Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Poria cocos Extract from Mushrooms Stimulates Aquaporin-3 via the PI3K/Akt/mTOR Signaling Pathway
Authors Park SG, Jo IJ, Park SA, Park MC, Mun YJ
Received 4 July 2022
Accepted for publication 5 September 2022
Published 15 September 2022 Volume 2022:15 Pages 1919—1931
DOI https://doi.org/10.2147/CCID.S378545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Sung-Gu Park,1,* Il-Joo Jo,2,* Seol-A Park,3 Min-Cheol Park,1 Yeun-Ja Mun4
1Department of Oriental Medical Ophthalmology & Otolaryngology & Dermatology, College of Korean Medicine, Wonkwang University, Iksan, South Korea; 2Central Stroke Center of Korean medicine, College of Korean Medicine, Wonkwang University, Iksan, South Korea; 3Department of Beauty Design Graduate School, Wonkwang University, Iksan, South Korea; 4Department of Anatomy, College of Korean Medicine, Wonkwang University, Iksan, South Korea
*These authors contributed equally to this work
Correspondence: Yeun-Ja Mun, Department of Anatomy, College of Korean Medicine, Wonkwang University, Iksan, South Korea, Tel +82-63-850-6942, Email [email protected]
Background: Poria cocos (PC), a fungus, has been used for more than 2000 years as a food and medicine in China. PC and its components have various pharmacological effects on the skin, including immunomodulatory activities, barrier function improvement, and anti-tumor effects. However, the effect of PC in aquaporin-3 (AQP3) expression, which is essential for epidermal water permeability barrier maintenance, was not reported.
Methods: This study examined the mechanism through which the ethanol extract of the sclerotium of PC (EPC) promoted the expression of AQP3 in cultured human keratinocytes. Western blotting was used to investigate the expression of AQPs and the activation of phosphoinositide 3-kinase (PI3K)/Akt-related signaling molecules in HaCaT cells. Cells were treated with inhibitors of PI3K/Akt and mechanistic target of rapamycin (mTOR) prior to EPC treatment.
Results: EPC promoted the expression of AQP3 in HaCaT cells without affecting AQP1 and AQP2 expression. Phosphorylated Akt levels were increased by EPC treatment, and the inhibition of PI3K by LY2940002 resulted in a reduction in EPC-induced AQP3 expression. Furthermore, EPC stimulated the phosphorylation of p70S6K and AktSer473, which are downstream targets of mTORC1 and mTORC2, respectively. The mTOR complex inhibitors, rapamycin and Torin 1, partially reduced EPC-induced AQP3 expression.
Conclusion: These results suggest that EPC increased expression of AQP3, which is important for skin moisturization, by activating the PI3K/Akt/mTOR signaling pathway in human keratinocytes.
Keywords: Poria cocos, AQP3, PI3K/Akt, mTORC1, mTORC2
Introduction
The skin is the body’s first line of defense against the external environment.1 For this reason, the skin is responsible for providing a physical and water-impermeable barrier for the body as it responds to a variety of stresses such as microorganisms, sunlight or physical trauma.2 In particular, keratinocytes, which form the skin barrier, are the predominant cell type found in the epidermis and constituting approximately 90% of the epidermal cells.3 Keratinocytes could handle the inflammatory response as well as skin hydration to maintain the homeostasis of the skin barrier.4 Thus, to find out whether the drugs/or cosmetics could regulate skin hydration, the determination of water proteins in keratinocytes is essential.
