Back to Journals » OncoTargets and Therapy » Volume 13
POLE Mutation Characteristics in a Chinese Cohort with Endometrial Carcinoma
Authors Li Y, He Q , Li S, Wen X, Ye L, Wang K, Wan X
Received 17 April 2020
Accepted for publication 15 July 2020
Published 28 July 2020 Volume 2020:13 Pages 7305—7316
DOI https://doi.org/10.2147/OTT.S258642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Yiran Li1 *, Qizhi He2 *, Shuangdi Li,1 Xiaoli Wen,3 Lei Ye,1 Kai Wang,3 Xiaoping Wan1
1Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Department of Pathology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 3Clinical and Translational Research Center, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kai Wang Clinical and Translational Research Center, Shanghai First Maternity and Infant Hospital, Shanghai 200040, People’s Republic of China
Tel +86 (021)-5403-7438
Email [email protected]
Xiaoping Wan Department of Gynecology, Shanghai First Maternity and Infant Hospital, Shanghai 200040, People’s Republic of China
Tel +86 136-5191-7177
Email [email protected]
Objective: To study the characteristics of polymerase epsilon (POLE) exonuclease domain mutations in Chinese patients with endometrial carcinoma (EC).
Methods: This study analyzed data from 529 patients with EC in The Cancer Genome Atlas (TCGA) and 467 EC patients evaluated at the Shanghai First Maternity and Infant Hospital (SFMIH). POLE mutation heterogeneity was analyzed in paired curettage and hysterectomy samples from 120 SFMIH patients. Sanger sequencing identified mutations in the POLE exonuclease domain, and correlations between POLE mutation status and various clinicopathological features were determined by chi-squared testing and Cohen’s kappa analysis, with Kaplan–Meier survival curves generated to assess correlations between POLE mutation status and overall survival (OS).
Results: Thirty-five mutations were identified in 467 samples (7.5%), and novel mutations were detected in the SFMIH cohort. Compared to the TCGA cohort, the SFMIH cohort had fewer POLE mutations when matched by age (< 60) and histology (endometrioid) (p < 0.001 and p = 0.010, respectively). In our study cohort, POLE mutations were significantly associated with adjuvant treatment (p = 0.029), and patients with POLE mutations who underwent chemoradiotherapy had a poor OS (p < 0.0001). Notably, shorter OS was significantly associated with POLE mutations in hysterectomy samples from patients aged > 60 years or with stage I disease in the paired curettage-hysterectomy group.
Conclusion: The significant difference in POLE mutation profiles between the TCGA and SFMIH cohorts, as well as the poor consistency between the curettage and hysterectomy samples, suggests that different parameters need to be applied to determine the prognosis of patients with EC in China.
Keywords: endometrial carcinoma, polymerase epsilon mutation, POLE mutation, Chinese cohort, hysterectomy, curettage specimens
Introduction
Endometrial carcinomas (ECs) are categorized into two subtypes according to their clinicopathologic characteristics. Approximately 70% to 80% of ECs are type I, with estrogen-dependent ECs often accompanied by a favorable prognosis. Type-II tumors account for ~10% to ~20% of ECs and exhibit more aggressive biological behaviors and poor outcomes.1,2 Treatments are selected according to the histologic subtype and other clinicopathologic features associated with prognosis;2–6 therefore, proper subtype classification is critical for selecting appropriate therapy. In 2013, The Cancer Genome Atlas (TCGA) project characterized ECs into four separate groups based on genomic features. A novel “ultramutated” subgroup harboring mutations in the exonuclease domain of the polymerase epsilon (POLE) gene was identified, often manifested as a microsatellite stable super-mutant phenotype with high somatic mutation rate. For example, Kandoth et al found a high mutant prevalence of PTEN (94%), PIK3R1 (65%), PIK3CA (71%), FBXW7 (82%) and KRAS (53%) in 17 ultramutated samples.7 Roberts et al detected 30 POLE mutations (5.6%) in 535 endometrioid carcinomas, which was lower than that in TCGA.8 In the Church study, POLE mutations were detected in 48 out of 788 (6.1%) endometrial cancers. In FIGO grade 3 endometrioid carcinoma, the POLE mutation rate is high, ranging from 15%-22%.7,9,10 Furthermore, Hussein et al observed that the endometrial carcinoma with POLE mutation was mainly inclined toward endometrioid differentiation, 60% of which were high-grade endometrioid carcinoma.11 However, POLE mutations, mainly referring to POLE exonuclease domain mutations in the present study, may be associated with outcomes in which studies involving Chinese patients are lacking.
