Back to Journals » Journal of Blood Medicine » Volume 10
Population Pharmacokinetic Modeling Of On-Demand And Surgical Use Of Nonacog Beta Pegol (N9-GP) And rFIXFc Based Upon The paradigm 7 Comparative Pharmacokinetic Study
Authors Simpson ML, Kulkarni R , Escuriola Ettingshausen C, Medom Meldgaard R, Cooper DL , Klamroth R
Received 29 May 2019
Accepted for publication 9 September 2019
Published 13 November 2019 Volume 2019:10 Pages 391—398
DOI https://doi.org/10.2147/JBM.S217539
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Mindy L Simpson,1 Roshni Kulkarni,2 Carmen Escuriola Ettingshausen,3 Rikke Medom Meldgaard,4 David L Cooper,5 Robert Klamroth6
1Pediatric Hematology/Oncology, Rush University Medical Center, Chicago, IL, USA; 2Department of Pediatrics and Human Development, Michigan State University, East Lansing, MI, USA; 3HZRM – Haemophilia Centre Rhein-Main, Frankfurt-Mörfelden, Mörfelden-Walldorf, Germany; 4Novo Nordisk A/S, Søborg, Denmark; 5Novo Nordisk Inc, Plainsboro, NJ, USA; 6Department for Internal Medicine, Vascular Medicine and Haemostaseology, Vivantes Klinikum, Berlin, Germany
Correspondence: Mindy L Simpson
Pediatric Hematology/Oncology, Rush University Medical Center, 1653 W. Congress Parkway, Chicago, IL 60612, USA
Tel +1 312 563 3995
Email [email protected]
Aim/objective: Understanding pharmacokinetic (PK) differences between standard and extended half-life (EHL) products is important, particularly for factor IX (FIX), where differences are more significant than for factor VIII. Two single-dose PK trials showed N9-GP achieves higher FIX levels and greater area-under-the-curve than pdFIX, rFIX, and rFIXFc through higher recovery and longer terminal half-life. In paradigm 7, N9-GP demonstrated consistently favorable PK characteristics compared with rFIXFc. Collins et al explored population PK differences between N9-GP and pdFIX/rFIX based upon paradigm 1 data. This analysis uses population PK models based upon the paradigm 7 trial.
Methods: 15 patients (21–65 years) with hemophilia B received single 50-IU/kg doses of N9-GP and rFIXFc ≥21 days apart. A population PK model developed from single-dose PK profiles simulated plasma FIX activity following dosing for surgery and on-demand treatment of bleeds. Simulations explored doses and frequencies required to sustain target World Federation of Hemophilia (WFH) factor activity levels.
Results: PK profiles of N9-GP and rFIXFc were described by one- and three-compartment models, respectively. Simulations predicted significantly reduced dosing frequency and consumption for N9-GP than rFIXFc. For severe bleeds, a single N9-GP dose (80 IU/kg) is sufficient to maintain WFH-recommended FIX levels, whereas multiple rFIXFc doses are required. For surgery, redosing in the first week with N9-GP is modeled at day 6 vs rFIXFc dosing at 6, 30, 54, 78, and 126 hrs. For life-threatening bleeds, N9-GP is required at days 0, 3, 6, 13, and 18 vs rFIXFc redosing after 6 hrs with 10 additional doses at 24-, 48-, and 72 hr intervals.
Conclusion: PK modeling approaches based upon direct comparative studies offer insights into PK differences between EHL FIX products. Model simulations show N9-GP may allow on-demand treatment and perioperative management with 55–75% fewer injections and 65–74% lower overall factor concentrate consumption than rFIXFc.
Keywords: N9-GP, rFIXFc, population PK modeling, paradigm 7 trial
Plain Language Summary
This study compared the pharmacokinetics (PK) of two extended half-life products used in the treatment of hemophilia B: nonacog beta pegol (N9-GP) and rFIX Fc fusion protein (rFIXFc). Using data from patients participating in the paradigm 7 comparative PK study, a population PK model was used to simulate the plasma FIX activity following dosing for surgery and on-demand treatment of bleeds. The model was designed with reference to factor activity level targets in the World Federation of Hemophilia guidelines. The modeling simulations predicted significantly reduced dosing frequency and total FIX consumption for N9-GP than for rFIXFc in the three situations investigated: severe bleeds, life-threatening bleeds, and major surgery. While head-to-head prospective clinical studies are not standardly performed in patients with hemophilia, this modeling simulation offers insights into the potential clinical relevance of the PK differences between these two EHL FIX products. This analysis showed that N9-GP may allow on-demand treatment and perioperative management with 55–75% fewer injections and 65–74% lower overall factor concentrate consumption than rFIXFc.
