Back to Journals » OncoTargets and Therapy » Volume 12
Poor prognosis of pulmonary sarcomatoid carcinoma with KRAS mutation and ALK fusion
Authors Chen F, Gu Q, Hu C, Cai X, Lei S
Received 2 December 2018
Accepted for publication 21 February 2019
Published 1 May 2019 Volume 2019:12 Pages 3321—3325
DOI https://doi.org/10.2147/OTT.S196751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Fangmin Chen, Qihua Gu, Chengping Hu, Xiaoling Cai, Shuhua Lei
Department of Respiratory Medicine, Xiangya Hospital of Central South University, Changsha, People’s Republic of China
Abstract: Pulmonary sarcomatoid carcinoma (PSC) is a rare subtype of poorly differentiated non-small-cell lung cancer (NSCLC), and no effective treatment is available in clinical practice currently. In the present report, a 61-year-old male patient was hospitalized due to cough, dyspnea, and right chest pain. Computed tomography (CT) showed spot- and piece-shaped shadows. The patient became very weak and had breathing difficulty after preliminary anti-pneumonia treatment with cefoperazone–sulbactam. Physical examination revealed dull sound by percussion and decreased breath sounds in the right lateral lung areas by auscultation. A second CT scan revealed a large amount of pleural effusion, and the patient was diagnosed with bloody pleural effusion through pleural space puncture. Multiple nodular lesions were found in the right pleural cavity under thoracoscopy. PSC was confirmed by biopsy and histopathology in combination with immunohistochemistry (IHC). Single-photon emission CT (SPECT) scan indicated multiple bone metastases. KRAS exon 2 mutation and EML4-ALK fusion were identified in carcinoma tissue by IHC and amplification refractory mutation system (ARMS)-PCR. The patient received one cycle of first-line combination chemotherapy of cisplatin and paclitaxel liposomes. However, the patient did not respond to the platinum-based combination chemotherapy within 3 weeks and was thus administered oral crizotinib instead of chemotherapy. Unfortunately, he still had rapid disease progression and died 2 weeks after the initiation of crizotinib treatment. Collectively, our results suggest that a PSC patient with coexistent KRAS mutation and ALK rearrangement would not benefit from chemotherapy and tyrosine kinase inhibitor (TKI) treatment.
Keywords: pulmonary sarcomatoid carcinoma, KRAS mutation, ALK rearrangement, chemotherapy, crizotinib, targeted therapy
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare subtype of poorly differentiated non-small-cell lung cancer (NSCLC) and is defined as carcinoma with pleomorphic, sarcomatoid, or sarcomatous elements. It can be divided into five histological types as follows: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma.1 As a rare form of lung malignancy, PSC has a significantly worse prognosis compared with other forms of NSCLC.2 Chemotherapy and oral administration of small molecule tyrosine kinase inhibitors (TKIs) are commonly used in the treatment of NSCLC. Before choosing chemotherapy or molecular targeted therapy, genes such as EGFR, ALK, ROS1, and KRAS should be analyzed. Although the incidence rate of ALK rearrangement in NSCLC is <5% and that of KRAS mutation ranges from 24% to 27.6%,3–5 ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS.3,4,6 Only a few NSCLC patients have both KRAS mutation and ALK rearrangement, and concomitant KRAS mutation and ALK rearrangement in PSC has not been reported so far.
NSCLC accompanied by ALK rearrangements is highly sensitive to ALK TKIs, such as crizotinib and ceritinib.7–9 However, KRAS mutations may decrease the efficacy of chemotherapy and oral molecular targeted therapy to NSCLC.10–13 It has been reported that PSC is highly resistant to conventional first-line chemotherapy.14–16 However, the response of KRAS mutation and ALK fusion-positive PSC to chemotherapy and molecular targeted therapy remains largely unexplored. In the present work, we report a case of PSC with coexistent KRAS exon 2 mutation and EML4-ALK fusion, who failed to respond to chemotherapy and crizotinib.