Multiple studies identified POLE mutations in several types of malignant tumors, especially in ECs, colorectal carcinomas, and gliomas.9,12-14 POLE encodes DNA polymerase epsilon, which is responsible for chromosomal DNA replication during cell division.15,16 Polymerase proofreading represents one of the primary mechanisms ensuring DNA replication fidelity.17,18 Moreover, inactivation or suppression of the proofreading capacities of DNA polymerases can cause a dramatic increase in the number of spontaneous mutations.11,19 In ECs, mutations in the exonuclease domain of POLE were previously identified primarily in hotspot regions, including exons 9, 13, and 14.10,20 POLE mutations tend to occur in patients with FIGO grades 2–3, and POLE mutant FIGO grade 3 ECs are less prone to recurrence.10 A study of 534 endometrioid carcinomas showed that 30 (5.6%) were POLE mutations, and the recurrence rate was approximately 3.4%, while the POLE wild type had a recurrence rate of 17%.21 At the same time, Stenzinger et al found that the prognosis of colorectal cancer patients with POLE mutations was not significantly different from that of wild-type patients, but the mortality rate of patients with adjuvant or palliative chemotherapy was significantly higher than that of POLE wild type for terminal stage.22
In this study, we compared POLE mutation frequencies and clinicopathologic features between a TCGA database cohort, a cohort of predominantly non-Asian people, and a Chinese cohort of patients with EC and analyzed the effects of POLE mutations on overall survival (OS) in these two cohorts. Additionally, we determined the consistency of POLE mutation status between curettage specimens and hysterectomy specimens and examined the associations between mutation statuses and OS.
Materials and Methods
Patient Cohorts
In this study, we used the following two cohorts: our local cohort of patients with EC from Shanghai First Maternity and Infant Hospital (SFMIH) and the TCGA cohort. Specimens from the SFMIH cohort, including 467 primary tumor samples and 120 paired curettage specimens, were derived from EC patients treated at the department of gynecology (SFMIH) between 2007 and 2018. Clinical data were obtained by retrospective chart review, disease-specific death was defined as death due to EC and excluded death from other causes, and OS was defined as the time from diagnosis to death. Ethical approval was received from the institutional research ethics board. Current guidelines for adjuvant chemotherapy and radiotherapy (NCCN clinical practice guidelines (2018)23) rely on the International Federation of Gynecology and Obstetrics (FIGO) grade, FIGO stage, histotype, and lymphovascular-invasion (LVI) status. For the TCGA cohort, we downloaded the clinical and POLE mutation data from the EC interface of the TCGA portal (https://portal.gdc.cancer.gov/projects/TCGA-UCEC), resulting in data from 529 patients with ECs, 51 of whom harbored POLE mutations.
DNA Isolation
Before DNA extraction, samples are enriched by macrodissection to increase the content of tumor cells. DNA was isolated using a GeneRead DNA kit (Qiagen, Hilden, Germany) from tumor-rich regions (at least 50% tumor cells) of formalin-fixed, paraffin-embedded samples, as assessed by two independent pathologists. In samples with mutations, nontumor endometrium was also extracted from the same slide and underwent the same determination of somatic mutations.
Targeted Sequencing and Analysis
Mutations in the exonuclease domain of POLE (amino acids 268–471) were previously identified mainly in exons 9, 13, and 14.10,20 The primer sets used covered these regions and are described later in this subsection. Polymerase chain reaction (PCR) amplifications were performed using 2×Taq master mix (Vazyme Biotech, Nanjing, China), and PCR products were purified using a QIAquick gel extraction kit (Qiagen) according to the manufacturer’s instructions, followed by Sanger sequencing. The primers used were as follows: POLE-Exon 9 forward, 5′-GTGTTCAGGGAGGCCTAATG-3′ and reverse, 5′-CCATCCCAGGAGCTTACTTC-3′; POLE-Exon 13 forward, 5′-CCTGGCTTCTGTTCTCATTCT-3′ and reverse 5′-GATGTGGCTCACATGCCT-3′; and POLE-Exon 14 forward 5′-GACCCTGGGCTCTTGATTT-3′ and reverse 5′-GGACATCCACCTCCATTCAG-3′. Sequencing reactions were performed as previously described,24 and all mutation-positive samples were resequenced to confirm the mutational status.
