Back to Journals » OncoTargets and Therapy » Volume 11
POEMS syndrome complicated with multiple ischemic vascular events: case report and review of literature
Authors Găman M, Vlădăreanu AM, Dobrea C, Onisâi M , Marinescu C, Cîşleanu D, Ciufu C, Vasile D , Bumbea H, Voican I
Received 13 July 2017
Accepted for publication 9 May 2018
Published 27 September 2018 Volume 2018:11 Pages 6271—6276
DOI https://doi.org/10.2147/OTT.S146221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Mihaela Găman,1,2 Ana-Maria Vlădăreanu,1,2 Camelia Dobrea,1 Minodora Onisâi,1,2 Cristina Marinescu,1,2 Diana Cîşleanu,1,2 Cristina Ciufu,1,2 Daniela Vasile,1,2 Horia Bumbea,1,2 Irina Voican2
1Department of Hematology, University of Medicine and Pharmacy Carol Davila Bucharest, Bucharest, Romania; 2Department of Hematology, University Emergency Hospital Bucharest, Bucharest, Romania
Abstract: POEMS syndrome (acronym consisting of: polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) is an uncommon disorder associated with an underlying plasma cell dyscrasia. There is no single specific test for POEMS, and due to its rarity and heterogeneity, patients are often mis- or underdiagnosed. Castleman disease (CD) is a rare lymphoproliferative disorder, closely related to POEMS syndrome; ~11%–30% of POEMS patients are associated with concomitant CD. In contrast to frequently published reports on vascular events in POEMS syndrome affecting coronary arteries or lower limbs, cases of cerebrovascular events are rarely mentioned in literature. We hereby report a patient with POEMS syndrome accompanied by CD who presented recurrent strokes and splenic infarction.
Keywords: POEMS, chronic inflammatory demyelinating polyradiculopathy, M protein, thrombotic risk, stroke, recurrent ischemic events
Introduction
POEMS syndrome is an uncommon disorder associated with an underlying plasma cell dyscrasia. The acronym derives from its main features: polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes. Additional findings include: papilledema, extravascular volume overload (ascites, edema, and pleural effusion), abnormal pulmonary function, elevated vascular endothelial growth factor (VEGF) levels, fever, sclerotic bone lesions, erythrocytosis, thrombocytosis, and predisposition toward thrombosis.1,2
The disorder was first named osteosclerotic myeloma, Crow–Fukase syndrome, or Takatsuki syndrome.3,4 Later, Scheinker reported a case with solitary plasmacytoma, sensorimotor polyneuropathy, and skin lesions.5 POEMS acronym was used by Bardwick et al, when 2 cases having features mentioned before were reported.6 The median age of diagnosis is in the fifth and sixth decade of life.1
Castleman disease (CD) is a rare lymphoproliferative disorder with giant angiofollicular lymph node hyperplasia. The most frequent symptoms and clinical features are fatigue, weight loss, night sweats, fever, lymphadenopathy, and hepatosplenomegaly.7 CD and POEMS syndrome are closely related. Approximately 11%–30% of POEMS patients are associated with CD.8 This subtype was first described by Dispeenzeri et al a few years ago.9
Arterial and venous thromboses, mainly involving the coronary and lower limbs arteries, have been reported in POEMS patients.10,11 There are only few reports on POEMS syndrome associated with CD complicated with cerebrovascular events.12–18 In this paper, we present a patient diagnosed with this subtype with thrombotic complications, recurrent strokes, and splenic infarction.
