Back to Journals » Clinical Epidemiology » Volume 9
Positive predictive values of ICD-10 codes to identify incident acute pancreatitis and incident primary malignancy in the Scandinavian national patient registries among women with postmenopausal osteoporosis
Authors Munch T , Christensen LB, Adelborg K, Tell GS , Apalset EM, Westerlund A, Lagerros YT , Kahlert J, Xue F, Ehrenstein V
Received 18 April 2017
Accepted for publication 13 July 2017
Published 17 August 2017 Volume 2017:9 Pages 411—419
DOI https://doi.org/10.2147/CLEP.S139895
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Irene Petersen
Troels Munch,1 Lotte B Christensen,1 Kasper Adelborg,1 Grethe S Tell,2 Ellen M Apalset,2,3 Anna Westerlund,3,4 Ylva T Lagerros,5,6 Johnny Kahlert,1 Fei Xue,7 Vera Ehrenstein1
1Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark; 2Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; 3Department of Rheumatology, Bergen Group of Epidemiology and Biomarkers in Rheumatic Disease, Haukeland University Hospital, Bergen, Norway; 4Department of Medicine Solna, Centre for Pharmacoepidemiology, Karolinska Institutet, Stockholm, Sweden; 5Department of Medicine Solna, Clinical Epidemiology Unit, Karolinska Institutet, Stockholm, Sweden; 6Department of Medicine, Clinic of Endocrinology, Metabolism and Diabetes, Karolinska University Hospital, Sweden; 7Center for Observational Research, Amgen Inc., Thousand Oaks, CA, USA
Background: Validation of definitions used to identify conditions of interest is imperative to epidemiologic studies based on routinely collected data. The objective of the study was thus to estimate positive predictive values (PPVs) of International Classification of Diseases, 10th Revision (ICD-10) codes to identify cases of incident acute pancreatitis leading to hospitalization and incident primary malignancy in the Scandinavian (Denmark, Norway, and Sweden) national patient registries in women with postmenopausal osteoporosis (PMO).
Methods: This validation study included postmenopausal (defined as 55 years or older) women with osteoporosis, identified between 2005–2014. Potential cases were sampled based on ICD-10 codes from the three national patient registries. Cases were adjudicated by physicians, using medical record review as gold standard. PPVs with corresponding 95% CIs were computed.
Results: Medical records of 286 of 325 (retrieval rate 88%) women with PMO were available for adjudication. Acute pancreatitis leading to hospitalization had a PPV of 87.6% (95% CI: 80.8%–90.2%). Incident primary malignancy had a PPV of 88.1% (95% CI: 81.3%–92.7%). The PPVs did not vary substantially across the three countries.
Conclusion: ICD-10 codes to identify acute pancreatitis leading to hospitalization, and incident primary malignancy in the Scandinavian national patient registries had high PPVs among women with PMO. This allows identification of cases of acute pancreatitis and incident primary malignancy with reasonable validity and to use these as outcomes in comparative analyses.
Keywords: validation, primary malignancy, acute pancreatitis, positive predictive value, postmenopausal women
Introduction
Pharmacoepidemiological observational studies using data from routine clinical settings are essential for monitoring long-term safety of medical treatment or drug use. To enable detection of rare events, such monitoring often relies on large health and administrative databases from routine clinical practice covering large patient populations over long periods of time.1–5 Such large datasets can potentially be achieved by merging datasets across nations.6 Combining data from the Nordic countries, with a total population of approximately 25 million persons, has previously been shown to provide valid and reliable data for assessing drug use.1 Three key features render Nordic countries (which, in addition to Denmark, Norway, and Sweden, include Finland and Iceland) attractive for epidemiologic research and for monitoring drug safety: 1) the long tradition of routine and prospective registration of health events (such as hospitalizations, surgeries, and treatments) and other key events to epidemiological research (such as emigration and death); 2) the universal health care coverage of the entire population; and 3) the possibility to link individual records across various registries using each person’s unique personal identification number.7–9
However, epidemiologic studies relying on routinely collected health data require valid algorithms to identify exposures, covariates, and outcomes of interest in order to provide valid estimates when analyzing absolute risks of adverse events, comparative effectiveness, or safety of treatment.10,11
Osteoporosis is a major health problem, especially common among postmenopausal women (denoted postmenopausal osteoporosis [PMO]). The consequence of the condition is fractures, which on an individual level leads to impaired quality of life and on a societal level to substantial costs related to treatment and increased need of care.12 Several drugs for treatment and prevention of osteoporosis have been introduced over the past 2 decades.12 Acute pancreatitis and primary malignancy are among the potential adverse events of these drugs.13–15
The objective of this study was thus to estimate the positive predictive values (PPVs) of International Classification of Diseases, 10th Revision (ICD-10) codes for incident acute pancreatitis leading to hospitalization and incident primary malignancy (excluding non-melanoma skin cancer) among women with PMO in the national patient registries of the three Scandinavian countries: Denmark, Norway, and Sweden.