Introduction
Congenital hemophilia B is an X-linked disorder resulting from missing or reduced amounts of clotting factor IX (FIX). Severity is classified based upon residual FIX activity with <1% severe, 1–5% moderate and >5–40% mild.1 In comparison with hemophilia A (factor VIII [FVIII] deficiency), where half the patients have severe disease marked by frequent spontaneous bleeding (typically in joints), only one third of patients with hemophilia B have severe disease. Consequently, the majority have mild-to-moderate hemophilia where bleeding has traditionally been associated more commonly with trauma and surgery.
Routine replacement of factor (prophylaxis) is recommended for those with severe disease or frequent bleeding,1 and it is not surprising that the percentage of patients on routine prophylaxis varies across hemophilia severities. Data from the United States suggest that routine prophylaxis is prescribed for 80.7% of those with severe hemophilia, 20.9% for moderate hemophilia, and 7.4% with mild hemophilia.2 However, increasing epidemiological evidence suggests that patients with mild-to-moderate hemophilia have fewer bleeding episodes when baseline factor levels reach 15–20%.3,4 Consequently, treatment of bleeding when it occurs (on-demand) and preventing bleeding during surgery are also important for the majority of patients with mild-to-moderate hemophilia B. Further, evolving psychosocial research suggest that those with mild-to-moderate hemophilia B may be less familiar with intravenous self-infusions and need to rely on family and healthcare practitioners to treat bleeds when they happen.
With plasma-derived and early recombinant FIX (rFIX) products that had a standard half-life (SHL) of about 18 hrs, two or more infusions were often required to treat bleeding; in clinical trials leading to registration, 61.4–75% of adults/adolescents received only one dose.5–7 Further, following initial high bolus doses, multiple and frequent additional doses or continuous infusions of FIX were used for perioperative management.8–10 The development of three extended half-life (EHL) FIX products with different prolongation mechanisms and pharmacokinetic (PK) profiles provided the potential to reduce the frequency of administration for routine prophylaxis, but also to impact on-demand treatment of bleeds and perioperative management.
Therefore, it is important to understand the PK profile differences between SHL and EHL products, and particularly between EHL FIXs, where the differences between products are more significant than for EHL FVIII products. Two single-dose PK trials showed that recombinant glycoPEGylated FIX (N9-GP, Rebinyn®/Refixia®, Novo Nordisk, Bagsværd, Denmark) achieves higher FIX levels and greater area under the plasma concentration-time curve (AUC) than plasma-derived FIX (pdFIX), rFIX (BeneFix®, Pfizer Inc, New York, NY, USA), and rFIX Fc fusion protein (Alprolix®, Bioverativ Therapeutics, Inc, Waltham, MA, USA) through higher recovery and longer terminal half-life. In paradigm 7, a 50-IU/kg dose based upon rFIXFc dosing recommendations was used for both products to allow comparison of all dose-dependent PK parameters.11 N9-GP demonstrated consistently favorable PK characteristics compared with rFIXFc based on both one-stage clotting assay and chromogenic assay. Based upon FIX activity levels with a one-stage assay, N9-GP had 4.4× higher AUC, 2.2× higher incremental recovery at 30 mins post-dosing, 5.8× higher FIX activity at 168 hrs, and 1.2× longer half-life.11 The findings are consistent with published data for each compound.12,13
The impact of PK differences between N9-GP and pdFIX/rFIX for on-demand and surgical use was first explored by Collins et al14 based on data from the paradigm 1 PK trial. The present analysis uses population PK models to explore the PK differences between N9-GP and rFIXFc that were observed in the paradigm 7 PK trial.
