Back to Journals » Open Access Surgery » Volume 12
Port-less technique (PLT) in pediatric video-assisted thoracoscopic surgery (VATS): a 10-year experience at National University of Malaysia
Authors Abdul Aziz DA , Osman M , Abdullah MF, Lim F, Teo R, Cheah FC , Ishak S , Jaafar R , Tang SF , Abdul Aziz B, Abdul Latif H, Abdul Latiff Z
Received 3 December 2018
Accepted for publication 21 February 2019
Published 29 April 2019 Volume 2019:12 Pages 7—11
DOI https://doi.org/10.2147/OAS.S195184
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Luigi Bonavina

Dayang Anita Abdul Aziz,1 Marjmin Osman,1 Mohd Fadli Abdullah,1 Felicia Lim,2 Rufinah Teo,2 Fook Choe Cheah,3 Shareena Ishak,3 Rohana Jaafar,3 Swee Fong Tang,3 Bilkis Abdul Aziz,3 Hasniah Abdul Latif,3 Zarina Abdul Latiff3
1Department of Surgery, UKM Medical Centre, 56000 Cheras, Kuala Lumpur, Malaysia; 2Department of Anaesthesia, UKM Medical Centre, 56000 Cheras, Kuala Lumpur, Malaysia; 3Department of Pediatrics, UKM Medical Centre, 56000 Cheras, Kuala Lumpur, Malaysia
Background: In pediatric patients, video-assisted thoracoscopic surgery (VATS) is usually carried out using three to five working ports. The port-less technique (PLT) means only one or two ports are used; in most cases only the telescope would require a port. At our center, the VATS services were started in 2008, initially using the standard three-ports technique but shortly after this was replaced with PLT for all neonatal and pediatric VATS. The rationale of doing PLT was so that working instruments could move easier in the pediatric thoracic cavity. Furthermore, budget constraints did not allow us to purchase trocars of different sizes.
Patients and methods: A review of all PLT cases was carried out at our institution from January 2008 to September 2018. We documented the diagnosis and type of surgery performed, age at surgery, number of ports used, conversion rate, morbidity and mortality as well as gross chest wall growth.
Results: A total of 46 PLT cases were carried out; 16 were in neonates (34.7%). Conversion to thoracotomy occurred in five patients (10.8%). Diagnosis ranged from congenital anomalies like esophageal atresia to infective cause like empyema thoracis. Immediate morbidity occurred in four patients (8.7%) and there was one perioperative mortality (2.2%). The majority of PLTs (54%) were using two ports, and another 46% of PLTs were successfully carried out using one port. All neonatal and infant PLT cases were using one port (46%). Maximum follow-up was for 5 years and gross growth of chest wall was good.
Conclusion: PLT is a feasible and safe technique for a variety of cases for neonatal and pediatric surgical intrathoracic pathology. We recommend PLT for all neonatal and pediatric VATS.
Keywords: pediatric, thoracoscopy, VATS, port-less
Introduction
Pediatric video-assisted thoracoscopic surgery (VATS) is more challenging than laparoscopic surgery especially when performed in neonates, as the rib cage is not as expandable as the abdomen. Most pediatric VATS is carried out using three to five working ports.1–5 The author started using only one working port for neonatal VATS in 2008 and was able to perform all VATS cases using one, or a maximum of two working ports in the last 10 years. The author coined the term port-less technique (PLT) to describe the use of minimal number of working ports or trocars in pediatric minimally invasive surgery (MIS) including VATS. As a small center actively practicing advanced pediatric MIS in the South East Asia region (ASEAN), the author faced limited financial assistance to purchase various sizes of instruments and working ports. At the author’s center, for pediatric MIS, only 3-mm and 5-mm instruments were used. The trocar used for 0° telescope was the 6 mm Hasson.
PLT is not unique for VATS alone; the most common abdominal laparoscopic cases at our center being carried out using PLT was laparoscopic herniotomy.6 Other pediatric laparoscopic procedures performed using PLT at our center were laparoscopic duodeno-duodenostomy, laparoscopic Ladd procedure, laparoscopic gastrostomy, laparoscopic excision of choledochal cyst and hepatico-duodenostomy, laparoscopic pyloromyotomy, laparoscopic pyeloplasty, laparoscopic Fowler Stephens orchidopexy, laparoscopic reduction of intussusception and laparoscopic biopsy.
