Back to Journals » OncoTargets and Therapy » Volume 8
Positive correlation between expression level of mitochondrial serine hydroxymethyltransferase and breast cancer grade
Authors Yin K
Received 8 February 2015
Accepted for publication 12 March 2015
Published 14 May 2015 Volume 2015:8 Pages 1069—1074
DOI https://doi.org/10.2147/OTT.S82433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Daniele Santini
Ke Yin
Department of Thyroid and Breast Surgery, Ningbo First Hospital, Ningbo, People’s Republic of China
Abstract: Metabolic reprogramming plays an essential role in supporting the survival and proliferation of cancer cells. Serine hydroxymethyltransferase (SHMT) directs serine to the metabolism of one-carbon unit and the synthesis of thymidilate as a key factor in this metabolic shift. Although the mitochondrial isoform of SHMT (SHMT2) has been proven to be a crucial factor in the serine/glycine metabolism in several cancer cell types, the expression pattern of SHMT2 and the correlation of expression level of SHMT2 and other clinicopathological parameters in clinical breast cancer remain to be explored. In this research, 76 breast cancer patients who underwent modified radical mastectomy were enrolled for immunohistochemical analysis of the expression level of SHMT2 in their cancerous breast tissues for comparison with that in matching, distant noncancerous tissues. The results showed that SHMT2 was not expressed in the distant noncancerous cells. In contrast, SHMT2 protein could be stained in all breast cancer samples at varying degrees. Higher level of SHMT2 was expressed in grade III breast cancer cells than that those in grade I–II (P<0.05). In conclusion, SHMT2 was highly expressed in breast cancer cells, and the expression level of SHMT2 was positively correlated with breast cancer grade, suggesting that SHMT2 could be a target for anticancer therapies.
Keywords: SHMT2, breast cancer, histological grading, predictive biomarkers
Introduction
Breast cancer is the most common malignant tumor in women, with a rapidly increasing incidence in recent years.1,2 Despite advancements in early detection and treatment of breast cancer, it is still the leading cause of death among all cancer-related deaths in women worldwide.3,4 Clinically, tumor stage and histological grade have been useful for evaluating and predicting breast cancer progression.5 And different tumor markers, such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor-2 (HER2), could lead to different responses to clinical treatment and different prognoses.6–12 Early detection, which could guide therapy, is still the key to survival of patients. Thus, identification and evaluation of novel tumor markers will be helpful for early detection of breast cancer and the development of novel therapeutic targets for treatment of breast cancer patients.
At present, increasing evidences have implicated the essential role of metabolic reprogramming in supporting the survival and proliferation of cancer cells.13–15 Particularly, it is recently been confirmed that hyperactivation of serine/glycine biosynthetic pathway drives tumorigenesis.16–20 The serine synthesis pathway utilizes the glycolytic intermediate glycerate-3-phosphate, which is catalyzed to yield serine by phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), and phosphoserine phosphatase (PSPH).16,17,21 And, serine can be transformed into glycine by serine hydroxymethyltransferase (SHMT).22,23 Although the mitochondrial isoform of SHMT (SHMT2) has been shown to be a crucial factor for the serine/glycine metabolism of several cancer cell types,24–27 the expression pattern of SHMT2 and the correlation of expression level of SHMT2 and other clinicopathological parameters in clinical breast cancer remain to be explored.
In this study, we evaluate the expression level of SHMT2 protein in breast cancer cells and distant normal tissue samples by immunohistochemical streptavidin peroxidase-conjugated (SP) method in order to figure out whether this protein is clinically associated with breast cancer. Furthermore, the correlation between the expression level of SHMT2 and clinicopathological parameters from breast cancer patients was demonstrated.
Materials and methods
Materials
Fresh biopsy specimens of breast cancer tissue and normal breast tissue from the incisal margin were collected from 76 patients with breast cancer who underwent radical surgery at Ningbo First hospital. None of the patients, aged 32–73 years (mean age, 49), had received any chemotherapy, radiotherapy, or other adjuvant therapy before the operation. This study was approved by The Ethics Committee of Ningbo First hospital and all patients provided informed consent. Fifty-six specimens were confirmed pathologically as infiltrating lobular carcinoma (ILC), and the other 20 specimens were invasive ductal carcinoma (IDC). Tumors were diagnosed and classified according to the American Joint Committee on Cancer-breast cancer the tumor node metastasis (TNM) staging system28 and the World Health Organization breast cancer histology classifications.29
Immunohistochemical streptavidin peroxidase-conjugated method (SP method)
All fresh specimens were fixed with formalin and embedded in paraffin according to the standard protocol. Tissue sections were deparaffinized and rehydrated routinely and then subjected to antigen retrieval by placing slides in 1× citrate buffer for 15 minutes at 100°C in a microwave oven. After treatment with 3% H2O2 for 30 minutes, the sections were incubated with 20% normal serum for 50 minutes and then with the primary antibody overnight at 4°C. The primary antibodies were ER (ab32063, Abcam, Cambridge, UK), PR (ab32085, Abcam), HER2 (ab8282, Abcam), and SHMT2 (ab180786, Abcam). On the following day, the sections were washed with PBS thrice and then processed using an ultrasensitive TM S-P kit (Maixin Biotechnology, Fuzhou, People’s Republic of China). After the washes in PBS, the color reaction was conducted using a 3,3′-diaminobenzidine kit (Maixin Biotechnology). The sections were counter-stained with hematoxylin and covered with a coverslip.