Statistical Analysis
Among the two study cohorts and the different tumor types, the chi-squared test or Fisher’s exact test was used to assess significant differences in POLE exonuclease domain-mutation status. Kaplan–Meier curves and log-rank statistics were applied to determine differences in the univariate OS analyses. Cohen’s kappa analysis was performed to determine the consistency of POLE mutation status between hysterectomy and curettage specimens. For all analyses, p < 0.05 was considered statistically significant, and all statistical tests were two sided. Statistical analyses were performed using SPSS (v.20.0; IBM Corp., Armonk, NY, USA).
Results
Characteristics of the EC-Patient Cohorts
We retrospectively analyzed 467 EC patients at SFMIH, the majority of whom (69.6%) were <60 years of age (32.9% of the TCGA cohort were <60 years of age). In total, 91% of the patients in the SFMIH cohort had early-stage disease (defined as patients with FIGO stage I or II disease), which was greater than this proportion in the TCGA cohort (72%). The SFMIH cohort included a lower proportion of patients with high-grade tumors than the TCGA cohort (55.8% TCGA vs 13.3% SFMIH), including grade 3 endometrioid, serous, mixed, and clear-cell carcinomas. Other notable differences between the SFMIH cohort and the TCGA patient population were found in the tumor histotypes, with endometrioid and serous carcinomas accounting for 85.2% and 7.3% of the patients in the SFMIH cohort compared with 74.9% and 21.0% in the TCGA cohort, respectively (Table 1). These findings suggested that the clinical characteristics of EC patients in the TCGA cohort differed from those in the SFMIH cohort.
 |
Table 1 Clinical Features of Patients in the TCGA and SFMIH Cohorts |
Comparison of POLE Mutations Between the TCGA and SFMIH Cohorts
Despite commonalities in the frequent mutations found in the POLE exonuclease domain between both cohorts, we found notable differences in clinical features and outcomes. Among the 467 patients with EC in the SFMIH cohort and the 529 patients in the TCGA cohort, we identified 35 and 55 mutations, respectively, in POLE exons 9, 13, and 14 (Figure 1A). Twenty-four novel mutations were identified in the POLE exonuclease domain (amino acids 268–471) in the SFMIH cohort, including missense mutations and a truncating mutation. Only three hotspot mutations within the POLE exonuclease domain were found in both cohorts, suggesting that the POLE mutations in the SFMIH cohort differed from those in the TCGA cohort (Figure 1B and Table 2).
 |
Table 2 Sites of Mutations in the POLE Exonuclease Domain in ECs |
 |
Figure 1 Mutations in the POLE exonuclease domain in ECs. (A) Mutations in the POLE exonuclease domain in the TCGA and SFMIH cohorts. (B) Hotspot POLE mutations in the SFMIH cohort. |
Several studies, including TCGA, had shown that POLE mutations were associated with outcomes. Before assessing the effect of POLE mutations on overall survival, baseline variables were examined using multivariate analysis, the results of which showed no significant differences in the clinical characteristics within each group of the SFMIH cohort (Table 3). We performed a detailed stratified analysis to further explore the differences in POLE mutations between the two study cohorts. In the SFMIH cohort, younger patients (age <60) and those with the endometroid subtype of EC had significantly fewer POLE mutations than their counterparts in the TCGA cohort (p < 0.001 and p = 0.01, respectively) (Table 4). Because previous findings showed that POLE mutations were associated with a favorable prognosis, we compared the effects of POLE mutations on OS in both cohorts. The presence of POLE mutations in the TCGA cohort remained a significant favorable prognostic factor for OS (p = 0.010) in both the age <60 years subtype (p < 0.0001) and the endometrioid tumor subtype (p < 0.0001). In the SFMIH cohort, the OS analysis grouped according to age and histotype did not show significant differences (Figure 2A and B).