Methods
Study design and population
This was a population-based validation study, in which medical record review served as the gold standard. Based on ICD-10 diagnosis codes, algorithms were developed to identify potential cases of acute pancreatitis leading to hospitalization or primary malignancy in the national patient registries of Denmark, Norway, and Sweden. Potential cases were identified in the patient registries among women with PMO seen in hospitals in selected geographic areas in each country. In Denmark, potential cases were selected among women with PMO who had been treated in hospitals in the Central Denmark Region (one of five regions in Denmark, covering 1.2 million inhabitants), with Aarhus University Hospital being the largest. In Norway, potential cases had been treated in large hospitals in ten counties in the western, southern, and central parts of Norway. Eleven hospitals were included in the study, with Oslo, St Olav’s and Haukeland, both university hospitals, being the largest. In Sweden, potential cases were identified from nine hospitals serving the Stockholm or an adjacent area, with Karolinska University Hospital being the largest one. PMO was defined among women 55 years or older by the presence of an ICD-10 code indicating osteoporosis, osteoporotic (fragility) fracture, or anatomic therapeutic chemical code indicating dispensation of osteoporosis medications (Table S1). Women with a diagnosis of Paget’s disease, any malignancy (except non-melanoma skin cancer) or pancreatitis within 12 months prior to the date when they fulfilled the PMO eligibility criteria were excluded (Table S2). Potential incident cases of acute pancreatitis or primary malignancy were sampled from the patient registries in the following periods: between 1 January 2005 and 31 December 2014 in Denmark, between 1 January 2008 and 31 December 2013 in Norway, and between 26 May 2010 and 31 December 2012 in Sweden. The study periods were chosen across the countries to achieve at least 50 retrievable potential cases of each condition in each country.
Since women with a prior malignancy diagnosis were excluded from the study and only first events during the study period were considered, “primary malignancy” will be used throughout the text to denote potential cases of incident primary malignancy as identified by the case identification algorithm. Similarly, patients with prior pancreatitis were also excluded in order to assess incident pancreatitis requiring hospitalization, henceforth denoted as “acute pancreatitis leading to hospitalization”.
In Denmark, we selected all women with PMO and with a diagnosis of incident acute pancreatitis treated at the Aarhus University Hospital during 2005–2012 (N=23) and randomly sampled patients from other hospitals in the Central Region treated between 26 May 2010 and 31 December 2012 (N=27). As records of these initially sampled women could not be obtained for logistical reasons, we further included all potential cases of acute pancreatitis among PMO women treated at Aarhus University Hospital in 2013 and 2014 (N=10). Thus, a total of 60 records of women with PMO and with a diagnosis of pancreatitis were subject to the medical chart review. Regarding incident primary malignancies, we randomly sampled 50 patients diagnosed at Aarhus University Hospital between 26 May 2010 and 31 December 2012, and an additional 20 patients diagnosed in 2013 and 2014 to reach a total of 70 potential cases, correcting for potential non-retrieval.
