840–848. | |
Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52(8):2386–2395. | |
Wei L, Fleming BC, Sun X, et al. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. J Orthop Res. 2010;28(7):900–906. | |
Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. J Orthop Res. 2000;18(1):109–115. | |
Shoemaker SC, Markolf KL. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee. Effects of partial versus total excision. J Bone Joint Surg Am. 1986;68(1):71–79. | |
Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40(2):215–222. | |
Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50(9):2811–2819. | |
von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. | |
Meuffels DE, Favejee MM, Vissers MM, Heijboer MP, Reijman M, Verhaar JA. Ten year follow-up study comparing conservative versus operative treatment of anterior cruciate ligament ruptures. A matched-pair analysis of high level athletes. Br J Sports Med. 2009;43(5):347–351. | |
Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17(2):195–200. | |
Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament injury, reconstruction and osteoarthritis. Curr Opin Orthop. 2005;16(5):354–362. | |
Wojtys EM, Huston LJ. Longitudinal effects of anterior cruciate ligament injury and patellar tendon autograft reconstruction on neuromuscular performance. Am J Sports Med. 2000;28(3):336–344. | |
Corrigan JP, Cashman WF, Brady MP. Proprioception in the cruciate deficient knee. J Bone Joint Surg Br. 1992;74(2):247–250. | |
Beard DJ, Kyberd PJ, Fergusson CM, Dodd CA. Proprioception after rupture of the anterior cruciate ligament. An objective indication of the need for surgery? J Bone Joint Surg Br. 1993;75(2):311–315. | |
Borsa PA, Lephart SM, Irrgang JJ, Safran MR, Fu FH. The effects of joint position and direction of joint motion on proprioceptive sensibility in anterior cruciate ligament-deficient athletes. Am J Sports Med. 1997;25(3):336–340. | |
Ageberg E. Consequences of a ligament injury on neuromuscular function and relevance to rehabilitation – using the anterior cruciate ligament-injured knee as model. J Electromyogr Kinesiol. 2002;12(3):205–212. | |
Lysholm M, Ledin T, Odkvist LM, Good L. Postural control – a comparison between patients with chronic anterior cruciate ligament insufficiency and healthy individuals. Scand J Med Sci Sports. 1998;8(6):432–438. | |
Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. | |
Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91 Suppl 1:95–101. | |
Cattano NM, Barbe MF, Massicotte VS, et al. Joint trauma initiates knee osteoarthritis through biochemical and biomechanical processes and interactions. OA Musculoskeletal Medicine. 2013;1:3–8. | |
Kramer WC, Hendricks KJ, Wang J. Pathogenetic mechanisms of posttraumatic osteoarthritis: opportunities for early intervention. Int J Clin Exp Med. 2011;4(4):285–298. | |
Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12(3):211. | |
Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. | |
Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–162. | |
Crawford DC, Miller LE, Block JE. Conservative management of symptomatic knee osteoarthritis: a flawed strategy? Orthop Rev (Pavia). 2013;5(1):e2. | |
Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487–1497. | |
Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606–2612. | |
Lonner JH, Hershman S, Mont M, Lotke PA. Total knee arthroplasty in patients 40 years of age and younger with osteoarthritis. Clin Orthop Relat Res. 2000(380):85–90. | |
W. Dahl A, Robertsson O, Lidgren L, Miller L, Davidson D, Graves S. Unicompartmental knee arthroplasty in patients aged less than 65. Acta Orthop. 2010;81(1):90–94. | |
Hui C, Salmon LJ, Kok A, et al. Long-term survival of high tibial osteotomy for medial compartment osteoarthritis of the knee. Am J Sports Med. 2011;39(1):64–70. | |
Amendola A. Unicompartmental osteoarthritis in the active patient: the role of high tibial osteotomy. Arthroscopy. 2003;19 Suppl 1:109–116. | |