Aquaporins (AQPs), also called water channels, are a family of transmembrane proteins that mainly transport water across biological membranes and are involved in many physiological functions, including renal water balance, epithelial fluid secretion, cell migration, brain oedema and metabolism in adipocytes.5,6 Among them, several of the mammalian aquaporins such as AQP1, and AQP2 appear to be highly selective for the passage of water, whereas others (recently termed aquaglyceroporins such as AQP3, AQP7 and AQP9) also transport glycerol.7 Especially, AQP3, an aquaglyceroporin, is most abundantly expressed in keratinocytes of the basal layer of the human epidermis and is associated with keratinocyte migration, and proliferation.8–10
Poria cocos, a medicinal mushroom belonging to the Polyporaceae family, is widely used in nephrosis, insomnia, and diarrhea.11–13 Previous studies have reported that several compounds derived from P. cocos such as polysaccharides and triterpenoids have been reported to exhibit antitumor, anti-inflammatory, and anti-allergic activities, as well as immunomodulatory activities.11,14,15 In addition, it has been shown that the sclerotium of P. cocos has a skin-whitening effect.16
However, the moisturizing activity of P. cocos has not been reported. Therefore, in this study, we investigated whether the ethanol extract of the sclerotium of P. cocos (EPC) regulates AQP3 expression in cultured keratinocytes. In addition, we also demonstrated regulatory mechanisms of EPC by evaluating the activation of PI3K/Akt/mTOR signaling pathway.
Materials and Methods
Preparation of P. cocos Extracts
P. cocos Wolf was purchased from Omniherb (Youngcheon, Kyungbook, South Korea). The fungus (500 g) was soaked in 3 L of 100% ethanol for 3 days at room temperature with sonication for 60 min once a day. The solution was filtered using a standard sieve (No. 270, 53 µm), evaporated to dryness at 40 °C under vacuum (Eyela N-11, Japan), and freeze-dried (PVTFD10RS, IlShin BioBase, Korea). The amount of ethanol extract obtained was 24.9 g (yield: 6.98%), which was stored. The extract was dissolved in dimethyl sulfoxide (DMSO), and DMSO was used at a final concentration range of less than 0.01%.
Cell Culture and Drug Treatment
HaCaT cell lines originating from human keratinocytes were purchased from the Korea cell line bank (Seoul, South Korea). HaCaT cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Welgene, Korea) supplemented with 10% fetal bovine serum (FBS, Gibco Inc., USA), 100 units/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained at 37 °C in a humidified incubator with 5% CO2, and the medium was changed every 48 h. All experiments were performed in plastic tissue culture flasks obtained from SPL Life Science (Pocheon-si, Gyeonggi-do, South Korea). The cells were serum-starved for 24 h before incubation with EPC. LY294002, Torin 1 (Cell Signaling Technology, Dallas, TX, USA) and rapamycin (Sigma-Aldrich, St.Louis, MO, USA) were added 2 h prior to EPC treatment.
Cell Viability Analysis
Cell viability was determined using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded in 24-well plates at a density of 1×104 cells/well. After incubation with various concentrations (6.25, 12.5, 25, 50, 100 and 200 µg/mL) of EPC, MTT (0.5 mg/mL, Sigma) was added to each culture well and further incubated at 37 °C for 2 h. The medium was removed, 200 µL of DMSO was added, and the mixture was shaken for 10 min to dissolve the formazan crystals. The absorbance of the solubilized formazan was measured at 570 nm using a spectrofluorometer (F-2500, Hitachi, Japan).
Immunocytochemistry
HaCaT cells were plated in a chamber slide and incubated with EPC (50 μg/mL) for 24 h at 37°C. Then, the cells were fixed in 4% paraformaldehyde for 15 min at RT and washed 3 times with PBS. The cells were treated with 0.1% TritonX-100 for 15 min at RT. After washing, non-specific binding sites were blocked with serum (3% BSA) for 1 h at RT and incubated with AQP3 antibody (1:200) at 4°C overnight. The cells were then washed and incubated with AlexaFluor®488 goat anti-rabbit IgG (1:1,000; cat. no. A-11011) for 2 h at RT in a darkened room. For nuclear staining, the cells were incubated with DAPI (cat. no. H-1200; Vector Laboratories, Newark, CA, USA) at 1:1000 for 5 min at RT. The slide was finally washed with PBS and mounted for microscopic examination. Images were visualized using a confocal laser microscope (Olympus, Japan).