Fifteen hospitals in Norway were asked to contribute to the validation study; eleven agreed to participate. The potential cases of primary malignancy were retrieved from four hospitals for convenience reasons. Potential cases of acute pancreatitis were obtained from all eleven hospitals that had agreed to participate. The cases sampled were diagnosed in the period between 1 January 2008 and 31 December 2013.
In Sweden, we primarily selected patients from Stockholm hospitals. Since there were fewer than 50 patients each of acute pancreatitis and primary malignancy treated in Stockholm, the remaining patients were randomly sampled from hospitals in adjacent areas. Consequently, among women with PMO, we included all patients hospitalized with acute pancreatitis in Stockholm (N=43) and an additional seven patients from Uppsala University Hospital. For primary malignancy, we included all patients diagnosed in Stockholm hospitals (N=48) and two patients diagnosed in the Norrtälje Hospital. However, seven of the patients sampled for primary malignancy validation were erroneously sampled based on a code for secondary malignancy. These were excluded and thus only 43 cases of primary malignancy were validated in Sweden.
Case identification
The case identification algorithm for incident acute pancreatitis leading to hospitalization, required a primary discharge ICD-10 diagnostic code of acute pancreatitis recorded during an inpatient hospital stay. For primary malignancy, a primary or secondary diagnosis of primary malignancy recorded during inpatient hospital stays or during outpatient visits within specialist care was required (Table S3).
Medical record review
For each potential case, medical records (paper charts or electronic medical records) were obtained from or reviewed directly at the hospital which treated the patients. Physicians reviewed all medical records and adjudicated all cases. The adjudicating physicians confirmed the case status in three categories, according to predefined clinical criteria (Figures S1 and S2): 1) definite case, 2) definite non-case, 3) insufficient information. For acute pancreatitis leading to hospitalization we required confirmation of both the diagnosis and it being the primary cause for hospitalization. If neither definite case nor definite non-case status could be assigned by adjudicators, the case was categorized as having insufficient information and excluded from PPV calculation. A screenshot of the data collection form can be seen in the Supplementary materials (Figures S1 and S2).
Statistical analysis
We compiled descriptive data obtained from the patient registries on the patients’ age, country of residence, case year (i.e., calendar year of admission), the specialty of the treating hospital department, and comorbidity. The PPVs were calculated as the proportion of retrievable potential cases identified in the patient registries which were classified as definite cases by medical record review. All PPVs were reported with 95% CIs calculated using the Wilson score interval method.16 Analyses stratified by country were conducted separately for acute pancreatitis and incident primary malignancies. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Overall, 325 potential cases were sampled from the patient registries in the three Scandinavian countries. Of those, 286 medical records were available for review, corresponding to a retrieval rate of 88%. Of the potential cases, 145 were acute pancreatitis leading to hospitalization (mean age 77 [SD: 11] years) (Table 1) and 141 primary malignancy (mean age 77 [SD: 9.0] years) (Table 2). Sixteen potential cases of acute pancreatitis leading to hospitalization (11.0%) and 15 potential cases of primary malignancy (10.6%) had medical records with insufficient information and were excluded from the PPV calculations.
Overall, for acute pancreatitis leading to hospitalization, adjudication confirmed 113 of 129 cases, resulting in a PPV of 87.6% (95% CI: 80.8%–90.2%) (Table 3). Norway reported the highest PPV at 92.5% (95% CI: 80.1%–97.4%) and Denmark the lowest at 82.5% (95% CI: 68.1%–91.3%). Adjudication of primary malignancy confirmed 111 of 126 cases, resulting in an overall PPV of 88.1% (95% CI: 81.3%–92.7%) (Table 3). Norway reported the highest PPV at 95.0% (95% CI: 83.5%–98.6%) and Sweden the lowest at 80.0% (95% CI: 65.2%–89.5%).