Methods
Data
paradigm 7 (Modeling Dataset)
paradigm 7 (NCT03075670) was a multicenter, multinational, open-label, randomized, crossover trial (conducted between March 7 and December 8, 2017).11 The trial consisted of one screening visit and two PK sessions, each comprising eight visits over 10 days. After randomization, patients received a single 50-IU/kg dose of either N9-GP or rFIXFc; at least 21 days after this first dose, patients received a single dose of the other product. Each dose administration was preceded by a minimum 96 hr washout period from nonmodified FIX products (if administered); the use of commercial EHL rFIX products was not permitted during the trial. Blood samples were collected for PK assessment at 14 time points over each 10-day PK session: predose, 10 and 30 mins, and 1, 3, 6, 8, 24, 48, 96, 144, 168, 192 and 240 hrs’ postdose.
paradigm 2 (Validation Dataset)
paradigm 2 (NCT01333111) was a multinational, single-blind trial in which 59 patients with hemophilia B (FIX ≤2 IU/dL) were randomized to one of two N9-GP prophylaxis arms (10 and 40 IU/kg once weekly).15 In the trial, which was conducted from April 2011 to April 2013, PK assessments were performed at trial initiation and after 12–44 weeks of prophylaxis with 10 IU/kg (n=7) and 40 IU/kg (n=10). The PK assessments included seven sampling points up to 168 hrs’ postdose. For model validation, the single dose 40-IU/kg PK sessions at enrollment in the phase 3 trial were used.
Modeling Strategy
Model selection was guided by visual inspection of goodness-of-fit plots, plausibility of parameter estimates, and changes in –2 Log Likelihood (–2LL). For nested models, the change in –2LL is assumed to be χ2 distributed. A P-value of 0.001 was used in model extension/reduction criteria throughout the analysis; hence, a drop in –2LL of at least 10.83 was required for the addition of a single parameter to be a significant improvement in model fit. Classical goodness-of-fit plots, such as observed values vs population predictions, observed values vs individual predictions, and individual residual errors vs time or vs concentrations, were used for graphical assessment of the model fit. The population model estimated the typical values of the model parameters and the interpatient variability of these parameters.
Interindividual Variability
The differences between individual parameters were described using random effects. These were assumed to be normally distributed with mean zero and a variance, which was estimated. The distribution of the individual parameters around the typical population value was assumed to be log-normal.
Residual Error Model
An additive model was used to describe the residual error of the log-transformed data. The residual error was assumed to arise from a distribution with mean zero and a variance, which was estimated.
Linear one-, two- and three-compartmental models were tested in the model development. Model development was based on data from paradigm 7. To validate the model, the final version was used to simulate single-dose PK data from paradigm 2. Data analysis was performed using SAS software, version 9.4 (SAS Institute Inc, Cary, NC, USA).
Pharmacokinetic Simulations To Compare rFIXFc And N9-GP
The developed PK population models were used to simulate plasma FIX activity following dosing with N9-GP and rFIXFc for major surgery and on-demand treatment of bleeds. For N9-GP, the model was based on the dosing amount recommended in the prescribing information: 40 IU/kg for mild/moderate bleeds; 80 IU/kg for severe and life-threatening bleeds with additional doses of 40 IU/kg as necessary; 80 IU/kg initial dose for major surgery and subsequent doses of 40 IU/kg in the postoperative period. For rFIXFc, the prescribing information provides formulas to calculate dosing based upon desired FIX levels and provides guidelines for FIX targets that mimic WFH guidelines.1
Simulations were performed to explore N9-GP and rFIXFc frequencies required to sustain target FIX levels derived from WFH guidelines:1 severe bleed >50% for 3 days; life-threatening bleed (eg, intracranial hemorrhage) 60–80% for the first 7 days, then >30% until 21 days; and major surgery >40% for 3 days, >30% for 3 days, then >20% until day 14.
While initial modeling was done to optimize FIX profiles to comply with WFH guidelines,1 additional sensitivity analyses were conducted to assess the impact of further aligning the dosing intervals to those recommended in prescribing information for N9-GP for major surgery (additional doses of 40 IU/kg every 1–3 days during the first week and weekly thereafter).
Results
Pharmacokinetic Model
The PK of N9-GP following intravenous bolus doses was described by a linear one-compartmental model, with first-order elimination. The PK of rFIXFc was described by a linear three-compartmental model with first order elimination, which is in accordance with the previous publication.16
The PK models were able to describe the data well. For both N9-GP and rFIXFc, the majority of data (95% for N9-GP and 87% for rFIXFc) are within the 95% confidence interval (CI) of the predictions (Figures 1 and 2). The mean of the observations is within the 95% CI of the predictions at all time points. Goodness-of-fit plots are provided as Supplementary Figures 1 and 2.
 |
Figure 1 Predictions of FIX activity with N9-GP compared with observed activity in paradigm 7. Abbreviation: FIX, factor IX. |
 |
Figure 2 Predictions of FIX activity with rFIXFc compared with observed activity in paradigm 7. Abbreviations: FIX, factor IX; rFIXFc, recombinant FIX Fc fusion protein. |
To validate the final model, it was used to predict the FIX activity from another trial (paradigm 2) where patients received a single 40-IU/kg dose of N9-GP. The mean of the observations at all time points is within the 95% CI of the population prediction and the majority of data (85%) are within the CI of the predictions (Supplementary Figure 3).