The stained tissue sections were reviewed and scored independently by two pathologists (Dr Jian Wang and Ming Li). The percentage of SHMT2 positive cells was rated as follows: −, ≤5% positive tumor cells; +, 5%–30% positive cells; ++, 30%–55% positive cells; and +++, >55% positive cells. ER and PR positivity was defined as strong nuclear staining in at least 3/8 of the tumor cells reviewed. HER2/neu positivity was defined as strong (3+) membranous staining in at least 10% of tumor cells, whereas scores of 0 to 2+ were regarded as negative.
Statistical analysis
Data were analyzed by SPSS 19.0 statistical software (IBM Corporation, Armonk, NY, USA). Measurement data were analyzed by Student’s t-test, while categorical data were analyzed by the chi-square test. P<0.05 was considered as significant.
Results
Differential expression level of SHMT2 protein in breast cancer and its distant noncancerous tissues
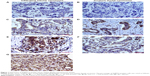
We first detected expression level of SHMT2 protein by immunohistochemical staining in breast cancer cells and the matching distant normal tissue samples from 76 patients of which 56 were ILC and 20 were IDC. The results of SHMT2 staining were scored as none (−), weak (+), moderate (++), and strong (+++) according to the assessment of two independent pathologists. No SHMT2 protein was expressed in the distant noncancerous cells (Table 1, Figure 1A and B). In contrast, SHMT2 protein was stained in all breast cancer samples to varying degrees (Table 1, Figure 1C–G). In most of ILC cases, SHMT2 protein was expressed moderately or strongly (++, 28/56, 50.00%; +++, 21/56, 37.50%), while in all of IDC cases, SHMT2 protein was expressed weakly or moderately (+, 12/20, 57.20%; ++, 8/20, 38.10%). Overall, the results demonstrated that SHMT2 is overexpressed in breast cancer cells.
Correlation between expression level of SHMT2 and clinicopathological parameters
We then conducted an association analysis between the expression level of SHMT2 protein and clinicopathological parameters from breast cancer patients (Table 2). The results showed that more SHMT2 was expressed in grade III breast cancer than grade I–II (P<0.05). It suggested that the expression level of SHMT2 was positively correlated with breast cancer grade. However, there was no association between expression level of SHMT2 and other clinicopathological parameters, such as age, tumor size, TNM stage, lymph node status, vascular invasion status, and biomarkers (ER, PR, HER2) (Table 2).
Discussion
In this study, we detected the expression level of SHMT2 protein in breast cancer and distant normal tissue samples in different cancer types, including ILC and IDC which account for 88% of breast cancer.30 And, we found that SHMT2 is overexpressed in all of the breast cancer samples. Our results is consistent with some previously published work: first, Jain et al19 found that SHMT2 was commonly overexpressed in several types of cancer cells, especially in rapidly proliferating cells; second, Paone et al26 showed that the expression level of SHMT2 was increased in the lung cancer tissue by an average of 2.22-fold. As we know, serine and glycine provide the essential precursors for the biosynthesis of proteins, nucleic acids, and lipids and also are required for the maintenance of cellular redox state. SHMT2 is a key protein in this pathway, and its main function is to catalyze the interconversion of serine and glycine and to generate one-carbon units from serine, which are exported as folate into the cytosol to support one-carbon metabolism.31–33 Therefore, SHMT2 should have a pivotal role in proliferating cells, including cancer cells. Since SHMT2 is overexpressed in all of the breast cancer samples, SHMT2 could serve as a diagnostic marker in the future.
We also conducted an association analysis between the expression level of SHMT2 protein and clinicopathological parameters from breast cancer patients. We found a positive association between the expression level of SHMT2 and breast cancer grade. We found that higher level of SHMT2 was expressed in high histological grade breast cancer compared with low grade tumors. The histological grading system in breast cancer is based on differentiation of tumor cells, which is an important factor in predicting prognosis of breast cancer patients and tumor aggressiveness. Thus, we speculate that breast cancer with higher expression level of SHMT2 in the high histological grade might be more likely to recur and/or have a worse prognosis. In fact, Jain et al19 found that higher expression level of genes in mitochondrial glycine biosynthetic pathway was associated with greater mortality in breast cancer patients. And recently, Lee et al25 and Antonov et al24 reported that elevated expression level of SHMT2 was found to be associated with worse prognosis in human cancer. It supported that the development of molecular therapies focused on SHMT2 or components of glycine biosynthetic pathway. Besides that, based on our clinical experiences, malignancy of breast cancer is closely associated with tumor size, TNM stage, lymph node metastasis, vascular invasion, and expression of other biomarkers (such as ER, PR, and HER2). However, we failed to find any statistical significance between the expression level of SHMT2 and these prognostic factors. This indicates that SHMT2 functions and whether SHMT2 could serve as a prognostic marker or not warrant further investigation in breast cancer.
