Back to Journals » Infection and Drug Resistance » Volume 12
Post-splenectomy sepsis: preventative strategies, challenges, and solutions
Authors Luu S , Spelman D, Woolley IJ
Received 22 May 2019
Accepted for publication 30 July 2019
Published 12 September 2019 Volume 2019:12 Pages 2839—2851
DOI https://doi.org/10.2147/IDR.S179902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Sarah Luu,1 Denis Spelman,2,3 Ian J Woolley3–5
1Australian Centre for Blood Diseases, Monash University, Melbourne, Victoria, Australia; 2Department of Infectious Diseases and Microbiology, Alfred Health, Melbourne, Victoria, Australia; 3Spleen Australia, Alfred Health, Melbourne, Victoria, Australia; 4Monash Infectious Diseases, Monash Health, Clayton, Victoria, Australia; 5Centre for Inflammatory Diseases, Monash University, Clayton, Victoria, Australia
Correspondence: Ian J Woolley
Monash Infectious Diseases, Monash Medical Centre, Clayton Rd, Victoria 68, Australia
Tel +61 39 594 4533
Fax +61 39 594 4564
Email [email protected]
Abstract: Removal of the spleen had already been established as a routine technique to treat splenic trauma and other diseases affecting the spleen before the anatomy, physiology, and function of the spleen were known in the mid-twentieth century. It is now widely accepted that the splenectomized individual is at increased risk for infection, in particular, overwhelming post-splenectomy infection (OPSI). OPSI is a syndrome of fulminant sepsis occurring in splenectomized (asplenic) or hyposplenic individuals that is associated with high mortality and morbidity. Poorly opsonized bacteria such as encapsulated bacteria, in particular, Streptococcus pneumoniae, are often implicated in sepsis. The spleen is a reticuloendothelial organ that facilitates opsonization and phagocytosis of pathogens, in addition to cellular maintenance. Splenectomy is associated with an impairment in immunoglobulin production, antibody-mediated clearance, and phagocytosis, leading to an increased risk of infection and sepsis. Early identification of the at-risk patient, early blood cultures prior to antibiotic administration, urgent blood smears and fast pathogen-detection tests, and sepsis bundles should be utilized in these patients. Prompt management and aggressive treatment can alter the course of disease in the at-risk splenectomized patient. Overwhelming post-splenectomy infection can be prevented through vaccination, chemoprophylaxis, and patient education. This article evaluates post-splenectomy sepsis by summarizing the anatomy and function of the spleen, physiological changes after splenectomy that predispose the splenectomized patient to infection, and current management and prevention strategies.
Keywords: splenectomy, sepsis, OPSI, asplenism
Introduction
Splenectomy had been performed for centuries prior to the establishment of the spleen’s physiology and function. The first documented splenectomy was performed in 1549 to remove a symptomatic enlarged spleen.1 During the nineteenth century, it was realized that splenectomy was an ineffective treatment for leukemia but could be utilized to treat penetrating injuries to the left upper quadrant of the abdomen. By the mid-twentieth century, it was a therapy for idiopathic/immune thrombocytopenia purpura (ITP).2 Morris and Bullock in 1919 were the first to provide evidence that splenectomy was associated with increased number of infections and impaired immunity to fight infection by infecting non-splenectomized and splenectomized rodents with the bacillus of rat plague.3 Reports of overwhelming post-splenectomy infection (OPSI) began to appear and it became clear that splenectomy was associated with impaired antibody synthesis and production, in addition to other immunological impairments.2,4 Greater understanding of the spleen’s anatomy and physiology in the last century has altered the approach to diseases involving the spleen and development of strategies to prevent post-splenectomy sepsis and the outcomes associated with OPSI.
There are many challenges in optimizing management and prevention of OPSI. Current strategies for prevention of infection involve patient education, vaccination, and antibiotic prophylaxis, in addition to ongoing involvement with a clinical registry such as the Spleen Australia registry. Limited effectiveness of vaccines and antibiotics in addition to variable adherence and compliance to recommendations are both factors that limit optimal protection and prevention of infection. In this review, we describe existing strategies for prevention and management, the challenges in prevention and management, and the solutions required to improve our approach to post-splenectomy sepsis.
Background
Splenectomy
Splenectomy is performed as a life-saving procedure in traumatic events, a therapeutic procedure in hematological conditions, a life-preserving procedure in malignant conditions, and a tactic for diagnosis of disease. The incidence of splenectomy is approximately 6.4–7.1 per 100,000 per year around the world.5–8 It is most commonly performed for trauma and hematological conditions. Approximately one-quarter of splenectomies are performed in a trauma setting, often where trauma to the abdomen has led to rupture of the spleen and consequent internal bleeding with life-threatening hemodynamic instability.7 Splenectomy for trauma is on the decline with increasing use of alternative procedures. Another 25% of splenectomies are performed in the setting of hematological diseases such as immune thrombocytopenia purpura, sickle cell disease, thalassemia, and hereditary spherocytosis.7,9,10 In addition to its use in the treatment of local malignancy, it is sometimes performed following iatrogenic injury during an unrelated abdominal procedure.7 Splenic function can be absent (asplenia) following surgical splenectomy or at birth (congenital asplenia) as a consequence of genetic variations such as isolated congenital asplenia and asplenia syndrome.11,12 Splenic function can also be impaired (hyposplenism) secondary to medical conditions, including but not limited to celiac disease, inflammatory bowel disease, and systemic lupus erythematosus.13 The anatomical structure of the spleen facilitates its physiological functions of filtration and cellular quality surveillance, and in the absence of the spleen, impairment in these functions predisposes these individuals to infection.
Splenic anatomy
The spleen is highly vascular: filtering 150 mL of blood per minute. Its unique architecture and circulation facilitate its functions of culling senescent erythrocytes, removing intra-erythrocytic inclusions and removing pathogens from the bloodstream.
Vasculature and innervation
The spleen receives its vascular supply from the splenic artery, a branch of the coeliac trunk, and collaterals from surrounding organs. The splenic artery enters at the splenic hilum and branches sequentially into central arteries and arterioles, terminating once splenic parenchyma is encapsulated in a lobulated fashion. Splenic microcirculation is characterized by an open circulation where central arterioles end in the splenic cords. This traps blood in a high pressure and low flow environment. This mechanism slows the filtration of blood, allowing for optimal pathogen clearance through increased exposure of foreign or unwanted material to splenic macrophages. This mechanism also traps damaged or senescent cells that have lost their membrane flexibility as blood must squeeze past gaps in the endothelium of venous sinuses to return to the circulation. During this process, the splenic macrophages also have the ability to remove intra-erythrocytic inclusions (“pitting”) such as Howell–Jolly bodies, erythrocyte “pits” and siderocytes without destruction of the entire erythrocyte.13–16 Many authorities have described the presence of a closed circulation in addition to an open circulation.13,17 However, Steiniger and colleagues (2011)18 noted that other than the initial study by Weidenreich and colleagues (1901),19 repeated anatomical studies have not been able to prove the presence of the closed circulation suggesting that it may not exist. Regardless of its presence, blood leaves the spleen through venous sinuses.