Discussion
Based on ICD-10 codes in national patient registries, we found high PPVs of acute pancreatitis leading to hospitalization (87.6% [95% CI: 80.8%–90.2%]) and primary malignancy (88.1% [95% CI: 81.3%–92.7%]) among women with PMO in Denmark, Norway, and Sweden.
To the best of our knowledge, no prior studies have validated acute pancreatitis in a Norwegian population. However, the PPV of acute pancreatitis has been investigated in the Danish and Swedish national patient registries. In accordance with our findings, a Swedish study of 530 randomly sampled patients hospitalized with acute pancreatitis in 1998 or 2007, found a PPV of 84% for acute pancreatitis as primary discharge diagnosis when adjudicated against medical records.17 Similarly, a Danish study randomly sampled 99 patients hospitalized with acute pancreatitis from 1981 to 2000 and found a PPV of 82%.18
A plethora of studies have validated individual malignancy diagnoses in the Danish National Patient Register using medical records or clinical databases such as cancer registries as the gold standard:19 high PPVs were generally reported (>70%, except for bone metastasis among prostate cancer patients).19 Among these studies, two validated codes of any primary malignancy, demonstrating PPVs of 98.0% (95% CI: 89.4%–99.9%)20 and 91.7% (95% CI: 91.3%–92.1%).21 The slightly lower PPV found in our study is likely explained by our restriction to incident primary malignancies. A single study validating ICD-10 codes of solid tumors in the Norwegian Patient Registry utilizing the Cancer Registry of Norway as gold standard found PPVs ranging from 75% (prostate) to 99% (breast cancer) for incident cancers in 2008.22 This also corresponds well with our finding. To our knowledge, no relevant validation study of the coding of malignancy diagnoses in the patient register has been conducted in Sweden. The difference in PPV across the three countries may be explained by slightly differing country-specific coding practices.
Limitations
This study was conducted among women with PMO in selected areas and hospitals of Denmark, Norway, and Sweden. Given the homogenous populations as well as universal income-independent access to health care and uniform health care delivery in the Scandinavian countries, results of this validation study are likely generalizable to the overall postmenopausal (female) population in each country, although variation by geography and hospital size cannot be ruled out. By design, this study assessed PPVs and could not assess sensitivity because of a lack of an independent sample of true pancreatitis or malignancy cases. The task of the reviewers was to confirm fulfillment of the diagnostic criteria, as such, they were not blinded. However, it is unlikely that this would have resulted in any substantial bias given the predefined criteria to confirm case status. Further, a small risk of misclassification of osteoporosis status might have occurred, as the codes to identify external cause of fracture were not available in Denmark or Norway. However, the resulting over-ascertainment of osteoporotic fractures is unlikely to be substantial based on available knowledge. Most fracture types among the elderly are related to low bone mineral density, even if they are precipitated by a trauma.23
Conclusion
We found high PPVs of ICD-10 codes for acute pancreatitis leading to hospitalization and incident primary malignancy (excluding non-melanoma skin cancer) recorded in the Scandinavian national patient registries among postmenopausal osteoporotic women. As such, these ICD-10 codes can be used to identify cases in future epidemiological studies with reasonable confidence. This allows for use of these as outcomes in comparative analyses, although estimates of absolute risks may be overestimated.
Ethics statement
In Denmark, this study received the required approval from the Danish Data Protection Agency (record number 2010–41-5171) and by the Data Protection Board of the Danish Central Region (record number 1-16-02-1-08). In Norway, this study received the mandatory approval from the Regional Committee for Medical and Health Research Ethics (REC West) (record number 2010/2616/REK vest). In Sweden, the Stockholm County Regional Ethics Review Board approved the study (record number 2014/1250-31/4). Further, approval of access was obtained from individual hospitals and/or departments in all three countries. Data were handled in accordance with Danish, Norwegian, and Swedish law respectively, including anonymization of data collected.
Availability of data and material
The datasets generated and/or analyzed during the current study are not publicly available as they contain sensitive information, but are available from the corresponding author on reasonable request.
Acknowledgments
We thank the research nurses, Hanne Moeslund Madsen and Henriette Kristoffersen, for practical help with the study.