Pharmacokinetic Simulations To Compare rFIXFc And N9-GP
The final population PK model was used to simulate plasma FIX activity following dosing with N9-GP and rFIXFc for major surgery and on-demand treatment of bleeds. Simulations predicted significantly reduced dosing frequency and total FIX consumption for N9-GP vs rFIXFc (Table 1).
 |
Table 1 Simulated Number Of Doses And Total Consumption Of N9-GP And rFIXFc For On-Demand Treatment And Surgery |
For severe bleeds, a single dose of N9-GP (80 IU/kg) is sufficient to maintain FIX levels according to WFH guidelines,1 whereas multiple doses of rFIXFc (initial dose of 80 IU/kg with three subsequent doses of 50 IU/kg) are required (Figure 3; Supplementary Figures 4A and B for CIs). Even with reduced overall consumption and number of doses, N9-GP achieved higher FIX activities (min–max) over the treatment period of 3 days (N9-GP: 0.73–1.36 IU/mL; rFIXFc: 0.46–0.88 IU/mL) (Table 2).
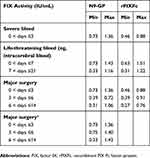 |
Table 2 Simulated FIX Activity Ranges |
 |
Figure 3 Simulated dosing for severe bleeds to achieve FIX activities meeting WFH guidelines:1 N9-GP 80 IU/kg and rFIXFc 80 IU/kg followed by 50 IU/kg. Abbreviations: FIX, factor IX; rFIXFc, recombinant FIX Fc fusion protein; WFH, World Federation of Hemophilia. |
For life-threatening bleeds, N9-GP 80 IU/kg is required at day 0, followed by doses of 40 IU/kg at days 3, 6, 13, and 18. For rFIXFc, a 110 IU/kg initial dose is required, followed by three doses of 90 IU/kg and seven of 80 IU/kg at intervals of 6 hrs, then 24, 48, and 72 hrs (Figure 4; Supplementary Figures 5A and B for CIs). FIX activities (min–max) achieved by the model simulations were similar for N9-GP (0–7 days [0.73–1.43 IU/mL], >7–21 days [0.33–1.16 IU/mL]) to those with rFIXFc (0–7 days [0.63–1.51 IU/mL], >7–21 days [0.31–1.22 IU/mL]) (Table 2).
 |
Figure 4 Simulated dosing for life-threatening bleeds to achieve FIX activities meeting WFH guidelines:1 N9-GP 80 IU/kg followed by 40 IU/kg and rFIXFc 110 IU/kg followed by 3 doses of 90 IU/kg and 7 doses of 80 IU/kg. Abbreviations: FIX, factor IX; rFIXFc, recombinant FIX Fc fusion protein; WFH, World Federation of Hemophilia. |
For major surgery, following an initial N9-GP 80 IU/kg dose, redosing with 40 IU/kg is modeled at day 6 post-surgery and then another dose at day 12. Following an initial rFIXFc dose of 80 IU/kg, eight additional 50-IU/kg doses were required at 6, 30, 54, 78, 126, 174, 222, 270 hrs (Figure 5; Supplementary Figures 6A and B for CIs). FIX activities (min–max) achieved by the model-simulated dosing regimen were higher in the first 3 days for N9-GP (0–3 days [0.73–1.36 IU/mL], >3–6 days [0.39–0.72 IU/mL], >6–14 days [0.31–1.06 IU/mL]) than those with rFIXFc (0–3 days [0.46–0.88 IU/mL], >3–6 days [0.39–0.91 IU/mL], >6–14 days [0.27–0.76 IU/mL]) (Table 2).