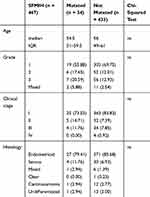 |
Table 3 Patient Characteristics at Baseline in the SFMIH Cohort |
 |
Table 4 Demographic and Clinical Characteristics of Mutations in the POLE Exonuclease Domain in the TCGA and SFMIH Cohorts |
These results suggested that POLE mutations of EC patients from China are unique and might not be a favorable prognostic factor for OS. The low incidence might weaken the positive effect of POLE mutation on the prognosis of EC.
The Clinical Significance of POLE Mutations in EC in the SFMIH Cohort
We then determined associations between mutations in the POLE exonuclease domain and patient clinicopathologic characteristics in the SFMIH cohort (Table 3). We followed the NCCN clinical practice guidelines (2018) to determine the treatment regimen for patients.23 After stratifying according to age group, we found no significant difference in POLE mutations between younger (<60 years) and older (≥60 years) subgroups. All POLE-mutated tumors were stage I, stage II, or stage III, with no POLE mutations identified in stage IV tumors. The POLE mutation rate according to the stage of EC was as follows: 6.44% in stage I, 13.51% in stage II, and 10.53% in stage III, with no significant difference among the groups (p = 0.275) (Table S1). Surprisingly, the frequency of POLE mutations was significantly higher in patients who received adjuvant chemoradiotherapy than in patients who did not, which was a novel characteristic of the SFMIH cohort (Table S1). We then investigated whether mutations in the POLE exonuclease domain correlated with differences in the OS of EC patients receiving adjuvant therapy. Among patients who underwent chemoradiotherapy, those harboring POLE mutations had a significantly worse prognosis than those without POLE mutations (p < 0.05; Figure 3).
 |
Figure 3 Among patients who underwent chemoradiotherapy, those harboring POLE mutations had a significantly worse prognosis than those without POLE mutations. |
Mutations in the POLE Exonuclease Domain in Hysterectomy and Curettage Specimens
We randomly selected 120 curettage specimens paired with hysterectomy specimens from EC patients from the SFMIH cohort for POLE mutation detection. Sixteen tumors (13.3%) were found to harbor mutations in the POLE exonuclease domain in the definitive hysterectomy specimens, whereas 23 curettage specimens (19.2%) harbored POLE mutations (Table 5). Comparison of the curettage samples with the hysterectomy samples revealed similarities with respect to histological subtype and grade, although identification of POLE mutations was inconsistent between the two groups (Table 5). We performed survival analysis using a univariate model according to POLE mutations in the subgroups of older patients, patients with stage I disease, patients with superficial myometrial invasion, and patients who underwent chemotherapy. Interestingly, patients in the older subgroup, as well as the subgroup with stage I disease and POLE mutations in the hysterectomy specimens but not in the curettage specimens, had significantly worse OS than patients in the same subgroup without POLE mutations (Figure 4A and B). Furthermore, OS was not associated with POLE mutations in either the curettage or hysterectomy specimens of patients with myometrial invasion and a history of chemotherapy (Figure 4C and D).
 |
Table 5 Concordance of Tumor Features in Hysterectomy and Preoperative Curettage Specimens from the SFMIH Cohort |
Discussion
In this study, we identified somatic mutations in the POLE exonuclease domain in 7.28% of a large independent cohort of 467 patients with EC from SFMIH, China. We identified characteristics distinguishing the SFMIH cohort from the TCGA cohort, including age, stage, grade, and histotype, that might be related to differences in race between the two cohorts.25 In the TCGA cohort, 55 POLE mutations were located in exons 9, 13, and 14, whereas 35 were detected in the SFMIH cohort. Furthermore, most hotspot mutations found in the TCGA cohort were not detected in our cohort, but we detected 24 novel somatic POLE mutations in the SFMIH cohort. Additionally, we explored differences in POLE mutations between subgroups of the TCGA and SFMIH cohorts, with the results confirming that in younger (<60 years) and endometrioid-histotype subgroups, the SFMIH cohort had fewer POLE mutations than the TCGA cohort. Moreover, previous studies investigating a TCGA cohort of patients with ECs showed that POLE mutations occurred predominantly in endometrioid tumors. In the SFMIH cohort, POLE mutations were found at higher frequencies in serous tumors than in endometrioid tumors, which was unexpected.