In addition to its arterial and venous vasculature, the spleen is innervated by parasympathetic and sympathetic fibers. Under adrenergic stimulation, the spleen can contract and expel a sequestered population of platelets, erythrocytes, and granulocytes from the parenchyma into the circulation.20–23
Parenchyma
The spleen consists of red pulp, white pulp, and an intermediate marginal zone (Figure 1). Upon repeated analysis of human spleens, Steiniger and colleagues (1997) noted that many descriptions of human splenic micro-anatomy13,17 reflect murine splenic micro-anatomy.24 This is presumably based on the assumption that the anatomy of the spleen in the human and mouse is the same. However, studies performed by Steiniger and colleagues,24 and Van Krieken and Te Velde,25,26 have shown that although the human and murine spleen share some similarities, there are many differences in their architecture and distribution of cells.
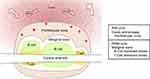 |
Figure 1 Splenic anatomy. |
Splenic red pulp in both humans and mice consists of splenic cords and venous sinuses. The red pulp constitutes 75% of the total splenic volume and is involved in maintenance of erythrocytes, sequestration/storage of cells including erythrocytes, granulocytes and platelets, and storage of iron. In the red pulp of the human spleen, there is a perifollicular zone, a compartment where erythrocytes accumulate outside of the marginal zone.24
The white pulp is primarily lymphocytic and consists of the remaining 25% of the splenic volume. In the human spleen, it consists primarily of B-lymphocytes (follicles) and some T-lymphocytes. Typical descriptions of splenic anatomy describe a peri-arteriolar lymphoid sheath (PALS; T-cell dominant), the follicle (B-cells), and the marginal zone. However, although this structure is true for murine spleens, in the human there are not continuous T-cell sheaths which encapsulate the central arterioles. Rather, the human white pulp consists predominantly of B-cells with follicles interrupting the T-cell dominant zones and encapsulating central arterioles.
The marginal zone is typically described as a layer that surrounds the T-cell and B-cell dominant zones, situated between the red and white pulp. It is thought to allow lymphocytes to continuously pass between the blood in the red pulp and the lymphocytic white pulp. The marginal sinus, the intermediate layer of the marginal zone in the mouse, is not present in humans. Rather, the perifollicular zone in the human red pulp is thought to play the role of the murine marginal sinus.24
Splenic development and changes with aging
The spleen arises from mesoderm in the dorsal mesogastrium during gestation. Accessory spleens, present in 14.5% of the population,27 arise where condensations of mesoderm fail to fuse.
There is poor protection to encapsulated bacteria during the early and late years of life and this may be associated with splenic cellular maturity. Cellular maturation of the spleen occurs during the first two decades of life. Initially, there is a high concentration of follicles in the spleen which decreases with age. After the third decade, where follicles peak in size, follicular cellularity reduces until they are atrophic by the eighth decade of life, suggesting that immunological splenic function deteriorates with age.28
Spleen function
Immunological function
The spleen can initiate immune responses to blood-borne antigens, produce antibodies, and clear antibody-mediated pathogens. The spleen consists of cells involved in both innate and adaptive immunity. The structure and cellular constituents of the spleen that provide splenic immunological function have been recently reviewed.29 Splenic macrophages have the ability to detect and remove bacteria in the circulation. Red pulp macrophages filter the blood and remove bacteria, damaged erythrocytes, and erythrocyte inclusions. Marginal zone macrophages remove cellular debris in the marginal zone and tingible body macrophages remove B-cell debris in the germinal center of the follicle. In addition to macrophages, there are also dendritic cells, natural killer cells, and monocytes that are involved in inducing T cell responses to pathogens.29
The white pulp of the spleen is B-cell dominant (follicles) with some T cell zones. Splenic B cells are required to produce specific antibodies for immunity (affinity maturation) and to enhance cytotoxic T-cell activity. Some bacteria such as encapsulated bacteria require opsonization to facilitate phagocytosis. In this case immunoglobulin (Ig)-M memory B cells, present where there is a functioning spleen, produce IgM that acts as an opsonin to facilitate the clearance of the polysaccharide-encapsulated bacteria. The spleen also produces and maintains levels of tuftsin which is involved in stimulating phagocytosis.30 The cellular structure of the spleen is designed not only to effectively provide immunity, but it also effectively removes damaged erythrocytes from the circulation.
Hematological function
Erythrocytes must squeeze through endothelial gaps to exit the splenic cords and enter the venous sinuses for drainage. This slowed mechanism allows for splenic macrophages to remove any undesired intra-erythrocytic inclusions and phagocytose defective or old erythrocytes with reduced membrane flexibility that are unable to enter into the venous sinuses.
The spleen also sequesters blood cells including platelets. The spleen is thought to pool approximately one-third of the total platelet volume in addition to sequestration of erythrocytes and granulocytes.
Tests of splenic function evaluate the capacity of the spleen to remove intra-erythrocytic inclusions such as Howell–Jolly bodies and erythrocyte pits, in addition to its ability to maintain IgM memory B cell population.
Tests of splenic function
Howell–Jolly bodies can be seen on a routine peripheral blood smear (Giemsa-Wright stain) where splenic function is impaired. This is a simple and quick qualitative test to evaluate splenic function. When present on film, it is a good marker to identify an individual with impaired splenic function. After splenectomy, however, its presence does not always exclude the presence of ectopic splenic tissue.31
Pitted red cell (erythrocyte) counting is a more sensitive test of the erythrocyte maintenance function of the spleen as it can better evaluate the splenic function, even in the setting of ectopic splenic tissue.31 This test is performed using differential interference contrast (DIC) microscopy to count the proportion of erythrocytes that have pits (membrane vacuoles). A pitted red cell count of less than or equal to 4% is associated with normal splenic function, whilst a count above 4% is associated with impaired splenic function.13
To evaluate the immunological capacity of the spleen, IgM memory B cells can also be measured. After splenectomy, IgM memory B cells are absent or significantly reduced.33,34
In addition to these blood tests, splenic function can also be evaluated using nuclear medicine techniques such as scintigraphy. 99m-Technetium-labeled heat-denatured red cell scans or 99m-Technetium-labeled sulfur colloid scans can be utilized to evaluate the morphology and function of the spleen.