 |
Figure 5 Simulated dosing for major surgery to achieve FIX activities meeting WFH guidelines:1 N9-GP 80 IU/kg followed by 40 IU/kg and rFIXFc 80 IU/kg followed by 50 IU/kg. Abbreviations: FIX, factor IX; rFIXFc, recombinant FIX Fc fusion protein; WFH, World Federation of Hemophilia. |
Model simulations to WFH target FIX levels1 resulted in doses similar to the product labeling. However, additional sensitivity analyses based upon a dosing interval of 1–3 days in the first week (as per N9-GP prescribing information) shifted the timing of additional N9-GP doses for major surgery to days 3, 6, and 13 (Figure 6). Adding one additional dose and increasing the total dose to 200 IU/kg resulted in FIX activity curves predicted to raise FIX levels to a minimum of 0.73 IU/mL in the first week and a peak of up to 1.43 IU/mL (Table 2), providing FIX levels well in excess of the WFH guidelines1 and those simulated for rFIXFc.
 |
Figure 6 Sensitivity analysis for simulated dosing for major surgery to achieve FIX activities to meet WFH guidelines:1 N9-GP 80 IU/kg followed by 40 IU/kg every 1–3 days during the first week and every week during the second week. rFIXFc 80 IU/kg followed by 50 IU/kg. Abbreviations: FIX, factor IX; rFIXFc, recombinant FIX Fc fusion protein; WFH, World Federation of Hemophilia. |
Discussion
Treatment of acute bleeds with SHL pdFIX or rFIX has been reported to require more than one injection to achieve hemostasis in clinical trials. Even with the longer half-life of SHL FIX compared with FVIII, more enhanced on-demand strategies targeting decreased inflammation and prevention of joint damage by preventing rebleeding would require additional dosing over several days.17
Clinicians treat bleeds differently between patients, as well as in the same patient over time (eg, for rebleeds, target joint bleeds). While prescribing information of SHL and EHL FIX products prior to N9-GP suggested recommended target FIX levels informed by the WFH guidelines,1 it is unclear to what extent the dosing used in the real-world setting reflects these guidelines. To simulate possible on-demand scenarios, we followed the approach of Collins et al14 and considered hypothetical bleeds of different severities and used the WFH guidelines as a way to compare two EHL FIXs with very different PK profiles in their respective comparative studies with SHL FIX.
While it was not surprising in previous modeling that a severe bleed requiring one N9-GP dose of 80 IU/kg would require six doses of SHL FIX (rFIX: 350 IU/kg; pdFIX: 310 IU/kg), the current study suggests that achieving the 0.50-IU/mL level for 3 days with rFIXFc would still require four doses (230 IU/kg). For life-threatening bleeds treated for 21 days, the PK models suggested five N9-GP doses (80 IU/kg followed by four doses of 40-IU/kg at decreasing intervals; total consumption: 240 IU/kg) and 11 rFIXFc doses (110 IU/kg followed by three doses of 90 IU/kg and seven doses of 80 IU/kg doses; total consumption: 940 IU/kg). Here, results show a similar pattern to the prior modeling (rFIX/pdFIX, 28 doses: 1490 / 1450 IU/kg), with rFIXFc requiring more than twice the number of doses (11 vs five) and 4× the total consumption (940 vs 240 IU/kg) as N9-GP. In both simulations, the initial more rapid clearance from the plasma following infusion results in the need to redose, here with rFIXFc within 6 hrs, then 24, 48, and 72 hrs to maintain FIX levels.
As with the prior modeling study,14 simulations using the paradigm 7 PK profiles for N9-GP to cover major surgery showed that three infusions (160 IU/kg) would be sufficient over 2 weeks. In the sensitivity analysis, dosing over 1–3 days during the first week resulted in four total infusions (200 IU/kg). While clinical experience with multiple administration of SHLs in the first few days after surgery is supported by trials and these modeling simulations, this does to some extent carry through to the surgical studies including N9-GP, where investigator guidelines were similar to and mirrored by the recommendation in the prescribing information.18 In that study, the median preoperative dose was 81.7 IU/kg and all patients received only one dose on the day of surgery. However, despite having high median FIX levels of 1.43 IU/mL after the preoperative dose, 1.12 IU/mL at 24 hrs, and 0.73 IU/mL at 48 hrs (measured at 47–57 hrs), four patients received additional dosing prior to the second day at the Investigator’s discretion with FIX levels at 24 hrs of 0.84 IU/mL, 1.12 IU/mL, 1.31 IU/mL, and 1.34 IU/mL at redosing. As a result, a median of two injections were administered during days 1–6 (median: 84.1 IU/kg) and a median of three injections were administered over days 1–13 (median: 126.1 IU/kg).18 So, while the trial experience under protocol is reflected in the dosing recommendations in the prescribing information, the study data on FIX activities also confirm the results of the sensitivity analysis, demonstrating that the levels achieved by redosing in the first few days result in substantially higher FIX levels than those recommended in the WFH criteria1 and levels above 0.75 IU/mL for more than a week. Thus, the primary modeling based upon targeted levels represents a more realistic comparative scenario and one that would match clinical expectations for FIX levels.