Case presentation
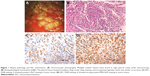
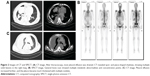
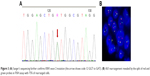
A 61-year-old male patient was hospitalized on February 5, 2018, because of cough and dyspnea. The patient had been ill for the past 15 days, exhibiting cough, dyspnea, and right chest pain. Computed tomography (CT) showed spot- and piece-shaped shadows. The patient was treated with cefoperazone–sulbactam in the early stage due to a preliminary diagnosis of pneumonia. However, anti-pneumonia therapy failed, and his condition worsened. He presented symptoms of extreme breathing difficulty and was hospitalized. Physical examination revealed dull sound by percussion and decreased breath sounds in the right lateral lung areas by auscultation. The second CT scan revealed a large amount of pleural effusion in the right lateral pleural cavity. He was diagnosed with bloody pleural effusion through pleural space puncture. Biopsy pathology and immunohistochemistry examination can be seen in Figure 1. Thoracoscopy revealed multiple nodular lesions in the right pleural cavity (Figure 1A). Lesion tissues were collected for biopsy. PSC was confirmed through biopsy and histopathology (Figure 1B). IHC showed tumor cells positive for CK7 (clone MX053; Figure 1C) while being negative for TTF-1 (clone MX011), Napsin A (clone MX015), CK5/6 (clone D5/16B4), Calretinin (clone SP13), WT1 (clone WT49), and MC (clone HBME-1). After thoracoscopy, CT revealed spot- and piece-shaped shadows and multiple solid lesions in the right lung as well as nodular pleural thickening (Figure 2A). Moreover, the enlargement of mediastinal lymph nodes was also observed. Single-photon emission CT (SPECT) scan revealed multiple bone metastases (Figure 2B). To make a treatment choice, nine genetic tests including EGFR, KRAS, ROS1, BRAF, NRAS, PIK3CA, HER-2, RET, and ALK were performed by amplification refractory mutation system (ARMS)-PCR. The ARMS-PCR revealed KRAS exon 2 mutation and EML4-ALK fusion, while the EGFR, ROS1, BRAF, NRAS, PIK3CA, HER-2, and RET genes were wild type. We further verified the KRAS exon 2 mutation by performing Sanger’s sequencing to analyze the KRAS gene sequence (an arrow shows code 12 GGT to GAT; Figure 3A). ALK rearrangement was also further confirmed by examining ALK expression (clone D5F3; Hoffman-La Roche Ltd., Basel, Switzerland) using IHC (Figure 1D) and FISH assay (Figure 3B). The patient was finally diagnosed as PSC T4N3M1C stage IVB KRAS(+) ALK(+). From February 14, 2018, to March 5, 2018, the patient received one cycle of first-line combination chemotherapy of cisplatin and paclitaxel liposomes. However, the platinum-based combination chemotherapy did not work within 3 weeks. The patient’s pleural effusion was still growing rapidly. He experienced breathing difficulty and exhibited a loss of appetite and fatigue. A repeat CT showed increased pleural effusion with further thickening of the pleura and presence of multiple nodules (Figure 2C). Based on the CT results, the patient was orally administered with crizotinib instead of chemotherapy. Unfortunately, he still exhibited rapid disease progression and died on April 2018, 2 weeks after the initiation of crizotinib treatment.
Discussion
PSC is a rare, poorly differentiated, and highly aggressive type of NSCLC.2 Radiotherapy, chemotherapy, and TKIs are commonly used in the treatment of advanced NSCLC. In patients with ALK fusion-positive NSCLC, small molecule TKIs are more efficient compared with the platinum-based combination chemotherapy. For example, NSCLC with ALK rearrangements is susceptible to the ALK TKI, crizotinib, and the overall response rate is as high as 70%–80%.9,17 Moreover, only few NSCLC patients have multiple oncogenic mutations, and coexistence of ALK rearrangement and KRAS mutation accounts for only ~13% of the multi-gene mutations.18 Therefore, crizotinib is the preferred therapeutic scheme for patients with ALK fusion-positive NSCLC.