Immunoblot Analysis
After treatment with EPC, cells were lysed with cold RIPA lysis buffer (EzRIPA lysis kit, ATTA, Japan) containing protease and phosphatase inhibitors. The HaCaT cell lysate was then centrifuged at 15,000 × g for 20 min at 4 °C. The resultant protein samples were separated using SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (Millipore, Burlington, Massachusetts, USA). The membrane was stained with ponceau solution to confirm uniform transfer of all proteins and then incubated in a blocking solution (TBS with 0.05% Tween 20) containing 5% non-fat dry milk or 5% bovine serum albumin at room temperature for 2 h. The membranes were then probed with antibodies against AQP-1 (1:1000), AQP-2 (1:1000), AQP-3 (1:1000), p-Akt (1:1000), Akt (1:1000), p-p70S6K (1:1000), p70S6K (1:1000), and β-actin (1:5000). Antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and Cell Signaling Technology (Dallas, TX, USA). The primary antibodies were diluted in TBST solution containing 5% (w/v) skim milk or 5% (w/v) BSA. The membranes were then incubated with a specific horseradish peroxidase-conjugated secondary antibody (Invitrogen Biotechnology, USA). Immunoreactive proteins were detected using the ECL Kit (Millipore, Burlington, Massachusetts, USA). The bands were analyzed using a Chemidoc image analyzer (Bio-Rad, Hercules, CA, USA). The signal intensity was quantified by ImageJ.
Liquid Chromatography–Mass Spectrometry Analysis (LC/MS)
The standard of pachymic acid (PA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The EPC and PA were dissolved in methanol. Thereafter, ultra-performance liquid chromatography was performed on the ACQUITY UPLC system (Waters, MA, USA) with an ACQUITY binary solvent manager pump (ACQ-BSM), a ACQUITY PDA detector (ACQ-PDA) and Waters micromass ZQ spectrometer (Waters) coupled with an electrospray ionization (ESI) interface and an ion trap mass analyzer. The EPC and pachymic acid were analyzed under the following conditions: column, ACQUITY UPLC BEH Shield RP18 (2.1 × 100 mm, 1.7 μm; USA); mobile phase, distilled water (solvent system A), and acetonitrile (CH3CN, solvent system B) in a gradient mode (B from 10% to 100% in 15 min); sample injection volume, 5 µL; flow rate, 0.3 mL/min; column temperature, 40°C, UV wavelength, 200 nm. The conditions of MS analysis in the negative ion mode were as follows: capillary voltages, –3.00 kV; cone voltage, –100 V; extractor voltage, –2 V; RF lens voltage, –0.2 V; source temperature 120°C, desolvation temperature 400°C, gas flow desolvation 600 L/hr, gas flow cone 30 L/hr.
Statistical Analysis
All analyses were performed in triplicate, and numerical data are presented as mean ± standard deviation (SD). Multiple comparisons were performed using one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05.
Results
EPC Promotes the Expression of AQP3 in Keratinocytes Without Affecting AQP1 and AQP2
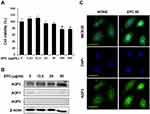
We first evaluated the cytotoxicity of EPC on HaCaT cells using an MTT assay as shown in Figure 1A. This figure shows HaCaT cells were treated with various concentrations of EPC (6.25, 12.5, 25, 50, 100 and 200 µg/mL) for 24 h. Because EPC did not show any cytotoxic effects at concentrations up to 50 µg/mL, we used EPC in the subsequent experiments at the concentration of 0–50 µg/mL. The effect of EPC on AQP1, AQP2 and AQP3 expression in cultured human keratinocytes is shown in Figure 1B. This figure shows HaCaT cells were treated with various concentrations of EPC (12.5, 25 and 50 µg/mL) for 24 h, and AQP expression was analyzed using Western blotting. As expected, AQP3 protein levels were significantly higher than other AQPs; AQP1 protein level was markedly lower than that of AQP3, and AQP2 was not detected in the cells. EPC induced AQP3 upregulation in a concentration-dependent manner without affecting the levels of AQP1 and AQP2. In addition, Figure 1C shows the immunocytochemistry analysis confirmed that intracellular AQP3 expression was visibly increased by ECT treatment. These results indicate that EPC promoted the expression of AQP3 in HaCaT cells without affecting AQP1 and AQP2 expression.