Conclusion
In conclusion, we found that SHMT2 was overexpressed in breast cancer cells, and the expression level of SHMT2 is positively correlated with breast cancer grade. We suggest that SHMT2 could be a target for anticancer therapies, and identification of selective SHMT2 inhibitors could be an innovative and successful approach.
Disclosure
The author reports no conflicts of interest in this work.
References
Dey S, Soliman AS. Cancer in the global health era: opportunities for the Middle East and Asia. Asia Pac J Public Health. 2010;22(3 Suppl):75S–82S. | ||
Wang YC, Wei LJ, Liu JT, Li SX, Wang QS. Comparison of cancer incidence between China and the USA. Cancer Biol Med. 2012;9(2):128–132. | ||
Rao R, Wiechmann L. Treatment of early breast cancer. Minerva Endocrinol. 2009;34(4):311–324. | ||
La Vecchia C, Bosetti C, Lucchini F, et al. Cancer mortality in Europe, 2000–2004, and an overview of trends since 1975. Ann Oncol. 2010;21(6):1323–1360. | ||
Chung C, Christianson M. Predictive and prognostic biomarkers with therapeutic targets in breast, colorectal, and non-small cell lung cancers: a systemic review of current development, evidence, and recommendation. J Oncol Pharm Pract. 2014;20(1):11–28. | ||
Peihong S, Perry F. Expression of nm23, MMP-2, TIMP-2 in breast neoplasm in Zhengzhou Center Hospital, China. Ethiop Med J. 2007;45(1):79–83. | ||
Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. | ||
Mohsin SK, Weiss H, Havighurst T, et al. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol. 2004;17(12):1545–1554. | ||
Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28(3):509–518. | ||
Ross JS, Linette GP, Stec J, et al. Breast cancer biomarkers and molecular medicine: part II. Expert Rev Mol Diagn. 2004;4(2):169–188. | ||
Allred DC. Issues and updates: evaluating estrogen receptor-alpha, progesterone receptor, and HER2 in breast cancer. Mod Pathol. 2010;23(Suppl 2):S52–S59. | ||
Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013;5(3):169–181. | ||
Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. | ||
Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12(10):685–698. | ||
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. | ||
Pollari S, Kakonen SM, Edgren H, et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res Treat. 2011;125(2):421–430. | ||
Possemato R, Marks KM, Shaul YD, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476(7360):346–350. | ||
Locasale JW, Grassian AR, Melman T, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43(9):869–874. | ||
Jain M, Nilsson R, Sharma S, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040–1044. | ||
di Salvo ML, Contestabile R, Paiardini A, Maras B. Glycine consumption and mitochondrial serine hydroxymethyltransferase in cancer cells: the heme connection. Med Hypotheses. 2013;80(5):633–636. | ||
Kit S. The biosynthesis of free glycine and serine by tumors. Cancer Res. 1955;15(11):715–718. | ||
Garrow TA, Brenner AA, Whitehead VM, et al. Cloning of human cDNAs encoding mitochondrial and cytosolic serine hydroxymethyltransferases and chromosomal localization. J Biol Chem. 1993;268(16):11910–11916. | ||
Anderson DD, Stover PJ. SHMT1 and SHMT2 are functionally redundant in nuclear de novo thymidylate biosynthesis. PloS One. 2009;4(6):e5839. | ||
Antonov A, Agostini M, Morello M, Minieri M, Melino G, Amelio I. Bioinformatics analysis of the serine and glycine pathway in cancer cells. Oncotarget. 2014;5(22):11004–11013. | ||
Lee GY, Haverty PM, Li L, et al. Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes. Cancer Res. 2014;74(11):3114–3126. | ||
Paone A, Marani M, Fiascarelli A, et al. SHMT1 knockdown induces apoptosis in lung cancer cells by causing uracil misincorporation. Cell Death Dis. 2014;5:e1525. | ||
Ye J, Fan J, Venneti S, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014;4(12):1406–1417. | ||
Benson JR, Weaver DL, Mittra I, Hayashi M. The TNM staging system and breast cancer. Lancet Oncol. 2003;4(1):56–60. | ||
Sinn HP, Kreipe H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care. 2013;8(2):149–154. | ||
Dixon AR, Ellis IO, Elston CW, Blamey RW. A comparison of the clinical metastatic patterns of invasive lobular and ductal carcinomas of the breast. Br J Cancer. 1991;63(4):634–635. | ||
Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. | ||
Stover PJ, Field MS. Trafficking of intracellular folates. Adv Nutr. 2011;2(4):325–331. | ||
Fu TF, Rife JP, Schirch V. The role of serine hydroxymethyltransferase isozymes in one-carbon metabolism in MCF-7 cells as determined by (13)C NMR. Arch Biochem Biophys. 2001;393(1):42–50. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.