Consequences of splenectomy
Although the function of the spleen is not imperative for survival, removal of the spleen is not without consequences. Prior to the twentieth century, the spleen was largely considered an obsolete organ. However, it is now evident that splenectomy is associated with an increased risk of infection and thromboembolism.8
After splenectomy, there are alterations to cell counts, cell quality, and immunological responses. Initially after splenectomy, a reactive thrombocytosis and leukocytosis can be seen. The reactive thrombocytosis often resolves after 6 to 12 months after splenectomy, but not uncommonly may persist. The leukocytosis is primarily granulocyte driven, as neutrophils are often elevated after splenectomy,35 and has seen to persist many years after splenectomy.36 Howell–Jolly bodies (the nuclear remnant of the erythrocyte predecessor), characteristic for asplenia, appear approximately 30 days after splenectomy.33 An increase in erythrocyte pits (Figure 2) can also be seen shortly after splenectomy.37 In addition to altered erythrocyte quality, lymphocyte cohorts proportions are altered. Although total B lymphocytes remain largely intact, there is a significant reduction in immunoglobulin (Ig)M memory B cells and switched B cell proportions may also be reduced. This occurs approximately 150 days post-splenectomy.33 This leads to a predisposition to infection by bacteria encapsulated by a polysaccharide capsule and reduction in the immunological response to polysaccharide vaccines.38,39 These hematological and immunological alterations predispose the splenectomized individual to infection.
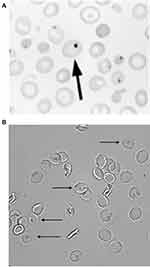 |
Figure 2 Erythrocyte tests of splenic function: (A) Howell–Jolly bodies (Reprinted with permission from Detection, Education and Management of the Asplenic or Hyposplenic Patient, February 1, 2001, Vol 63, No 3, American Family Physician Copyright © 2001 American Academy of Family Physicians. All Rights Reserved32) (B) Pitted erythrocytes.Notes: The arrow in Panel A shows the Howell-Jolly Body in the erythrocyte. The arrows in Panel B indicates the pitted erythrocytes. |
Infection following splenectomy
Removal of the body’s largest lymphoid organ is associated with increased risk of infection, among other complications including thromboembolism. Following splenectomy, individuals have an elevated risk of infection, in particular to encapsulated bacteria, Gram-negative pathogens such as Capnocytophagia carnimorsus and Bordetella holmesii,40,41 and intra-erythrocyte parasites such as Malaria and Babesia.42,43
Overwhelming post-splenectomy infection
In addition to their increased risk of infection, splenectomized individuals are at risk to OPSI. OPSI, although variously defined,44,45 is generally defined as a syndrome of fulminant sepsis that may initially present as generalized non-specific viral symptoms but very quickly deteriorates into a fulminant sepsis within 24–48 hrs. OPSI may initially present as a mild viral-like illness that can be difficult to diagnose early on. Symptoms of OPSI include fever, shakes, shivers, chills, diarrhea, and vomiting. As a syndrome, there is no specific diagnostic criteria for OPSI. However, the splenectomized individual with these symptoms should be considered at risk for OPSI. The incidence rate of OPSI among splenectomized individuals is 0.13 per 100 person years46 and is associated with significant morbidity and up to 50–70% mortality.47 Early identification and prompt management are suggested to reduce mortality rates.48
The risk of infections is highest in the first 2 to 3 years following splenectomy but remains elevated for the individual’s life.8,44,49,50 Young children (under the age of 2) and individuals splenectomized for hematological malignancy or malignant conditions are at highest risk for sepsis,44,51 whereas individuals splenectomized for trauma have the lowest risk. Presence of functional splenic tissue, commonly seen after splenectomy for trauma, may be protective against infection. Physiological alterations to immunity and use of therapeutic agents in the case of hematological disease and malignancy are likely to further increase the risk and propensity of severe infection.
OPSI is often caused by encapsulated bacteria (bacteria with a poorly opsonized polysaccharide capsule) such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b or Gram-negative bacteria such as Escherichia coli and Pseudomonas species (spp.). Sepsis is a syndrome of systemic inflammation caused by an infection. It is characterized by increased vascular permeability, dysregulated vascular tone (increased nitric oxide and prostaglandin), and activated inflammatory mechanisms including pyroptosis (pro-inflammatory cell death mechanism following inflammasome activation) that lead to elevated inflammatory mediators such as TNFα.52–54 Abolishment of the effective opsonizing filter function of the spleen leads to an increase in infections of poorly opsonized bacteria. Impaired clearance of the offending pathogen, due to delayed and impaired immunoglobulin production33,55,56 and reduced phagocytic function (absent splenic macrophages and reduced tuftsin production),30,55 increases both the rates of infection and the propensity for severe infection. The complement system in the splenectomized patient is largely intact as major complement proteins C3, C4, and transferrin remain at normal levels.55
In addition to these immunological changes, there is an alteration in the course of systemic inflammation in the splenectomized individual. The spleen plays an imperative role in the cholinergic anti-inflammatory pathway. Splenic macrophages are the largest producers of tumor necrosis factor (TNF) in sepsis. Upon vagal stimulation, production and secretion of TNF and other pro-inflammatory cytokines are attenuated and anti-inflammatory cytokines such as IL-10 are secreted. Following splenectomy, the cholinergic anti-inflammatory pathway is completely inhibited.57,58 Although its direct association has yet to be established, impairment in this anti-inflammatory pathway may play a role in the increased propensity for overwhelming post-splenectomy infection (OPSI). Further investigation is required to identify the pathogenesis of OPSI to identify additional targets for management.