PI3K/Akt Pathway is Involved in EPC-Induced AQP3 Upregulation
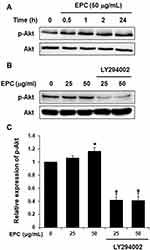
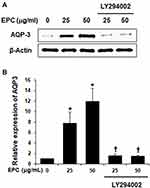
To examine whether EPC regulated AQP3 expression through activation of PI3K/Akt in HaCaT cells, we analyzed the effect of EPC on Akt phosphorylation. Figure 2A shows the phosphorylated Akt levels were increased following treatment with 50 µg/mL EPC for 0.5, 1, 2, 24 h. However, Figure 2B and C shows that the increase in AKT was suppressed by the PI3K inhibitor LY294002. Next, we evaluated whether the inhibition of endogenous PI3K signaling in HaCaT cells affects EPC-induced AQP3 regulation. Figure 3A and B shows the expression levels of AQP3 in HaCaT cells after treatment with EPC at 25 and 50 μg/mL in the presence or absence of LY294002. The inhibition of PI3K caused a noticeable decrease in EPC-induced AQP3 expression. These results support the involvement of the PI3K/Akt pathway in the upregulation of AQP3 by EPC in HaCaT cells.
EPC Activates mTOR Signaling in Keratinocytes
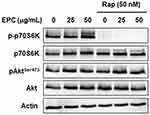
mTOR is one of the downstream components of the PI3K/Akt pathway and exists in two functionally and structurally distinct protein complexes, mTORC1 and mTORC2.9,10 To determine whether mTORC1 or mTORC2 activation is involved in AQP3 upregulation by EPC in HaCaT cells, phosphorylation of the downstream targets mTORC1 and mTORC2 was examined. p70S6K and AktSer473 are the respective mTORC1 and mTORC2 markers. Figure 4 shows the EPC increased the phosphorylation of p70S6K and AktSer473. Figure 4 shows the rapamycin, a mTORC1 inhibitor, completely blocked the EPC-induced increase in p70S6K phosphorylation. However, it did not affect the phosphorylation of the mTORC2 target AktSer473. This result suggests that EPC activates mTORC1 and mTORC2 signaling complexes.
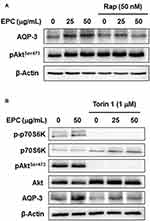
Inactivation of mTOR Signaling Partially Blocks EPC-Induced AQP3 Upregulation
Based on the above results, we assessed the potential involvement of mTOR in the ability of EPC to stimulate AQP3 expression in HaCaT cells using mTOR inhibitors. Figure 5A shows the inhibition of mTORC1 signaling with rapamycin partially down-regulated the EPC-induced AQP3 expression, but had no effect on AktSer473 phosphorylation. Moreover, Figure 5B shows the Torin 1 blocking two mTOR complexes, an inhibitor of mTOR downstream targets, significantly reduced AQP3 expression increased by EPC. As expected, in Figure 5B, activation of p70S6K and AktSer473 was completely blocked by Torin 1. These results indicate that upregulation of AQP3 by EPC in HaCaT cells can be regulated by activation of mTOR complexes.
Analysis of Active Candidate Components from EPC
To determine the active ingredient from EPC, we performed an optimized HPLC using PA based on the reports reported so far.17–22 As shown in Figure 6, analysis by UPLC of EPC provided major peaks at 4.8 min, and this peak was identified as PA based on the comparison of the retention time in chromatograms of compounds.