To maintain WFH-recommended FIX levels for surgery, rFIXFc would require fewer doses than previously simulated for rFIX/pdFIX but would require 3× as many doses (nine vs three) and a higher total dose (480 IU/kg) compared with N9-GP; these results remain clinically different even with the sensitivity analysis for N9-GP. The modeling for rFIXFc is consistent with the data reported in its phase 3 surgery trial with a median infused dose of 90.9 IU/kg, with patients reported to receive 1–3 infusions on the day of surgery and 2–3 during postoperative days 1–3. The median total consumption over 14 days was 432.3 IU/kg, with a maximum of 1084.7 IU/kg.19
A potential limitation of this modeling approach is that the data used were single-dose PK data in a nonbleeding state. The FIX activities maintained after infusions in an actively bleeding patient or surgical setting may be slightly lower. While it is not known exactly how much FIX is consumed at the site of bleeding during a bleed or procedure, population-based modeling has been able to predict doses and, in the case of this model in the surgical setting, correlates with the experience in phase 3 surgical trials of N9-GP and rFIXFc. Any differences in FIX levels due to consumption would also be seen across all FIX products, so improved incremental recovery and half-life should still impact the burden of treatment.
As we continue to develop new products and assess their use in more severe bleeds and surgical settings, and as we combine non-factor and factor products in such settings to control bleeding but avoid thrombosis, it is important to recognize the ongoing need to better understand and generate evidence on target factor activities. WFH guideline recommendations on treatment of different types of bleeding episodes range from level 2–5 evidence, and separate guidelines exist for developed countries (nonconstrained factor availability) and developing countries (limited factor availability). Ultimately, patients are all different and even orthopedic procedures in patients with hemophilia are all potentially very different, making it very difficult to gather sufficient evidence to inform guidelines. Whether considering intensity and duration of treatment for an intracerebral hemorrhage to prevent reoccurrence, or for a total knee replacement to avoid postoperative bleeding and potential for late infection, the benefit/risk is likely to remain on the side of treating to higher activity levels than what might be minimally necessary.
Conclusions
While head-to-head prospective clinical studies are not standardly performed in patients with hemophilia, PK modeling approaches based upon direct PK comparisons in PK studies can offer insights into the implications of PK differences between EHL FIX products. These data show N9-GP may allow on-demand treatment and perioperative management with 55–75% fewer injections and 65–74% lower overall factor concentrate consumption than rFIXFc. These data highlight the particular need to consider SHL/EHL and potential EHL/EHL differences for FIX products and consider product-specific PK profiles to prescribe treatment.
Abbreviations
–2LL, –2 Log Likelihood; AUC, area under the plasma concentration-time curve; CI, confidence interval; EHL, extended half-life; FIX, factor IX; FVIII, factor VIII; N9-GP, nonacog beta pegol (glycoPEGylated rFIX); OS, one-stage; pdFIX, plasma-derived factor IX; PK, pharmacokinetic; rFIX, recombinant factor IX; rFIXFc, recombinant FIX Fc fusion protein; SHL, standard half-life; WFH, World Federation of Hemophilia.
Acknowledgment
This paper was presented at the HTRS/NASTH 2019 Scientific Symposium as a poster. The poster’s abstract was published in “Abstracts” in Haemophilia July 2019.
Author Contributions
DLC wrote the manuscript draft. RMM performed the data analysis. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
MLS reports consulting honoraria from Bayer, Bioverativ/Sanofi, CSL Behring, Genentech, Octapharma and Takeda. She also served in the speaker bureau for Bayer and Novo Nordisk. RK served in the advisory boards for Bioverativ/Sanofi, BPL, Catalyst Bioscience and Biomarin, Genentech, Kedrion, Novo Nordisk, Octapharma, Pfizer, and Shire/Takeda. CEE reports consultant/speaker’s fees/research funding from Alnylam, Bayer, Biotest, CSL Behring, Freeline, Grifols, LFB, Novo Nordisk, Octapharma, Roche, Shire, and SOBI. RMM is an employee of Novo Nordisk A/S and owns stocks in Novo Nordisk. DLC is employee of Novo Nordisk Inc. RK reports research support/honoraria from Bayer, Biomarin, Biotest, Catalyst Bioscience, CSL Behring, Genentech, Grifols, LEO Pharma, Novo Nordisk, Octapharma, Pfizer, Roche, Shire/Takeda, and SOBI. The authors report no other conflicts of interest in this work.