Niinimaki TT, Eskelinen A, Ohtonen P, Junnila M, Leppilahti J. Incidence of osteotomies around the knee for the treatment of knee osteoarthritis: a 22-year population-based study. Int Orthop. 2012;36(7):1399–1402. | |
Dowd GS, Somayaji HS, Uthukuri M. High tibial osteotomy for medial compartment osteoarthritis. Knee. 2006;13(2):87–92. | |
Virolainen P, Aro HT. High tibial osteotomy for the treatment of osteoarthritis of the knee: a review of the literature and a meta-analysis of follow-up studies. Arch Orthop Trauma Surg. 2004;124(4):258–261. | |
Spahn G, Hofmann GO, von Engelhardt LV, Li M, Neubauer H, Klinger HM. The impact of a high tibial valgus osteotomy and unicondylar medial arthroplasty on the treatment for knee osteoarthritis: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(1):96–112. | |
Hellio Le Graverand-Gastineau MP. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage. 2009;17(11):1393–1401. | |
Köck FX, Beckmann J, Lechler P, Götz J, Schaumburger J, Grifka J. [The 2-year follow-up results of a patient-specific interpositional knee implant]. Orthopade. 2011;40(12):1103–1110. German. | |
Catier C, Turcat M, Jacquel A, Baulot E. The Unispacer unicompartmental knee implant: its outcomes in medial compartment knee osteoarthritis. Orthop Traumatol Surg Res. 2011;97(4):410–417. | |
Clarius M, Becker JF, Schmitt H, Seeger JB. The UniSpacer: correcting varus malalignment in medial gonarthrosis. Int Orthop. 2010;34(8):1175–1179. | |
Bailie AG, Lewis PL, Brumby SA, Roy S, Paterson RS, Campbell DG. The Unispacer knee implant: early clinical results. J Bone Joint Surg Br. 2008;90(4):446–450. | |
Sisto DJ, Mitchell IL. UniSpacer arthroplasty of the knee. J Bone Joint Surg Am. 2005;87(8):1706–1711. | |
Hallock RH, Fell BM. Unicompartmental tibial hemiarthroplasty: early results of the UniSpacer knee. Clin Orthop Relat Res. 2003;(416):154–163. | |
Allen MJ, Townsend KL, Bauer TW, Gabriel SM, O’Connell M, Clifford A. Evaluation of the safety of a novel knee load-bypassing device in a sheep model. J Bone Joint Surg Am. 2012;94(1):77–84. | |
Citak M, Kendoff D, PF OL, et al. Failed joint unloading implant system in the treatment of medial knee osteoarthritis. Arch Orthop Trauma Surg. 2013;133(11):1575–1578. | |
Clifford A, O’Connell M, Gabriel S, Miller LE, Block JE. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J Med Eng Technol. 2011;35(1):65–71. | |
Clifford AG, Gabriel SM, O’Connell M, Lowe D, Miller LE, Block JE. The KineSpring (®) Knee Implant System: an implantable joint-unloading prosthesis for treatment of medial knee osteoarthritis. Med Devices (Auckl). 2013;6:69–76. | |
Gabriel SM, Clifford AG, Maloney WJ, O’Connell MK, Tornetta Iii P. Unloading the osteoarthritic knee with a novel implant system. J Appl Biomech. 2013;29(6):647–654. | |
Hayes DA, Miller LE, Block JE. Knee osteoarthritis treatment with the KineSpring Knee Implant System: a report of two cases. Case Rep Orthop. 2012;2012:297326. | |
Li CS, Seeger T, Auhuber TC, Bhandari M. Cost-effectiveness and economic impact of the KineSpring® Knee Implant System in the treatment for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2629–2637. | |
Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93(1):1–24, xv. | |
Wilson DR, McWalter EJ, Johnston JD. The measurement of joint mechanics and their role in osteoarthritis genesis and progression. Med Clin North Am. 2009;93(1):67–82, x. | |
Radin EL, Burr DB. Hypothesis: joints can heal. Semin Arthritis Rheum. 1984;13(3):293–302. | |
Bowditch M, Miller LE, Block JE. Successful two-stage revision of a KineSpring® joint unloading implant: a case study. Int Med Case Rep J. 2012;5:91–95. | |
Zhao D, Banks SA, Mitchell KH, D’Lima DD, Colwell CW Jr, Fregly BJ. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J Orthop Res. 2007;25(6):789–797. | |
London NJ, Smith J, Miller LE, Block JE. Midterm outcomes and predictors of clinical success with the KineSpring Knee Implant System. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:19–28. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