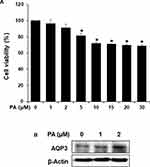
PA Promotes the Expression of AQP3 in Keratinocytes
We first evaluated the cytotoxicity of PA on HaCaT cells using an MTT assay is shown in Figure 7A. This figure shows the HaCaT cells were treated with various concentrations of PA (1, 2, 5, 10, 15, 20 and 30 µM) for 24 h. Because PA did not show any cytotoxic effects at concentrations up to 2 µM, we used PA in the subsequent experiments at the concentration of 0–2 µM. The effect of PA on AQP3 expression in cultured human keratinocytes is shown in Figure 7B. This figure shows the HaCaT cells were treated with various concentrations of PA (1 and 2 µM) for 24 h, and AQP3 expression was analyzed using Western blotting. As expected, PA induced upregulation of AQP3 in a concentration-dependent manner. These results indicate that PA upregulates the expression of AQP3 in HaCaT cells.
Discussion
In this study, we investigated the moisturizing effects and regulatory mechanisms of EPC in cultured human keratinocytes. We confirmed that AQP3 expression was significantly higher than that of AQP1 and AQP2 in cells and that the expression of AQP3 was significantly increased via EPC treatment. Moreover, the present study showed that EPC induced AQP3 expression through activation of the P3K/Akt/mTOR signaling pathway. These findings support the potential that EPC could lead to an increase in AQP3, which is important for maintaining the epidermal water permeability barrier, through PI3K/Akt/mTOR signaling pathway.
In this study, we could only detect the AQP3 not AQP1 and AQP2 (Figure 1). Among AQPs, AQP1 is mainly found on endothelial cells and fibroblasts of the human dermis, acts as a selective water channels, and up-regulated in fibroblasts during hypertonic stress.23,24 In similar, AQP2 is mainly expressed on kidney and fibroblasts,25 and rarely detected in keratinocytes.5,26,27 Because AQP1 and AQP2 is rarely detected in keratinocytes, we think that the AQP1 was not detected well in our study. However, EPC significantly up-regulated the AQP3 in keratinocytes, which suggests that it may facilitate the transport and metabolism of water and glycerol in the skin epidermis, and the function of the epidermal water permeability barrier.
In this study, we examined the role of the PI3K/Akt pathway in AQP3 expression in EPC-stimulated cells, because there are so many previous reports regarding PI3K/Akt pathway to induce AQP3.28–31 Previous reports suggested that the overexpression of Akt could contribute to the increase of AQP3, and consequently lead to autophagic responses,28 which means PI3K/AKT could mainly control the AQP3. In accordance with previous reports,28–31 we also found that the EPC treatment could increase the p-Akt, and EPC-induced AQP3 expression was noticeably decreased via treatment with the PI3K inhibitor, which support the involvement of the PI3K/Akt pathway in EPC-induced upregulation of AQP3. However, the increase rate of p-Akt is irrational to be a main regulator of AQP3. As shown in Figures 2 and 3, AQP-3 was increased about 12-fold increase, whereas p-AKT was increased about 20%. This might be a discrepancy that the increase of AQP-3 stem from p-ATK because AKT increase is relatively less than AQP-3. We think that AKT pathway might contribute the increase of AQP-3 at least partly, not the mainly. There are possibilities that Ras, ERK, AMPK could be mediated to induce AQP-3.28,32,33 Although we cannot perform the experiments of other pathway right now, we will examine the various mechanisms in next further experiments of active ingredients of PC.