Survival analysis showed that no significant prognostic impact of POLE mutations on OS was identified within the young and endometrioid subgroups of the SFMIH cohort, which differed from previous reports, which presented better outcomes for patients with POLE mutations.26–28 The higher frequency of low-risk (Grades 1 and 2) patients in the Chinese cohort (typically with good clinical outcomes), together with the pathogenicity of POLE EDM, could account for the discrepancies with previous literature regarding survival of patients with POLE mutated EC.
Interestingly, we found that among patients who received adjuvant chemoradiotherapy according to the treatment guidelines, those with POLE mutations had a significantly higher mortality rate than those without POLE mutations. This result is consistent with an in vitro study that showed that POLE mutant tumor cells have a significantly higher resistance rate to platinum drugs than the POLE wild-type cell line (p < 0.004),29 indicating that POLE mutations contribute to the insensitivity to traditional chemotherapy.30 These data support the possibility that POLE-mutated tumors, due to their defective polymerase proofreading capability, continue to acquire mutations during cell division; with the high tumor mutation burden, large amounts of tumor neoantigens are produced and may lead to insensitivity to adjuvant therapy. Studies have shown that POLE mutations do not play a significantly positive role in adjuvant treatment, and our results revealed a worse OS with adjuvant therapy.31 In summary, further studies should investigate whether POLE mutations have prognostic or predictive implications in these patients and, if so, the nature of the underlying biological mechanism. In the present study, we did not find an association between POLE mutation status and progression-free survival (PFS) due to the relatively small number of patients who experienced recurrence; therefore, a longer follow-up time will be needed to address the significance of the prognostic impact of POLE mutations on PFS.
One novel objective of this study was to determine whether curettage and hysterectomy specimens were consistent in terms of the identified somatic mutations in the POLE exonuclease domain and whether such mutations in the curettage specimens could be used for predictions of prognosis. Various studies showed inconsistencies in tumor typing and histological grading between preoperative and hysterectomy specimens.32,33 The present study showed good consistency for both pathological grade and histological type in paired samples from 120 patients with ECs. Unexpectedly, we identified more POLE mutations in curettage specimens than in hysterectomy specimens by Sanger sequencing, although a recent report showed that using DNA-based techniques to evaluate molecular alterations had high concordance rates between preoperative specimens and definitive hysterectomy specimens.34,35 To determine whether the POLE mutations in curettage specimens differed from those in hysterectomy specimens, we grouped patients according to clinical characteristics for detailed stratified analyses. Our data indicated that POLE mutations result in preoperative tumors not accurately predicting the final POLE mutation results, especially in patients aged >60 or in those with stage I tumor, exhibiting superficial myometrial invasion, or having received adjuvant chemotherapy. Although this result is somewhat puzzling and counterintuitive, expression inconsistencies between curettage and hysterectomy specimens were not uncommon. For example, β-catenin staining was observed in the curettage specimens but not in the hysterectomy specimens36 from patients with sporadic ECs. The reason behind this discordance needs to be further clarified. Recently, many researchers have tried to utilize molecular biomarkers in curettage specimens to predict the behavior of EC cells. However, the high inconsistency rate shown here indicates that this approach should be used very cautiously.
Furthermore, we also observed that identification of POLE mutation status could facilitate accurate patient counseling and help determine further treatment, thereby enabling tailoring of the extent of surgery and/or adjuvant therapies to the individual patient profile. Based on these findings, we explored whether POLE mutations in curettage or hysterectomy specimens correlated with OS. Importantly, we found that POLE mutations in hysterectomy specimens but not in curettage specimens were a significant predictor of poor OS among older patients and those with stage I disease. These findings suggested that identifying POLE mutations in curettage specimens was not helpful for determining the extent of treatment. However, this is the first study investigating the correlation of POLE mutations with prognosis in curettage specimens from subgroups of patients with different clinical features, and more studies are needed to determine their clinical significance.
Clinical Perspectives
The Cancer Genome Atlas (TCGA) project characterized endometrial cancer harboring polymerase epsilon (POLE) gene mutations as a “ultramutated” subgroup, with favorable outcomes. In the present study, total of 467 patients with EC were screened for the presence of mutations in the POLE exonuclease domain within exons 9, 13, and 14 in China. In contrast to the data obtained for the TCGA cohort, in which most patients were non-Asian, we found that POLE mutations might not be prognostic factors of poor OS for endometrioid patients with ECs in China. Moreover, we found that, for EC patients requiring chemotherapy combined with radiotherapy in China, POLE mutations might lead to a worse prognosis. This study represents the first analysis of correlations between POLE mutations in hysterectomy specimens with EC prognosis, but our results require further validation to clarify the role of POLE mutations in ECs in China.