References
1. Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47. doi:10.1111/j.1365-2516.2012.02909.x
2. American Thrombosis & Hemostasis Network. Factor IX Deficiency. Research Report. American Thrombosis & Hemostasis Network; 2018.
3. den Uijl IE, Fischer K, Van Der Bom JG, et al. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41–44. doi:10.1111/j.1365-2516.2010.02383.x
4. Soucie JM, Monahan PE, Kulkarni R, Konkle BA, Mazepa MA. The frequency of joint hemorrhages and procedures in nonsevere hemophilia A vs B. Blood Adv. 2018;2(16):2136–2144. doi:10.1182/bloodadvances.2018020552
5. Shire. RIXUBIS prescribing information. Available from: https://www.shirecontent.com/PI/PDFs/RIXUBIS_USA_ENG.pdf.
6. Pfizer Inc. BENEFIX prescribing information. Available from: http://labeling.pfizer.com/showlabeling.aspx?id=492.
7. Aptevo BioTherapeutics LLC. IXINITY prescribing information. Available from: https://www.ixinity.com/sites/default/files/IXINITY_PI_Aptevo.pdf.
8. Roth DA, Kessler CM, Pasi KJ, et al. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood. 2001;98(13):3600–3606. doi:10.1182/blood.v98.13.3600
9. Ragni MV, Pasi KJ, White GC, et al. Use of recombinant factor IX in subjects with haemophilia B undergoing surgery. Haemophilia. 2002;8(2):91–97.
10. Quon DV, Logan L. Safety and efficacy of plasma-derived coagulation factor IX concentrate (AlphaNine(R) SD) in patients with haemophilia B undergoing surgical intervention: a single institution retrospective analysis. Haemophilia. 2011;17(1):e196–e201. doi:10.1111/j.1365-2516.2010.02354.x
11. Escuriola Ettingshausen C, Hegemann I, Simpson ML, et al. Favorable pharmacokinetics in hemophilia B for nonacog beta pegol versus recombinant factor IX-Fc fusion protein: a randomized trial. Res Pract Thromb Haemost. 2019;3(2):268–276. doi:10.1002/rth2.12192
12. Negrier C, Knobe K, Tiede A, Giangrande P, Moss J. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2011;118(10):2695–2701. doi:10.1182/blood-2011-02-335596
13. Shapiro AD, Ragni MV, Valentino LA, et al. Recombinant factor IX-Fc fusion protein (rFIXFc) demonstrates safety and prolonged activity in a phase 1/2a study in hemophilia B patients. Blood. 2012;119(3):666–672. doi:10.1182/blood-2011-07-367003
14. Collins PW, Moss J, Knobe K, et al. Population pharmacokinetic modeling for dose setting of nonacog beta pegol (N9-GP), a glycoPEGylated recombinant factor IX. J Thromb Haemost. 2012;10(11):2305–2312. doi:10.1111/jth.12000
15. Collins PW, Young G, Knobe K, et al. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124(26):3880–3886. doi:10.1182/blood-2014-05-573055
16. Diao L, Li S, Ludden T, et al. Population pharmacokinetic modelling of recombinant factor IX Fc fusion protein (rFIXFc) in patients with haemophilia B. Clin Pharmacokinet. 2014;53(5):467–477. doi:10.1007/s40262-013-0129-7
17. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. doi:10.1056/NEJMoa067659
18. Escobar MA, Tehranchi R, Karim FA, et al. Low-factor consumption for major surgery in haemophilia B with long-acting recombinant glycoPEGylated factor IX. Haemophilia. 2017;23(1):67–76. doi:10.1111/hae.13041
19. Powell JS, Apte S, Chambost H, et al. Long-acting recombinant factor IX Fc fusion protein (rFIXFc) for perioperative management of subjects with haemophilia B in the phase 3 B-LONG study. Br J Haematol. 2015;168(1):124–134. doi:10.1111/bjh.13112
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