Challenges
Prompt diagnosis and management of OPSI can prevent deterioration and fatality. Early diagnosis requires health care workers to be aware of OPSI and its outcomes, to be able to quickly identify an at-risk patient or OPSI in an otherwise unsuspected patient, and to quickly initiate treatment. If a history of splenectomy cannot be obtained from the patient, collateral history from family or the presence of a surgical scar can help identify the at-risk individual. In the setting where a history of impaired splenic function is not clearly detailed, identifying the syndrome of OPSI is imperative. Overwhelming infection presents with signs of severe infection and systemic inflammation (high fever, altered mental state, vomiting, and diarrhea) beyond the individuals’ expected immunological capacity, in addition to faster deterioration and higher requirements for therapy. In this individual, a blood film should be performed and reviewed urgently to evaluate for the presence of Howell–Jolly bodies, as identifying their immunocompromised state alters the course of management. In addition to the identification of the patient and the risk of OPSI, first dose of antibiotics is the most important step to survival. Swift management by applying aggressive fluid therapy and monitoring are also imperative. Early identification of the implicated organism can help guide antibiotic therapy.
Not only is it important to identify and aggressively treat these patients, it is also imperative that such infections are prevented. Prevention of infections involves vaccination, antibiotic prophylaxis, and patient education. These strategies were formed on the basis of existing practices and new evidence in the literature. Some of these recommendations are less well supported by good quality evidence and the effectiveness of these strategies is unclear. Poor uptake and adherence of prevention strategies is another barrier encountered in preventing infection. Many studies have evaluated education levels and uptake of vaccinations in splenectomized individuals and have reported sub-optimal proportions of uptake. Combating OPSI through prevention requires active involvement from both patients and health care providers to ensure uptake of immunization and adherence to antibiotics, in addition to further research to evaluate current and new strategies to prevent infection.
Solutions
Management and treatment
Sepsis in the splenectomized patient should be managed promptly and aggressively to prevent the fulminant course of disease. The course of OPSI is fulminant and deteriorates more rapidly than expected. Sepsis management bundles exist to expedite initial management.59 In this setting, collecting two sets of blood cultures prior to administration of antibiotics is imperative to identify the offending pathogen. Performing a buffy coat or gram stain on blood film urgently can expedite identification of the pathogen and direct a more specific and appropriate antibiotic course. Aggressive fluid therapy and intensive monitoring is imperative in these individuals. Use of blood purification therapies with adjunctive adsorbent treatment should also be considered to reduce inflammatory mediators and improve hemodynamic stability as its use appears beneficial in splenectomized individuals with pneumococcal sepsis.60,61 Where risk of OPSI is known, such as in the splenectomized individual, adherence to prevention strategies is recommended to prevent OPSI.
Preventing infections
Educating patients in addition to utilizing vaccination and antibiotic prophylaxis is imperative in preventing infections in the splenectomized individual. As mentioned earlier, existing recommendations (Table 1) are based upon limited evidence. There is further scope to optimize these strategies through new vaccines and/or investigating new pharmacological agents that may combat the intrinsic impairment in immunological function after splenectomy. In optimizing strategies, the use of a clinical registry has been shown to be effective in preventing adverse outcomes associated with splenectomy. Here we describe the currently available strategies to prevent infections and the literature that supports its use.
 |
Table 1 Medical prevention for splenectomized individuals |
Patient education
Patient education is considered an integral factor, if not the most important factor, for preventing OPSI. Splenectomized individuals should be educated about their post-splenectomy state, the increased risk of infection, how to prevent the risk of infection, and what to do in the case of illness. In addition, splenectomized individuals should seek medical advice prior to any travel, especially if traveling to a malaria-endemic country. Additional information that individuals with asplenism should be educated with are listed in Table 2.
 |
Table 2 Information for splenectomized individuals |
Studies performed by Hegarty and colleagues (2000) and El Alfy (2004) have shown that a large proportion of splenectomized individuals had limited knowledge about asplenia and the associated potential complications.62,63 In addition, El Alfy and colleagues revealed that those who had better knowledge had lower risk of OPSI.63
A systematic review of online resources for asplenic patients revealed websites only covered a portion of all the information that a patient should know.64 Limited access to information and recommendations is a barrier to improving patient knowledge. This emphasizes the importance for medical professionals to educate themselves and their asplenic patients.
Vaccination
Splenectomized individuals have impaired immunological memory due to the absence of IgM memory B cells and impaired ability to opsonize and clear encapsulated bacteria. Vaccination against S. pneumoniae, N. meningitidis, and H. influenzae type b (Hib) can help prevent OPSI by establishing immunological memory. Initial vaccinations should be administered either 14 days prior to planned splenectomy or 14 days after an urgent splenectomy to ensure an adequate response.
Vaccination with conjugated vaccines or combination schedules with polysaccharide vaccines aim to ensure adequate immunological memory and broad coverage of serotypes. Conjugated vaccines establish better immunological responses in individuals with asplenia as compared to polysaccharide vaccines. Polysaccharide vaccine-initiated immunological memory relies on the thymus-independent (T-cell independent) pathway which is significantly impaired following splenectomy. Conjugated vaccines, however, use the thymus-dependent (T-cell dependent) pathway to establish immunological memory which remains intact.38 Whilst mucosally active vaccines are currently under investigation to induce immunity against S. pneumoniae and N. meningitidis serotype B, their role in the asplenic individual has yet to be evaluated.65,66
Pneumococcus can be targeted using a combination schedule of the 13-valent conjugated vaccine (PCV13) and augmenting it with the 23-valent polysaccharide vaccine (PPV23). This is aimed to ensure both effective and broad coverage of pneumococcal serotypes. Broader-coverage conjugated vaccines, that are currently being formulated and tested, will provide better immunological responses over a broader range of pneumococcal serotypes. Conjugated meningococcal vaccines are recommended in individuals with asplenia as a quadrivalent (serotypes A, C, W, and Y) vaccine and, a more recently available, serotype B vaccine. Hib vaccines are often conjugated to diphtheria or other proteins and can be safely administered in individuals with asplenia.
Although influenza itself does not pose significant risk to the individual with asplenia, there is a heightened risk associated with secondary bacterial infection. Thus, seasonal (annual) influenza vaccination is also recommended.
There is slight variability in recommendations in vaccination of individuals with asplenia (Table 3). Most recent guidelines67–70 recommend PCV13 administration prior to administration with PPV23. In upkeeping with new vaccines and evidence, these same guidelines also recommend initial and booster doses of the quadrivalent conjugated meningococcal vaccine (MenACWY) and the recombinant meningococcal B vaccine (MenBV) in splenectomized adult patients. However, guidelines from the Green Book (United Kingdom) (2016) do not recommend administration of PCV13, administration of the booster dose of MenACWY, or administration of MenBV in individuals with asplenia. Other than variations in pneumococcal and meningococcal vaccine recommendations, all guidelines are consistent in recommending a single dose of Hib vaccine and seasonal annual influenza vaccine.
 |
Table 3 Vaccination recommendations |
Antibiotics
As the infection risk is highest in the initial years after splenectomy, all splenectomized patients are recommended to take daily antibiotic prophylaxis for the initial few years. Australian Antibiotic Guidelines and Spleen Australia recommend 3 years of daily antibiotic prophylaxis initially after splenectomy.68,69 Guidelines elsewhere vary in their recommendations for duration of daily antibiotic use post-splenectomy.74 Individuals who are at high risk for infections due to other comorbidities are recommended to take daily lifelong antibiotics.