In the present case, the patient, diagnosed with PSC positive for KRAS exon 2 mutation and EML4-ALK fusion, could not receive surgery or radiation as he was in late-stage disease. PSC is a rare type of lung cancer, and PSC with coexistent KRAS mutation and ALK fusion has never been reported before. There is no experience of success or guideline for treatment of such disorders in clinical practice. Referring to the commonly used treatment for NSCLC, the patient should be treated with crizotinib because of positive EML4-ALK fusion.19 However, NSCLC may fail to respond to molecular targeted therapy because of adverse TP53 and KRAS mutations.13 Moreover, in this patient, PSC progressed rapidly. If the patient failed to respond to oral administration of TKI crizotinib, he might never have another chance of chemotherapy. Therefore, after fully considering the benefits and potential risks, the patient preferred combination chemotherapy of cisplatin and paclitaxel. Unfortunately, the platinum-based combination chemotherapy failed, and the patient’s condition deteriorated rapidly. Therefore, the patient was orally administered with crizotinib instead of chemotherapy. However, he failed to respond to the oral crizotinib treatment and died in April 2018.
As a very rare disease, there is still a lack of effective treatment for PSC. Surgical treatment should be considered first in the early stages. Advanced PSC always has a very poor prognosis and responds poorly to conventional platinum-based chemotherapy.14,20 It has been reported that ALK inhibitors may be an effective treatment for ALK rearrangement in PSC.19 However, KRAS mutations can result in a detrimental impact on prognosis and responsiveness to platinum-based chemotherapy in NSCLC18,21 as well as in PSC.14–16 In the present report, the patient with coexistent KRAS mutation and ALK rearrangement failed to respond to platinum-based chemotherapy, and his condition deteriorated rapidly even when he was administered the ALK inhibitor, crizotinib. The results of treatment, in this case, were consistent with the literature that KRAS mutations can result in primary resistance to crizotinib.10–13 In recent years, high expression of PD-L has been reported in PSC,22,23 which can offer the therapeutic possibility of immunotherapy.23–25 In addition to tumor PD-L1 expression being linked to KRAS mutations, higher response rates to anti-PD-L1 antibody treatment are also reported in the KRAS-mutated NSCLC.25 Therefore, it is necessary to investigate the mechanisms underlying the PD1/PD-L1 immunological therapy in future studies.
Conclusion
PSC is a rare, highly malignant NSCLC with poor prognosis, and there is no available effective treatment in clinical practice. Here, we report a case of PSC positive for KRAS exon 2 mutation and EML4-ALK fusion, who failed to respond to platinum-based chemotherapy. The patient’s condition deteriorated rapidly even with ALK inhibitor, crizotinib treatment. Our results suggest that a PSC patient with coexistent KRAS mutation and ALK rearrangement would not benefit from chemotherapy and TKI treatment.
Consent
Written informed consent from the patient and approval of the medical ethics committee of Xiangya Hospital (ethical number: 201803294) have been obtained to publish the article.
List of abbreviations
ARMS, amplification refractory mutation system; CT, computed tomography; IHC, immunohistochemistry; NSCLC, non-small-cell lung cancer; PSC, pulmonary sarcomatoid carcinoma; SPECT, single-photon emission CT; TKI, tyrosine kinase inhibitor.
Acknowledgments
We would like to thank the authority of bronchoscopy room of Xiangya Hospital for providing technical support. The authors are also grateful to Dr Richard Liang from Scientific Writing Solutions, USA, for his help in language editing. This work was supported by the National Key R&D Program of China (Grant No 2016YFC1303800).
Disclosure
The authors report no conflicts of interest in this work.