mTOR signaling is one of the known downstream targets of the PI3K/Akt pathway and exists as two structurally distinct multiprotein complexes, mTORC1 and mTORC2; they regulate different cellular processes.34,35 mTORC1 mediates cell growth, cell proliferation, protein synthesis, and autophagy. mTORC2 functions as a regulator of actin cytoskeleton. Especially, mTORC1 regulates keratinocyte proliferation in the basal layer, whereas mTORC2 controls epidermal stratification and terminal differentiation.36,37 In addition, it has been shown that mTORC1 contributes to keratinocyte proliferation following UVB radiation, whereas mTORC2 maintains the survival of DNA-damaged keratinocytes. The mTOR activator MHY1485 attenuated skin cell damage by UV radiation, mTOR inhibitors almost abolished MHY1485-induced cytoprotective effects.38,39 Thus, we investigated the roles of the mTORC1 and mTORC2 pathways in EPC-induced AQP3. Here, EPC promoted the phosphorylation of p70S6K and AktSer473, the downstream targets of mTORC1 and mTORC2, respectively. We confirmed that mTORC1 inhibitor rapamycin treatment partially down-regulated EPC-induced AQP3 expression as well as suppressing p70S6K activation. Torin 1, as a selective ATP-competitive mTOR inhibitor, inhibits mTORC1 and mTORC2. The effect of EPC on AQP3 expression was decreased by blocking both mTOR complex with Torin 1. These data suggest that the mTOR pathway is activated by EPC, leading to the upregulation of AQP3 expression.
Several previous studies have reported that dehydrotrametenolic acid, the lanostane-type triterpene acid isolated from P. cocos, improves skin barrier function and has anti-diabetic and anti-tumor effects. Thus, many studies have focused on the dehydrotrametenolic acid, the lanostane-type triterpene acid as active compounds of P. cocos. However, in this study, we firstly reported that PA is a main ingredient of EPC, and a main active compound to induced AQP3 (Figures 6 and 7). Indeed, there are reports that PA could be derived from EPC,17 and exhibit the anti-inflammatory activity. In addition, PA has been reported to possess anti-inflammatory, anti-cancer properties, and anti-emetic,18–22 and could protect the skin tumor formation in mouse following initiation with 7,12-dimethylbenz[a]anthracene (DMBA).20 Although we did not determine the detailed mechanisms of PA in AQP3, the novel findings of active ingredients of EPC could be helpful for further development of skin goods.
Conclusion
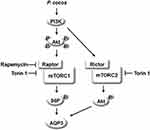
The summary is shown in Figure 8. EPC, and PA, a major physiologically active component of P. cocos, could promote the expression of AQP3. Figure 8 shows the EPC stimulated AQP3 expression through activation of PI3K/Akt in human keratinocytes. mTORC1 and mTORC2 pathways are activated by EPC, and the effect of EPC on AQP3 expression was decreased by blocking PI3K/Akt and both mTOR complex. These results suggest that EPC contributes to an increase in AQP3 levels, which is important for migration and proliferation of keratinocytes and the function of the epidermal water permeability barrier, by activating the PI3K/Akt/mTOR signaling pathway.
Data Sharing Statement
The original contributions presented in the study are included in the article material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
As this study does not involve humans or animals, no ethical approval is required.