Consent for Publication
The authors confirm that we have obtained written consent from the patients to publish this manuscript.
Abbreviations
POLE, polymerase epsilon; EC, endometrial carcinoma; TCGA, The Cancer Genome Atlas; SFMIH, Shanghai First Maternity and Infant Hospital; OS, overall survival.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
We confirm that this study was conducted in accordance with the Declaration of Helsinki. The written informed consent for the biological studies was obtained from each patient involved in the study, and the study was approved by the Ethics Committee of The First Maternity and Infant Hospital of Tongji University School of Medicine. Reference number: KS18108; Date of approval: May 4, 2018.
Acknowledgments
We thank all patients involved in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no potential conflicts of interest.
References
1. Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268–78. doi:10.1016/S1470-2045(13)70591-6
2. Robert J, Kurman M, Simon Herrington C, Young R. Who Classification of Tumours of Female Reproductive Organs. Lyon, France: WHO; 2014:69–73.
3. Benedet JL, Bender H, Jones H, Ngan HY, Pecorelli S. Figo staging classifications and clinical practice guidelines in the management of gynecologic cancers. Figo committee on gynecologic oncology. Int J Gynaecol Obstet. 2000;70:209–262.
4. Colombo N, Preti E, Landoni F, et al. Endometrial cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–8. doi:10.1093/annonc/mdt353
5. Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Portec study group. Post operative radiation therapy in endometrial carcinoma. Lancet. 2000;355:1404–1411. doi:10.1016/S0140-6736(00)02139-5
6. Kwon JS, Qiu F, Saskin R, Carey MS. Are uterine risk factors more important than nodal status in predicting survival in endometrial cancer? Obstet Gynecol. 2009;114:736–743. doi:10.1097/AOG.0b013e3181b96ec6
7. Kandoth C, Schultz N, Cherniack AD; Cancer Genome Atlas Research N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 497;2013:67–73. doi:10.1038/nature12113
8. Roberts SA, Gordenin DA. Hypermutation in human cancer genomes: footprints and mechanisms. Nat Rev Cancer. 2014;14:786–800. doi:10.1038/nrc3816
9. Church DN, Briggs SE, Palles C, et al. DNA polymerase epsilon and delta exonuclease domain mutations in endometrial cancer. Hum Mol Genet. 2013;22:2820–2828. doi:10.1093/hmg/ddt131
10. Meng B, Hoang LN, McIntyre JB, et al. Pole exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol. 2014;134:15–19. doi:10.1016/j.ygyno.2014.05.006
11. Hussein YR, Weigelt B, Levine DA, et al. Clinicopathological analysis of endometrial carcinomas harboring somatic pole exonuclease domain mutations. Mod Pathol. 2015;28:505–514. doi:10.1038/modpathol.2014.143
12. Palles C, Cazier JB, Howarth KM, et al. Germline mutations affecting the proofreading domains of pole and pold1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45:136–144. doi:10.1038/ng.2503
13. Yoshida R, Miyashita K, Inoue M, et al. Concurrent genetic alterations in DNA polymerase proofreading and mismatch repair in human colorectal cancer. Eur J Hum Genet. 2011;19:320–325. doi:10.1038/ejhg.2010.216
14. Erson-Omay EZ, Caglayan AO, Schultz N, et al. Somatic pole mutations cause an ultramutated giant cell high-grade glioma subtype with better prognosis. Neuro Oncol. 2015;17:1356–1364. doi:10.1093/neuonc/nov027
15. Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science. 2007;317:127–130. doi:10.1126/science.1144067
16. Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PM, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30:137–144. doi:10.1016/j.molcel.2008.02.022
17. Kunkel TA. DNA replication fidelity. J Biol Chem. 2004;279:16895–16898. doi:10.1074/jbc.R400006200
18. Reha-Krantz LJ. DNA polymerase proofreading: multiple roles maintain genome stability. Biochim Biophys Acta. 2010;1804:1049–1063. doi:10.1016/j.bbapap.2009.06.012