References
Beasley MB, Brambilla E, Travis WD. The 2004 world health organization classification of lung tumors. Semin Roentgenol. 2005;40(2):90–97. | ||
Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis. Surgery. 2012;152(3):397–402. doi:10.1016/j.surg.2012.05.007 | ||
Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–4281. doi:10.1158/1078-0432.CCR-13-0318 | ||
Sequist LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol. 2011;22(12):2616–2624. doi:10.1093/annonc/mdr489 | ||
Terra SB, Jang JS, Bi L, et al. Molecular characterization of pulmonary sarcomatoid carcinoma: analysis of 33 cases. Mod Pathol. 2016;29(8):824–831. doi:10.1038/modpathol.2016.89 | ||
Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. doi:10.1200/JCO.2009.22.6993 | ||
Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–673. doi:10.1158/2159-8290.CD-13-0846 | ||
Malik SM, Maher VE, Bijwaard KE, et al. U.S. food and drug administration approval: crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. 2014;20(8):2029–2034. doi:10.1158/1078-0432.CCR-13-3077 | ||
Kwak EL, Bang Y-J, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi:10.1056/NEJMoa1006448 | ||
Ulivi P, Chiadini E, Dazzi C, et al. Nonsquamous, non-small-cell lung cancer patients who carry a double mutation of EGFR, EML4-ALK or KRAS: frequency, clinical-pathological characteristics, and response to therapy. Clin Lung Cancer. 2016;17(5):384–390. doi:10.1016/j.cllc.2015.11.004 | ||
Mengoli MC, Barbieri F, Bertolini F, Tiseo M, Rossi G. K-RAS mutations indicating primary resistance to crizotinib in ALK-rearranged adenocarcinomas of the lung: report of two cases and review of the literature. Lung Cancer. 2016;93:55–58. doi:10.1016/j.lungcan.2016.01.002 | ||
Sahnane N, Frattini M, Bernasconi B, et al. EGFR and KRAS mutations in ALK-positive lung adenocarcinomas: biological and clinical effect. Clin Lung Cancer. 2016;17(1):56–61. doi:10.1016/j.cllc.2015.08.001 | ||
Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. doi:10.1158/1078-0432.CCR-11-2906 | ||
Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol. 2013;8(12):1574–1577. doi:10.1097/01.JTO.0000437008.00554.90 | ||
Lococo F, Gandolfi G, Rossi G, et al. Deep sequencing analysis reveals that KRAS mutation is a marker of poor prognosis in patients with pulmonary sarcomatoid carcinoma. J Thorac Oncol. 2016;11(8):1282–1292. doi:10.1016/j.jtho.2016.04.020 | ||
Mehrad M, Roy S, LaFramboise WA, et al. KRAS mutation is predictive of outcome in patients with pulmonary sarcomatoid carcinoma. Histopathology. 2018;73(2):207–214. doi:10.1111/his.13505 | ||
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi:10.1056/NEJMoa1408440 | ||
Guibert N, Barlesi F, Descourt R, et al. Characteristics and outcomes of patients with lung cancer harboring multiple molecular alterations: results from the IFCT study biomarkers France. J Thorac Oncol. 2017;12(6):963–973. doi:10.1016/j.jtho.2017.02.001 | ||
Chen X, Zhang Y, Lu J, et al. Pulmonary sarcomatoid carcinoma with ALK rearrangement: frequency, clinical-pathologic characteristics, and response to ALK inhibitor. Transl Oncol. 2017;10(2):115–120. doi:10.1016/j.tranon.2016.11.009 | ||
Ung M, Rouquette I, Filleron T, et al. Characteristics and clinical outcomes of sarcomatoid carcinoma of the lung. Clin Lung Cancer. 2016;17(5):391–397. doi:10.1016/j.cllc.2016.03.001 | ||
Johnson ML, Sima CS, Chaft J, et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer. 2013;119(2):356–362. doi:10.1002/cncr.27730 | ||
Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol. 2013;8(6):803–805. doi:10.1097/JTO.0b013e318292be18 | ||
Vieira T, Antoine M, Hamard C, et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer. 2016;98:51–58. doi:10.1016/j.lungcan.2016.05.013 | ||
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774 | ||
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi:10.1056/NEJMoa1501824 |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.