Methodology
To look at the success of pediatric PLT in VATS, a review of all PLT VATS cases from January 2008 to October 2018 was carried out. The 6 mm-Hasson trocar was used as main port for the telescope (
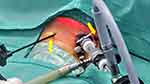 | Figure 1 Arrows (yellow) point to the 3 mm instruments inserted without usage of trocar, ie, port-less. |
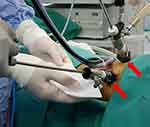 | Figure 2 Arrows (red) point to use of 2 working ports; upper one for the camera and the lower one for 5 mm suction and irrigation instrument, ie, port-less. |
Ethics
Reviews based on our existing audit is waived for ethical approval by UKM Medical Research and Ethics Committee. Reviews based on existing audit are waived for ethics approval as long as patient’s confidentiality is maintained. This study was performed in compliance with the Declaration of Helsinki.
Results
There were total of 50 pediatric VATS over the last 10 years, PLT was performed in 46 cases. Sixteen PLT was in neonates, 15 PLT was in infants (age 5 weeks to 1 year) and 25 PLT were in patients beyond one year old, with the oldest patient aged 10 years old. In the neonatal group, out of 16 neonates; there were eight esophageal atresia and tracheo-esophageal fistula (EA/TOF), six congenital diaphragmatic hernia (CDH), one right sided eventration of hemidiaphragm (EH) and one right bronchogenic cyst. In the infant group, out of five infants there were one bilateral empyema thoracis (ET), three EH and one delayed left CDH. In the older than 1-year-old group, out of 25 patients; 23 were unilateral ET, one congenital pulmonary airway malformation (CPAM) and one lung fibrosis from diagnostic lung biopsy (see Figure 3).
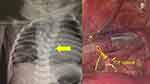
Conversion to open thoracotomy occurred in five cases (10.8%); two in patients with EA/TOF, one in patient with left CDH and two in patients with unilateral ET. In the two patients with EA/TOF, the first patient successfully had the fistula ligated thoracoscopically but we were unable to be anastomosed due to presence of severe thoracic scoliosis (see Figure 4). In the second EA/TOF patient, conversion was inevitable as he had VACTERL-H syndrome, the patient’s huge hydrocephalus-head made it difficult to keep his neck extended resulting in difficulty in maintaining the airway during VATS, hence conversion to thoracotomy was made to hasten the surgery. The other cases needed conversion for reasons not pertaining to PLT, eg, intraoperative bleeding in empyema thoracis and inexperienced surgeon in the CDH case.
Immediate morbidity occurred in four patients (8.7%); there were two anastomotic leaks in EA/TOF patients which were treated conservatively and two patients with mild subcutaneous emphysema post decortication of ET, which resolved on its own.
There was one death, ie, 2.2% peri-operative mortality rate. It occurred in a Down's syndrome newborn with EA/TOF; thoracoscopic ligation of fistula was carried out but patient deteriorated on the table, resulting in abandonment of procedure. The patient died 48 h post-surgery due to un-diagnosed complex heart disease. The pre-operative echocardiogram was reported as normal.
There were three patients with delayed morbidity (6.5%), they presented during infancy. All three patients had VATS repair of EA/TOF. The first two patients were the same ones with anastomotic leak who eventually developed esophageal stricture. However, they were successfully treated with esophagoscopy-guided balloon dilatation under general anesthesia. The third patient had recurrent fistula at four months old, successfully underwent bronchoscopy-guided tissue glue application to seal the fistula but after 3 months, the fistula recurred. Parents requested transfer to another center despite our plan for repeat glue application.