These antibiotic recommendations are based on two studies performed in children with hyposplenism secondary to sickle cell disease,75–77 where antibiotic prophylaxis reduced S. pneumoniae infections. There have been no studies that have evaluated the effect of daily antibiotic use for any other indication for splenectomy nor in splenectomized adults. In addition, there are multiple issues regarding long-term antibiotic use, including microbial resistance in addition to medication adherence.78 It remains unclear whether the benefit of antibiotic prophylaxis is suitable for other splenectomized groups and/or for adults. Some guidelines do not recommend an initial period and some recommend lifelong antibiotics for all individuals with asplenia.74,79 Most guidelines recommend an initial period of daily antibiotic use after splenectomy, and consideration for lifelong use based on risk for infection.
In addition to the use of daily antibiotics, splenectomized individuals are recommended to carry their own supply of high-dose antibiotics in case of emergency. These recommendations suggest that in the case of illness or symptoms associated with OPSI, patients should self-administer a high-dose of antibiotics and seek urgent medical attention.
Clinical registry
Studies have suggested that use of a clinical registry may improve uptake and adherence to recommended interventions, as well as patient education. Various registries for individuals with asplenia have previously existed, including formal registries such as those in the United Kingdom and informal registries.80–82 These registries have served various purposes including a review of vaccine immunity at registration (over a 2-year period of registration), adherence and uptake of preventative interventions and management and ongoing clinical management. The UK Department of Health provides education and management for individuals with asplenia, as evidenced by their publicly available brochures, recommendations, and wallet-sized alert card.83 Unlike the United Kingdom, the Spleen Australia, a clinical service based at The Alfred Hospital in Melbourne, provides education and ongoing management recommendations for patients and their physicians. Studies have shown that our local clinical registry, the Spleen Australia clinical registry, has a positive effect on patient uptake of ongoing (booster) vaccinations,84–86 is associated with a reduction in invasive pneumococcal disease,87 and is cost-effective in its activities.88 Improved uptake of vaccination with participation with a registry program has also been shown elsewhere.80 The Spleen Australia registry is for individuals with asplenia and hyposplenia in Australia. It was initially established in 2003, as the Victorian Spleen Registry. Over the last 16 years, it has grown and expanded to now include Queensland and Tasmania. With almost 10,000 registrants on the registry to date, the Spleen Australia registry proactively registers eligible patients onto the registry and provides education and advice about their long-term care associated with asplenia or hyposplenism.
Where are we now?
Although there may be variation in recommendations globally, all guidelines should target the three arms of prevention – patient education, vaccination, and antibiotic prophylaxis. However, there still remains a risk of OPSI even if all prevention interventions are taken. Infection rates are extremely low. Vaccines studies often evaluate effectiveness by serology and not clinical outcomes. Thus, there is limited evidence that determines the effectiveness of each intervention (or combined uptake of all interventions) in preventing infection.
The persistent risk of OPSI may also partly be explained by sub-optimal uptake of interventions and levels of patient education.62,63,89,90 Our experience with Spleen Australia has shown us the challenges in optimizing uptake with expensive vaccines, supporting ongoing adherence with updated recommendations, engaging long-term registrants, and back-tracking to find old splenectomy patients who have never received education about their risk of infection nor received vaccines for their post-splenectomy risk of infection.
Even with optimal uptake of interventions, vaccine failure (infection where the pathogen is a serotype that is covered by a vaccine that has been administered) may occur or a non-vaccine preventable pathogen may cause an infection. Early presentation to medical services in the case of illness is imperative in limiting the progression of sepsis in the splenectomized individual. Small drug molecules, including but not limited to TLR antagonists, have been investigated in their ability to inhibit or prevent the progression of sepsis with limited clinical success.91,92 Caspase-inhibitors, C5a antagonists, and COX-inhibitors, in addition to other small molecule drugs, are under investigation in their potential role in inhibiting sepsis.91–93 Early recognition of the risk of OPSI and prompt management using sepsis bundles and protocols is of the utmost importance.
Presence of preserved splenic tissue either protected using splenic preservation procedures, not removed at time of splenectomy or auto-transplanted may provide splenic immunological function. Individuals who receive splenic preservation procedures such as splenic artery embolization are considered immunocompetent, as they do not lose their population of IgM memory B cells.94 Splenic preservation procedures can be utilized as an alternative to splenectomy and are becoming increasingly used for higher-grade splenic injury to stop blood loss and salvage the spleen and its functions. Presence of residual functional splenic tissue after splenectomy may also provide splenic immunological function. This can occur in the form of an embryologically derived accessory spleen or spontaneous autotransplantation (splenosis). However, its immunological contribution remains unclear and appears variable.95 Autotransplantation of splenic tissue has been considered as an option to retain splenic tissue and there is work to evaluate its feasibility and contribution. However, there remain challenges around the surgical procedure including amount of volume of tissue to implant, location of implantation, handling of splenic tissue, and surgical procedure.96–98 Although splenic preservation procedures are a great and effective alternative to splenectomy, they can only be utilized in specific conditions such as trauma. Prevention of infection for individuals who receive splenectomy for hematological conditions or malignancy remains limited to vaccination and antibiotic use.
There remains scope to increase awareness of the impact and severity of OPSI amongst health professionals, patients, and in the general community. Individuals who have received a total splenectomy should be registered to a spleen registry if available. They should also receive initial and ongoing vaccines and take adequate antibiotic prophylaxis. Patients should have a thorough understanding of the risk of infection without a functioning spleen and should be fully aware of what they should do in the circumstance of illness. Prevention of infection and prompt management are imperative in ensuring the patient has the best chance of surviving OPSI unscathed. In addition, further research into the effectiveness of recommended interventions will better inform physicians to optimize management for these patients.
Conclusion
The body’s immunological armor is damaged with removal of the spleen, providing a gateway for infections with poorly opsonized bacteria. Sepsis in the splenectomized individual is often severe and associated with high morbidity and mortality. Ongoing prevention with vaccination, antibiotic prophylaxis, and patient education is imperative in reducing the risk of infection. Early identification and prompt management in the circumstance of infection provide the splenectomized individual the best chance of survival. Further research is required to optimize existing prevention strategies and to improve management reduce the impact of OPSI on the splenectomized individual.