Author Contributions
All authors made a significant contribution to the manuscript, including the conception, study design, execution, acquisition of data, analysis and interpretation. All authors participated in drafting and revising or critically reviewing the article, provided final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This study was supported by Wonkwang University in 2020.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Swaney MH, Kalan LR. Living in your skin: microbes, molecules, and mechanisms. Infect Immun. 2021;89(4):e00695–e00720. doi:10.1128/IAI.00695-20
2. Bollag WB, Aitkens L, White J, et al. HyndmanAquaporin-3 in the epidermis: more than skin deep. Am J Physiol Cell Physiol. 2020;318(6):C1144–C1153. doi:10.1152/ajpcell.00075.2020
3. El-Serafi AT, El-Serafi I, Steinvall I, et al. A systematic review of keratinocyte secretions: a regenerative perspective. Int J Mol Sci. 2022;23(14):7934. doi:10.3390/ijms23147934
4. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063–1072. doi:10.1111/j.1600-0625.2008.00786.x
5. Tricarico PM, Mentino D, Marco AD, et al. Aquaporins are one of the critical factors in the disruption of the skin barrier in inflammatory skin diseases. Int J Mol Sci. 2022;23:4020. doi:10.3390/ijms23074020
6. Ishibashi K, Kondo S, Hara S, et al. The evolutionary aspects of aquaporin family. Am J Physiol Regul Integr Comp Physiol. 2011;300(3):R566–R576. doi:10.1152/ajpregu.90464.2008
7. Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278(1):13–28. doi:10.1152/ajprenal.2000.278.1.F13
8. Chikuma MH, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med. 2008;86(2):221–231. doi:10.1007/s00109-007-0272-4
9. Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J Biol Chem. 2002;277(48):46616–46621. doi:10.1074/jbc.M209003200
10. Qin H, Zheng X, Zhong X, et al. Aquaporin-3 in keratinocytes and skin: its role and interaction with phospholipase D2. Arch Biochem Biophys. 2011;508(2):138–143. doi:10.1016/j.abb.2011.01.014
11. Rios JL. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011;77(7):681–691. doi:10.1055/s-0030-1270823
12. Lee SM, Lee YJ, Yoon JJ, et al. Effect of Poria cocos on hypertonic stress-induced water channel expression and apoptosis in renal collecting duct cells. J Ethnopharmacol. 2012;141(1):368–376. doi:10.1016/j.jep.2012.02.048
13. Feng YL, Lei P, Tian T, et al. Diuretic activity of some fractions of the epidermis of Poria cocos. J Ethnopharmacol. 2013;150(3):1114–1118. doi:10.1016/j.jep.2013.10.043
14. Bae MJ, See HJ, Choi G, et al. Regulatory T cell induced by poria cocos bark exert therapeutic effects in murine models of atopic dermatitis and food allergy. Mediators Inflamm. 2016;2016:3472608. doi:10.1155/2016/3472608
15. Tian H, Liu Z, Pu Y, et al. Immunomodulatory effects exerted by Poria cocos polysaccharides via TLR4/TRAF6/NF-κB signaling in vitro and in vivo. Biomed Pharmacother. 2019;112:108709. doi:10.1016/j.biopha.2019.108709
16. Kang KY, Hwang YH, Lee SJ, et al. Verification of the functional antioxidant activity and antimelanogenic properties of extract of Poria cocos mycelium fermented with Freeze-dried plum powder. Int J Biomater. 2019;2019:9283207. doi:10.1155/2019/9283207
17. Xia B, Zhou Y, Tan HS, et al. Advanced ultra-performance liquid chromatography-photodiode array-quadrupole time-of-flight mass spectrometric methods for simultaneous screening and quantification of triterpenoids in Poria cocos. Food Chem. 2014;152:237–244. doi:10.1016/j.foodchem.2013.11.151
18. Tai T, Akita Y, Kinoshita K, et al. Anti-emetic principles of Poria cocos. Planta Med. 1995;61(6):527–530. doi:10.1055/s-2006-959363
19. Giner EM, Manez S, Recio MC, et al. In vivo studies on the anti-inflammatory activity of pachymic and dehydrotumulosic acids. Planta Med. 2000;66(3):221–227. doi:10.1055/s-2000-8563