In the follow-up period, we lost two more EA/TOF patients. The cause of death in both patients were not related to PLT-VATS. Both patients were feeding normally. Both were also syndromic; one was EA/TOF with VACTERL who had developed one episode of adhesive obstruction after closure colostomy related to surgery for his anorectal anomaly, he died at home at 18 months of age from an unknown cause; the other was the EA/TOF with VACTERL-H, this patient died at 3 years of age secondary to complications from the ventriculo-peritoneal shunt.
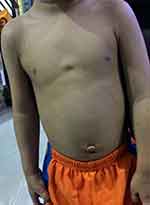
With regards to gross chest wall growth, maximum follow-up was for five years. All patients had no obvious chest deformity/asymmetry and their chest movement were equal bilaterally (Figure 5). All patients with CDH and EH did not have recurrences.
For assessment of the number of ports used in PLT-VATS; for all patients less than one year old, only one port was required (46%), including performing upper lobectomy in a day 5 of life baby with right bronchogenic cyst. For all cases in neonates and infants, wherever anastomosis was required, intra-corporeal suturing was performed and no sealing device was used for the vessels. However, two ports were used in all cases of PLT-VATS for all patients beyond one year old (54%), this was related to usage of 5-mm instruments like suction irrigation instrument and/or sealing devices.
Discussion
The use of PLT in abdominal MIS work at our center was further successfully advanced to thoracic MIS services. The range of cases were varied from congenital anomalies to infective causes. With success of these cases, we are convinced that surgeons do not need to use special 1 mm instruments in neonatal VATS or use trocars for 3 mm instruments; these would translate to less cost and overall budget. We also did not find any disadvantages or problem such as CO2 leak during surgery when using the 3 mm instruments directly into the thoracic cavity. Morbidity and mortality encountered in our series were not directly related to PLT. The overall outcome for PLT was also very favourable. In our series, there were only 4 cases of VATS which were not PLT, equal comparison was not possible but certainly we found that performing VATS without extra ports enable us to move the instruments with ease. To our knowledge, this is the first published work regarding usage of fewer number of ports and trocars in pediatric VATS.
Conclusion
The PLT in pediatric VATS is indeed a feasible technique. It can be used for a variety of cases in neonates, infants and older children. Pediatric MIS centers in developing nations do not need to overspend for different sizes of trocars and instruments. The technique is also safe. We highly recommend pediatric surgeons to use PLT for pediatric VATS.
Acknowledgment
The interim finding of this work was presented as oral presentation at Society of Laparoendoscopic Surgeons (SLS), Minimally Invasive Surgery Week 2018, 31 August 2018, Sheraton Times Square Hotel, New York USA;
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shah R, Suyodhan Reddy A, Dhende NP. Video assisted thoracic surgery in children. J Minim Access Surg. 2007;3(4):161–167. doi:10.4103/0972-9941.38910
2. Adams S, Jobson M, Sangnawakij P, et al. Does thoracoscopy have advantages over open surgery for asymptomatic congenital lung malformations? An analysis of 1626 resections. J Ped Surg. 2017;52:247–251. doi:10.1016/j.jpedsurg.2016.11.014
3. Rothenberg SS. Thoracoscopic repair of esophageal atresia and tracheo-esophageal fistula in neonates: evolution of a technique. J Laparoendosc Adv Surg Tech A. 2012;22(2):195–199. doi:10.1089/lap.2011.0063
4. Rothenberg SS, Middlesworth W, Kadennhe-Chiweshe A, et al. Two decades of experience with thoracoscopic lobectomy in infants and children: standardizing techniques for advanced thoracoscopic surgery. J Laparoendosc Adv Surg Tech A. 2015;25(5):423–428. doi:10.1089/lap.2014.0350
5. Lamas-Pinheiro R, Henriques-Coelho T, Fernandes S, et al. Thoracoscopy in the management of pediatric empyemas. Rev Port Pneumol. 2006;22(3):157–162. doi:10.1016/j.rppnen.2015.12.004
6. Aziz DAA, Osman M, Lim F, Teo R, Latiff ZA, Manaf RA. External squeeze test during pediatric laparoscopic hernia repair: a novel on-table assessment to ensure complete closure of patent processus vaginalis. Open Access Surg. 2018;11:1–3. doi:10.2147/OAS.S163265
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.