19. Rayner E, van Gool IC, Palles C, et al. A panoply of errors: polymerase proofreading domain mutations in cancer. Nat Rev Cancer. 2016;16:71–81. doi:10.1038/nrc.2015.12
20. Bellone S, Bignotti E, Lonardi S, et al. Polymerase epsilon (pole) ultra-mutation in uterine tumors correlates with t lymphocyte infiltration and increased resistance to platinum-based chemotherapy in vitro. Gynecol Oncol. 2017;144:146–152. doi:10.1016/j.ygyno.2016.11.023
21. Billingsley CC, Cohn DE, Mutch DG, Stephens JA, Suarez AA, Goodfellow PJ. Polymerase varepsilon (pole) mutations in endometrial cancer: clinical outcomes and implications for lynch syndrome testing. Cancer. 2015;121:386–394. doi:10.1002/cncr.29046
22. Stenzinger A, Pfarr N, Endris V, et al. Mutations in pole and survival of colorectal cancer patients–link to disease stage and treatment. Cancer Med. 2014;3:1527–1538. doi:10.1002/cam4.305
23. Koh WJ, Abu-Rustum NR, Bean S, et al. Uterine neoplasms, version 1.2018, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:170–199. doi:10.6004/jnccn.2018.0006
24. McIntyre JB, Nelson GS, Ghatage P, et al. Pik3ca missense mutation is associated with unfavorable outcome in grade 3 endometrioid carcinoma but not in serous endometrial carcinoma. Gynecol Oncol. 2014;132:188–193. doi:10.1016/j.ygyno.2013.11.015
25. Guttery DS, Blighe K, Polymeros K, Symonds RP, Macip S, Moss EL. Racial differences in endometrial cancer molecular portraits in the cancer genome atlas. Oncotarget. 2018;9:17093–17103. doi:10.18632/oncotarget.24907
26. McConechy MK, Talhouk A, Leung S, et al. Endometrial carcinomas with pole exonuclease domain mutations have a favorable prognosis. Clin Cancer Res. 2016;22:2865–2873. doi:10.1158/1078-0432.CCR-15-2233
27. Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28:836–844. doi:10.1038/modpathol.2015.43
28. Church DN, Stelloo E, Nout RA, et al. Prognostic significance of pole proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107:402. doi:10.1093/jnci/dju402
29. Bellone S, Centritto F, Black J, et al. Polymerase epsilon (pole) ultra-mutated tumors induce robust tumor-specific cd4+ t cell responses in endometrial cancer patients. Gynecol Oncol. 2015;138:11–17. doi:10.1016/j.ygyno.2015.04.027
30. Van Gool IC, Rayner E, Osse EM, et al. Adjuvant treatment for pole proofreading domain-mutant cancers: sensitivity to radiotherapy, chemotherapy, and nucleoside analogues. Clin Cancer Res. 2018;24:3197–3203. doi:10.1158/1078-0432.CCR-18-0266
31. Wortman BG, Nout RA, Bosse T, Creutzberg CL. Selecting adjuvant treatment for endometrial carcinoma using molecular risk factors. Curr Oncol Rep. 2019;21:83. doi:10.1007/s11912-019-0825-z
32. Thanachaiviwat A, Thirapakawong C, Leelaphatanadit C, Chuangsuwanich T. Accuracy of preoperative curettage in determining tumor type and grade in endometrial cancer. J Med Assoc Thai. 2011;94:766–771.
33. Francis JA, Weir MM, Ettler HC, Qiu F, Kwon JS. Should preoperative pathology be used to select patients for surgical staging in endometrial cancer? Int J Gynecol Cancer. 2009;19:380–384. doi:10.1111/IGC.0b013e3181a1a657
34. Stelloo E, Nout RA, Naves LC, et al. High concordance of molecular tumor alterations between pre-operative curettage and hysterectomy specimens in patients with endometrial carcinoma. Gynecol Oncol. 2014;133:197–204. doi:10.1016/j.ygyno.2014.02.012
35. Talhouk A, Hoang LN, McConechy MK, et al. Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: earlier prognostic information to guide treatment. Gynecol Oncol. 2016;143:46–53. doi:10.1016/j.ygyno.2016.07.090
36. Moreno-Bueno G, Hardisson D, Sanchez C, et al. Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene. 2002;21:7981–7990. doi:10.1038/sj.onc.1205924
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