20. Kaminaga T, Yasukawa K, Kanno H, et al. Inhibitory effects of lanostane-type triterpene acids, the components of Poria cocos, on tumor promotion by 12-O-Tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology. 1996;53(5):382–385. doi:10.1159/000227592
21. Gapter L, Wang Z, Glinski J, et al. Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem Biophys Res Commun. 2005;332(4):1153–1161. doi:10.1016/j.bbrc.2005.05.044
22. Li G, Xu ML, Lee CS, et al. Cytotoxicity and DNA topoisomerases inhibitory activity of constituents from the sclerotium of Poria cocos. Arch Pharm Res. 2004;27(8):829–833. doi:10.1007/BF02980174
23. Boury JM, Sougrata R, Tailhardat M, et al. Expression and function of aquaporins in human skin: is aquaporin-3 just a glycerol transporter? Biochim Biophys Acta. 2006;1758(8):1034–1042. doi:10.1016/j.bbamem.2006.06.013
24. Baey AD, Lanzavecchia A. The role of aquaporins in dendritic cell micropinocytosis. J Exp Med. 2000;191(4):743–748. doi:10.1084/jem.191.4.743
25. Zhang Z, Xu P, Xie Z, et al. Downregulation of AQP2 in the anterior vaginal wall is associated with the pathogenesis of female stress urinary incontinence. Mol Med Rep. 2017;16(3):3503–3509. doi:10.3892/mmr.2017.7014
26. Aburada T, Ikarashi N, Kagami M, et al. Byakkokaninjinto prevents body water loss by increasing the expression of kidney aquaporin-2 and skin aquaporin-3 in KKAy mice. Phytother Res. 2011;25(6):897–903. doi:10.1002/ptr.3358
27. Patel R, Heard LK, Chen X, et al. Aquaporins in the skin. Adv Exp Med Biol. 2017;969:173–191. doi:10.1007/978-94-024-1057-0_11
28. Malale K, Fu J, Qiu L, et al. Hypoxia-induced aquaporin-3 changes hepatocellular carcinoma cell sensitivity to sorafenib by activating the PI3K/Akt signaling pathway. Cancer Manag Res. 2020;12:4321–4333. doi:10.2147/CMAR.S243918
29. Xu H, Xu Y, Zhang W, et al. Aquaporin-3 positively regulates matrix metalloproteinases via PI3K/AKT signal pathway in human gastric carcinoma SGC7901 cells. J Exp Clin Cancer Res. 2011;30(1):86. doi:10.1186/1756-9966-30-86
30. Chandrashekar L, Rajappa M, Rajendiran KS, et al. Is vitiligo associated with systemic aquaporin-3 deficiency? Postepy Dermatol Alergol. 2021;38(2):156–158. doi:10.5114/ada.2021.104291
31. Rodríguez A, Catalán V, Ambrosi JG, et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab. 2011;96(4):E586–E597. doi:10.1210/jc.2010-1408
32. Wang W, Geng X, Lei L, et al. Aquaporin-3 deficiency slows cyst enlargement in experimental mouse models of autosomal dominant polycystic kidney disease. FASEB J. 2019;33(5):6185–6196. doi:10.1096/fj.201801338RRR
33. Hou SY, Li YP, Wang JH, et al. Aquaporin-3 inhibition reduces the growth of NSCLC cells induced by hypoxia. Cell Physiol Biochem. 2016;38(1):129–140. doi:10.1159/000438615
34. Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23(18):3151–3171. doi:10.1038/sj.onc.1207542
35. Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi:10.1126/science.1106148
36. Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7(1):13226. doi:10.1038/ncomms13226
37. Buerger C, Shirsath N, Lang V, et al. Inflammation dependent mTORC1 signaling interferes with the switch from keratinocyte proliferation to differentiation. PLoS One. 2017;12(7):e0180853. doi:10.1371/journal.pone.0180853
38. Carr TD, DiGiovanni J, Lynch CJ, et al. Inhibition of mammalian target of rapamycin (mTOR) suppresses UVB-induced keratinocyte proliferation and survival. Cancer Prev Res. 2012;5(12):1394–1404. doi:10.1158/1940-6207.CAPR-12-0272-T
39. Yang B, Xu QY, Guo CY, et al. MHY1485 ameliorates UV-induced skin cell damages via activating mTOR-Nrf2 signaling. Oncotarget. 2017;8(8):12775–12783. doi:10.18632/oncotarget.14299
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.