Support for this study was partially provided by a research grant from Amgen Inc. to and administered by Aarhus University Hospital. The study sponsor and the academic investigators collaborated on study planning, design, and reporting. The academic investigators designed data collection instrument, collected the data, and performed data analysis.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
FX is an employee of Amgen Inc., which manufactures medications for treatment of osteoporosis. The remaining authors are salaried employees of their respective academic institutions. The authors report no other conflicts of interest in this work.
References
Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86–94. | ||
Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. | ||
Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. | ||
Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291–303. | ||
Toh S, Platt R. Is size the next big thing in epidemiology? Epidemiology. 2013;24(3):349–351. | ||
Coloma PM, Schuemie MJ, Trifiro G, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011;20(1):1–11. | ||
sundhed.dk [homepage on the Internet]. Health care in Denmark. Available from: https://www.sundhed.dk/service/english/an-ehealth-nation/healthcare-in-dk/. Accessed March 10, 2016. Danish. | ||
sweden.se [homepage on the Internet]. Healthcare in Sweden. Available from: https://sweden.se/society/health-care-in-sweden/. Accessed July 20, 2017. | ||
legemiddelverket.no [homepage on the Inernet]. The Norwegian health care system and pharmaceutical system. Statens legemiddelverk; 2016. Available from: http://www.legemiddelverket.no/english/the-norwegian-health-care-system-and-pharmaceutical-system/sider/default.aspx. Accessed July 20, 2017. | ||
Ehrenstein V, Petersen I, Smeeth L, Jick SS, Benchimol EI, Ludvigsson JF, Sørensen HT. Helping everyone do better: a call for validation studies of routinely recorded health data. Clin Epidemiol. 2016;8:49–51. | ||
Lash TL, Olshan AF. EPIDEMIOLOGY announces the “Validation Study” submission category. Epidemiology. 2016;27(5):613–614. | ||
Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8:136. | ||
Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf. 2011;3:79–91. | ||
Ishtiaq S, Fogelman I, Hampson G. Treatment of post-menopausal osteoporosis: beyond bisphosphonates. J Endocrinol Invest. 2015;38(1):13–29. | ||
Xue F, Ma H, Stehman-Breen C, et al. Design and methods of a postmarketing pharmacoepidemiology study assessing long-term safety of Prolia® (denosumab) for the treatment of postmenopausal osteoporosis. Pharmacoepidemiol Drug Saf. 2013;22(10):1107–1114. | ||
Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22(158):209–212. | ||
Razavi D, Ljung R, Lu Y, Andren-Sandberg A, Lindblad M. Reliability of acute pancreatitis diagnosis coding in a National Patient Register: a validation study in Sweden. Pancreatology. 2011;11(5):525–532. | ||
Floyd A, Pederson L, Nielsen GL, Thorlacius-Ussing O, Sorensen HT. Secular trends in incidence and 30-day case fatality of acute pancreatitis in North Jutland County, Denmark: a register-based study from 1981–2000. Scand J Gastroenterol. 2002;37(12):1461–1465. | ||
Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. | ||
Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. | ||
Osterlind A, Jensen OM. Evaluering af cancerregistreringen i Danmark 1977. En praeliminaer evaluering af cancerregisterets og landspatientregisterets registrering af cancertilfaelde. [Evaluation of cancer registration in Denmark in 1977. Preliminary evaluation of cancer registration by the Cancer Register and the National Patient Register]. Ugeskr Laeger. 1985;147(31):2483–2488. Danish. | ||
Bakken IJ, Gystad SO, Christensen OO, et al. Comparison of data from the Norwegian Patient Register and the Cancer Registry of Norway. Tidsskr Nor Laegeforen. 2012;132(11):1336–1340. | ||
Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. |
Supplementary materials
  | Figure S1 Data entry form for acute pancreatitis. |
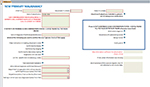  | Figure S2 Data entry form for incident primary malignancy. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