Acknowledgment
This work was supported by an Australian Government Research Training Program (RTP) Stipend.
Disclosure
Dr Sarah Luu reports grants from The Royal College of Pathologists of Australasia, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. McClusky DA, Skandalakis LJ, Colborn GL, Skandalakis JE. Tribute to a triad: history of splenic anatomy, physiology, and surgery—part 1. World J Surg. 1999;23(3):311–325.
2. McClusky DA, Skandalakis LJ, Colborn GL, Skandalakis JE. Tribute to a triad: history of splenic anatomy, physiology and surgery – part 2. World J Surg. 1999;23(5):514–526.
3. Morris DH, Bullock FD. The importance of the spleen in resistance to infection. Ann Surg. 1919;70(5):513–521. doi:10.1097/00000658-191911000-00001
4. King H, Shumacker HB
5. Yong M, Thomsen RW, Schoonen WM, et al. Mortality risk in splenectomised patients: a Danish population-based cohort study. Eur J Intern Med. 2010;21(1):12–16. doi:10.1016/j.ejim.2009.10.003
6. Thomsen RW, Schoonen WM, Farkas DK, Riis A, Fryzek JP, Sørensen HT. Risk of venous thromboembolism in splenectomized patients compared with the general population and appendectomized patients: a 10-year nationwide cohort study. J Thromb Haemost. 2010;8(6):1413–1416. doi:10.1111/j.1538-7836.2010.03849.x
7. Dendle C, Sundararajan V, Spelman T, Jolley D, Woolley I. Splenectomy sequelae: an analysis of infectious outcomes among adults in Victoria. Med J Aust. 2012;196(9):582–586.
8. Kristinsson SY, Gridley G, Hoover RN, Check D, Landgren O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica. 2014;99(2):392–398. doi:10.3324/haematol.2013.092460
9. Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018;131:1172–1182. doi:10.1182/blood-2017-09-742353
10. Sandusky WR, Leavell BS, Benjamin BI. Splenectomy: indications and results in hematologic disorders. Ann Surg. 1964;159(5):695–709. doi:10.1097/00000658-196405000-00007
11. Iijima S. Sporadic isolated congenital asplenia with fulminant pneumococcal meningitis: a case report and updated literature review. BMC Infect Dis. 2017;17(1):777. doi:10.1186/s12879-017-2896-5
12. Bhalla K, Singh J, Yadav J, Mehra S. Asplenia syndrome in a neonate: a case report. J Clin Diagn Res. 2016;10(6):SD05–SD06. doi:10.7860/JCDR/2016/18535.8028
13. Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378(9785):86–97. doi:10.1016/S0140-6736(10)61493-6
14. Nathan DG. Rubbish in the red cell. N Engl J Med. 1969;281(10):558–559. doi:10.1056/NEJM196909042811013
15. Crosby WH. Normal functions of the spleen relative to red blood cells: a review. Blood. 1959;14(4):399–408.
16. Crosby WH. Siderocytes and the spleen. Blood. 1957;12(2):165–170.
17. Hall JE, Guyton AC. Guyton and Hall Textbook of Medical Physiology.
18. Steiniger B, Bette M, Schwarzbach H. The open microcirculation in human spleens: a three-dimensional approach. J Histochem Cytochem. 2011;59(6):639–648. doi:10.1369/0022155411408315
19. Weidenreich F. Das gefässsystem der menschlichen Milz. Archiv für Mikroskopische Anatomie. 1901;58(1):247–376. doi:10.1007/BF02906775
20. Penny R, Rozenberg MC, Firkin BG. The splenic platelet pool. Blood. 1966;27(1):1–16.
21. Wadenvik H, Kutti J. The spleen and pooling of blood cells. Eur J Haematol. 68;41(1):1–5. doi:10.1111/j.1600-0609.1988.tb00861.x
22. Wadenvik H, Kutti J. The effect of an adrenaline infusion on the splenic blood flow and intrasplenic platelet kinetics. Br J Haematol. 1987;67(2):187–192. doi:10.1111/j.1365-2141.1987.tb02325.x
23. Ilardo MA, Moltke I, Korneliussen TS, et al. Physiological and genetic adaptations to diving in sea nomads. Cell. 2018;173(3):569–580.e515. doi:10.1016/j.cell.2018.03.054
24. Steiniger B, Barth PJ, Herbst B, Hartnell A, Crocker PR. The species-specific structure of microanatomical compartments in the human spleen: strongly sialoadhesin-positive macrophages occur in the perifollicular zone, but not in the marginal zone. Immunology. 1997;92(2):307–316. doi:10.1046/j.1365-2567.1997.00328.x
25. Van Krieken JHJM, Te Velde J. Normal histology of the human spleen. Am J Surg Pathol. 1988;12(10):777–785.
26. Van Krieken JHJM, Te Velde J. Immunohistology of the human spleen: an inventory of the localization of lymphocyte subpopulations. Histopathology. 1986;10(3):285–294.
27. Vikse J, Sanna B, Henry BM, et al. The prevalence and morphometry of an accessory spleen: A meta-analysis and systematic review of 22,487 patients. Int J Surg. 2017;45:18–28. doi:10.1016/j.ijsu.2017.07.045
28. Lizamma A, Rajan ML, Xavier B, Jacob P, Rani KD, Lakshmi G. Microscopic study of human spleen in different age groups. Int J Res Med Sci. 2015;3(7):1701–1706.
29. Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol. 2019;4:33. doi:10.1126/sciimmunol.aau6085
30. Zoli G, Corazza GR, D’amato G, Bartoli R. Splenic autotransplantation after splenectomy: tuftsin activity correlates with residual splenic function. Br J Surg. 1994;81(5):716–718. doi:10.1002/bjs.1800810530
31. Lammers AJ, de Porto AP, Bennink RJ, et al. Hyposplenism: comparison of different methods for determining splenic function. Am J Hematol. 2012;87(5):484–489. doi:10.1002/ajh.23154
32. Brigden ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician. 2001;63(3):499–506, 508.
33. Cameron PU, Jones PA, Gorniak M, et al. Splenectomy associated changes in IgM memory B cells in an adult spleen registry cohort. PLoS One. 2011;6(8):e23164. doi:10.1371/journal.pone.0023164
34. de Porto APNA, Lammers AJJ, Bennink RJ, Ten Berge IJM, Speelman P, Hoekstra JBL. Assessment of splenic function. Eur J Clin Microbiol Infect Dis. 2010;29(12):1465–1473. doi:10.1007/s10096-010-1049-1
35. Lipson RL, Bayrd ED, Watkins CH. The postsplenectomy blood picture. Am J Clin Pathol. 1959;32(6):526–532. doi:10.1093/ajcp/32.6.526
36. Rab M, Meerveld-Eggink A, van Velzen-Blad H, van Loon D, Rijkers G, de Weerdt O. Persistent changes in circulating white blood cell populations after splenectomy. Int J Hematol. 2018;107(2):157–165. doi:10.1007/s12185-017-2335-9
37. Zago MA, Covas DT, Figueiredo MS, Bottura C. Red cell pits appear preferentially in old cells after splenectomy. Acta Haematol. 1986;76(1):54–56. doi:10.1159/000206019
38. Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985;15(5):508–512. doi:10.1002/eji.1830150516
39. Zandvoort A, Timens W. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin Exp Immunol. 2002;130(1):4–11. doi:10.1046/j.1365-2249.2002.01953.x
40. Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol. 1996;12(5):521–533.
41. Shepard CW, Daneshvar MI, Kaiser RM, et al. Bordetella holmesii bacteremia: a newly recognized clinical entity among asplenic patients. Clin Infect Dis. 2004;38(6):799–804. doi:10.1086/381888
42. Chotivanich K, Udomsangpetch R, McGready R, et al. Central role of the spleen in malaria parasite clearance. J Infect Dis. 2002;185(10):1538–1541. doi:10.1086/340213
43. Rosner F, Zarrabi MH, Benach JL, Habicht GS. Babesiosis in splenectomized adults. Review of 22 reported cases. Am J Med. 1984;76(4):696–701. doi:10.1016/0002-9343(84)90298-5
44. Kyaw MH, Holmes EM, Toolis F, et al. Evaluation of severe infection and survival after splenectomy. Am J Med. 2006;119(3):276 e271–e277. doi:10.1016/j.amjmed.2005.07.044
45. Di Cataldo A, Puleo S, Li Destri G, et al. Splenic trauma and overwhelming postsplenectomy infection. Br J Surg. 1987;74(5):343–345. doi:10.1002/bjs.1800740504
46. Cullingford GL, Watkins DN, Watts AD, Mallon DF. Severe late postsplenectomy infection. Br J Surg. 1991;78(6):716–721. doi:10.1002/bjs.1800780626
47. Standage BA, Goss JC. Outcome and sepsis after splenectomy in adults. Am J Surg. 1982;143(5):545–548. doi:10.1016/0002-9610(82)90158-1
48. Brigden ML. Overwhelming postsplenectomy infection still a problem. West J Med. 1992;157(4):440–443.
49. Ejstrud P, Kristensen B, Hansen JB, Madsen KM, Schonheyder HC, Sorensen HT. Risk and patterns of bacteraemia after splenectomy: a population-based study. Scand J Infect Dis. 2009;32(5):521–525.
50. Green JB, Shackford SR, Sise MJ, Fridlund P. Late septic complications in adults following splenectomy for trauma: a prospective analysis in 144 patients. J Trauma. 1986;26(11):999–1004. doi:10.1097/00005373-198611000-00007
51. Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43(3):182–186. doi:10.1053/jinf.2001.0904
52. King EG, Bauzá GJ, Mella JR, Remick DG. Pathophysiologic mechanisms in septic shock. Lab Invest. 2014;94(1):4–12. doi:10.1038/labinvest.2013.110
53. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi:10.1056/NEJMra021333
54. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi:10.1038/nrmicro2070
55. El Akkad H, Sass W, Colberg A, Knippert A, Seifert J. New arguments to explain the high infection rate in posttraumatic spleenless patients. Zentralbl Chir. 1997;122(10):909–913.
56. Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197(7):939–945. doi:10.1084/jem.20022020
57. Huston JM, Ochani M, Rosas-Ballina M, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203(7):1623–1628. doi:10.1084/jem.20052362
58. Gigliotti JC, Okusa MD. The spleen: the forgotten organ in acute kidney injury of critical illness. Nephron Clin Pract. 2014;127(1–4):153–157. doi:10.1159/000363255
59. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925–928. doi:10.1007/s00134-018-5085-0
60. Leonardis F, De Angelis V, Frisardi F, et al. Effect of hemoadsorption for cytokine removal in pneumococcal and meningococcal sepsis. Case Rep Crit Care. 2018;2018:7.
61. Sinkovic A, Kit B, Markota A. Successful use of combined blood purification techniques in splenectomised patient with septic shock in streptococcus pneumoniae infection – a case report. BMC Infect Dis. 2018;18(1):433. doi:10.1186/s12879-018-3327-y
62. Hegarty PK, Tan B, O’Sullivan R, Cronin CC, Brady MP. Prevention of postsplenectomy sepsis: how much do patients know? Hematol J. 2000;1(5):357–359. doi:10.1038/sj/thj/6200056
63. El-Alfy MS, El-Sayed MH. Overwhelming postsplenectomy infection: is quality of patient knowledge enough for prevention? Hematol J. 2004;5(1):77–80. doi:10.1038/sj.thj.6200328
64. Downing MA, Omar AH, Sabri E, McCarthy AE. Information on the internet for asplenic patients: a systematic review. Can J Surg. 2011;54(4):232–236. doi:10.1503/cjs.005510
65. Heyderman RS, Davenport V, Williams NA. Mucosal immunity and optimizing protection with meningococcal serogroup B vaccines. Trends Microbiol. 2006;14(3):120–124. doi:10.1016/j.tim.2006.01.007
66. Gupalova T, Leontieva G, Kramskaya T, Grabovskaya K, Kuleshevich E, Suvorov A. Development of experimental pneumococcal vaccine for mucosal immunization. PLoS One. 2019;14(6):e0218679. doi:10.1371/journal.pone.0218679
67. Kroger ATDJ, Vázquez M. General best practice guidelines for immunization. In: ACIP, editor. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP); 2017. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html.
68. Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook. Canberra: Australian Government Department of Health, 2018. Available from: https://immunisationhandbook.health.gov.au. Accessed August 19, 2019.Australian Technical Advisory Group on Immunisation. Australian immunisation handbook. In: Health AGDo, editor. Canberra (Australia): Australian Government Department of Health; 2018.
69. Kanhutu K, Jones PA, Cheng AC, Grannell L, Best E, Spelman DW. Spleen Australia guidelines for the prevention of sepsis in patients with asplenia and hyposplenism in Australia and New Zealand. Intern Med J. 2017;47(8):848–855. doi:10.1111/imj.13348
70. Bonanni P, Grazzini M, Niccolai G, et al. Recommended vaccinations for asplenic and hyposplenic adult patients. Hum Vaccin Immunot. 2017;13(2):359–68. doi:10.1080/21645515.2017.1264797
71. Public Health England. Pneumococcal. In: Ramsay M, editor. The Green Book. 2nd ed. London: Public Health England; 2017:1–18. Public Health England. Green Book Chapter 25 Pneumococcal. 2nd ed. 2017:1–18.
72. Public Health England. Pneumococcal. In: Ramsay M, editor. The Green Book. 2nd ed. London: Public Health England; 2017:1–18.Public Health England. Green Book Chapter 25 Pneumococcal. 2nd ed. 2016:1–24.
73. Public Health England. Immunisation of individuals with underlying medical conditions. In: Ramsay M, editor. The Green Book. 2nd ed. London: Public Health England; 2016:1–9.Public Health England. Immunisation of Individuals with Underlying Medical Conditions. 2nd ed. 2016:1–9.
74. Norfolk and Norwich University Hospitals NHS Foundation Trust. Trust Guideline for Patients with an Absent or Dysfunctional Spleen (CA4012). Norwich: NNUH NHS FT; 2017.
75. Salamah MM. Oral penicillin prophylaxis in children with sickle cell anaemia in Saudi Arabia. N Engl J Med. 1987;316(5):274.
76. Colonna P, Ardjoun FZ. Oral penicillin prophylaxis in thalassaemia and in sickle cell anaemia. N Engl J Med. 1986;315(19):1230.
77. Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(25):1593. doi:10.1056/NEJM198605153142021
78. Lau JSY, Korman TM, Woolley I. Life-long antimicrobial therapy: where is the evidence? J Antimicrob Chemother. 2018;73(10):2601–2612. doi:10.1093/jac/dky174
79. Gloucestershire Hospitals. Hyposplenism. Trust Guideline. 2015:13. Available from: https://www.gloshospitals.nhs.uk/gps/antimicrobial-resources/adult-antibiotic-prophylactic-guidelines-specialty/splenectomy-guidelines/.
80. MacInnes J, Waghorn DJ, Haworth E. Management of asplenic patients in South Buckinghamshire: an audit of local practice. Commun Dis Rep CDR Rev. 1995;5(12):R173–R177.
81. Spickett G. Northern Region asplenia register - analysis of first two years. J Clin Pathol. 1999;52(6):424–429. doi:10.1136/jcp.52.6.424
82. Watson AR. Pretravel health advice for asplenic individuals. J Travel Med. 2006;10(2):117–121. doi:10.2310/7060.2003.9376
83. Public Health England. Splenectomy: leaflet and card. Health and Social Care Publications. 2015. Available from: https://www.gov.uk/government/publications/splenectomy-leaflet-and-card.
84. Wang J, Jones PA, Cheng AC, Leder K. Adherence to infection prevention measures in a statewide spleen registry. Med J Aust. 2014;200(9):538–540.
85. Premawardena C, Bowden D, Kaplan Z, Dendle C, Woolley IJ. Understanding of the significance and health implications of asplenia in a cohort of patients with haemaglobinopathy: possible benefits of a spleen registry. Hematology. 2017;39:1–5.
86. Luu S, Dendle C, Jones P, Ojaimi S, Woolley IJ. Impact of a spleen registry on optimal post-splenectomy vaccination and care. Hum Vaccin Immunother. 2018;14(12):2894–9. doi:10.1080/21645515.2018.1498282
87. Arnott A, Jones PA, Franklin LJ, Spelman DW, Leder K, Cheng AC. A registry for patients with asplenia/hyposplenism reduces the risk of infections with encapsulated organisms. Clin Infect Dis. 2018;67:557–561. doi:10.1093/cid/ciy141
88. Luu S, Jones P, Woolley I, Spelman D, Gold L. Initial modelling and updates on cost effectiveness from the first 10 years of a spleen registry. Aust N Z J Public Health. 2018;42(5):463–466. doi:10.1111/1753-6405.12832
89. Keenan RD, Boswell T, Milligan DW. Do post-splenectomy patients take prophylactic penicillin? Br J Haematol. 1999;105(2):509–510.
90. Boam T, Sellars P, Isherwood J, et al. Adherence to vaccination guidelines post splenectomy: a five year follow up study. J Infect Public Health. 2017;10(6):803–808. doi:10.1016/j.jiph.2017.01.006
91. Okeke EB, Uzonna JE. In search of a cure for sepsis: taming the monster in critical care medicine. J Innate Immun. 2016;8(2):156–170. doi:10.1159/000442469
92. Shukla P, Rao GM, Pandey G, et al. Therapeutic interventions in sepsis: current and anticipated pharmacological agents. Br J Pharmacol. 2014;171(22):5011–5031. doi:10.1111/bph.12829
93. Eisen DP, Moore EM, Leder K, et al. AspiriN To Inhibit SEPSIS (ANTISEPSIS) randomised controlled trial protocol. BMJ Open. 2017;7(1):e013636. doi:10.1136/bmjopen-2016-013636
94. Foley PT, Kavnoudias H, Cameron PU, Czarnecki C, Paul E, Lyon SM. Proximal versus distal splenic artery embolisation for blunt splenic trauma: what is the impact on splenic immune function? Cardiovasc Intervent Radiol. 2015;38(5):1143–1151. doi:10.1007/s00270-015-1162-8
95. Connell NT, Brunner AM, Kerr CA, Schiffman FJ. Splenosis and sepsis: the born-again spleen provides poor protection. Virulence. 2011;2(1):4–11. doi:10.4161/viru.2.1.14611
96. Weber T, Hanisch E, Baum R, Seufert R. Late results of heterotopic autotransplantation of splenic tissue into the greater omentum. World J Surg. 1998;22(8):883–889.
97. Lee SH, Kim DH, Hwang HK, Kang CM, Lee WJ. Spleen autotransplantation following laparoscopic distal pancreatosplenectomy and cholecystectomy. JOP. 2015;16(3):299–302.
98. Di Carlo I, Pulvirenti E, Toro A. A new technique for spleen autotransplantation. Surg Innov. 2012;19(2):156–161. doi:10.1177/1553350611419867
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