Case presentation
A 45-year-old female presented with significant weight loss (about 28% of body weight during the past year), night sweats, and severe pain in the lower extremities. She had no history of smoking, hypertension, hyperlipidemia, or alcohol consumption. The patient had been diagnosed 1 year before presentation with chronic inflammatory demyelinating polyradiculoneuropathy. She had been treated with acetylsalicylic acid, carbamazepine, and small doses of corticosteroids with no clinical improvement. On referral to our hospital, there was progressive weakness of left limbs and numbness. Physical examination showed a female with cachexia (BMI =16.8 kg/m2), facial lipoatrophy, skin hyperpigmentation, hypertrichosis, hyperhidrosis, sclerodermiform cutaneous thickening, white nails, Raynaud phenomenon, muscle atrophy, non-tender axillary lymph nodes measuring 2.0×1.5 cm, and hepatosplenomegaly. No signs of extravascular volume overload were noted. A neurological examination revealed left-side hemiparesis and sensory loss in the left limbs. Sensorimotor demyelinating and axonal polyneuropathy in upper (median and ulnar nerves) and lower limbs (peroneal, tibial, and sural nerves), prolonged distal motor latency, and slowed velocity of both motor and sensory nerve conduction were the main findings on nerve conduction studies. Ophthalmic fundus examination presented bilateral papilledema. CT scan identified a newly emerged area of infarction in the right frontal parietal occipital area.
Laboratory studies are presented in Table 1. Hypoparathyroidism and secondary amenorrhea (most likely a functional hypothalamic amenorrhea) were identified as endocrine abnormalities. The clinical and laboratory abnormalities constellation suggested POEMS syndrome. No tests for VEGF, interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), known to be involved in the pathogenesis, were available at our center.
  | Table 1 Summary laboratory tests at diagnosis |
On skeletal survey, lytic lesions with a sclerotic rim were present on proximal extremities. On thorax and abdomen CT scan, multiple enlarged axillary, mediastinal and mesenteric lymph nodes along the iliac vessels (maximum diameter of ~2 cm), minimum pleural effusion, hepatomegaly, and splenomegaly with chronic infarction were observed. Transthoracic echocardiography revealed normal left ventricle volume and ejection fraction, no pericardial effusion, no signs of pulmonary hypertension, but interventricular septal thickening with granular appearance suggestive of an infiltrative cardiomyopathy. A fat-pad abdominal biopsy specimen was negative for amyloid deposits. Due to patient’s poor clinical status, no pulmonary function tests were performed at baseline.
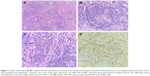
Axillary lymph node biopsy (Figure 1) revealed thickening of the mantle and marginal zone with small lymphocytes surrounding the germinal center, which were hyalinized and hypoplastic with “onion peel” appearance, and surrounded by blood vessels. Immunohistochemical analysis was positive for: CD3 in frequent parafollicular reactive T cell lymphocytes; CD 20 in reactive B cells; <5% Ki67 plasma cells with κ/λ ratio ~3/1. These histological findings were consistent with mixed type (the hyaline vascular type and plasma cell form of CD). Antibodies for human herpes virus 8 were negative. A diagnosis of POEMS associated with CD was established.
She was treated for plasma cell dyscrasia with CyBorD protocol (cyclophosphamide, bortezomib, and dexamethasone) ×4 cycles as induction therapy, along with supportive care (acetylsalicylic acid 75 mg daily, prophylactic low-molecular-weight heparin, calcium, vitamin D to correct hypoparathyroidism-induced hypocalcemia, and analgesics). Because of the preexisting POEMS neuropathy, bortezomib was administered subcutaneously. A partial improvement on neurological function (improved neuropathic pain, but the patient was still not able to walk without support) and clinical status (weight gain, muscle strength, and diminished night sweats) were noticed. The organomegaly and the endocrine abnormalities did not resolve.
After 4 cycles of CyBorD, a CT scan for evaluation was performed; it revealed enlarged lymph nodes >2 cm diameter (larger than the previous examination), bilateral pleural effusion, and hepatosplenomegaly with chronic spleen infarction. Laboratory screening presented thrombocytosis 600×109/L, higher inflammatory syndrome (fibrinogen 680 mg/dL, C reactive protein 26.53 mg/L), LDH 249 U/L (NR 81-234 U/L) and increasing level of free λ chains. Furthermore, she had neurological deterioration: aggravated neuropathic pain and sudden onset of speech difficulties. Cerebral CT scan showed a newly emerged area of infarction, involving the middle cerebral artery. All these findings (especially recurrent cerebrovascular ischemic attack) were suggestive of progressive disease and her treatment was changed to melphalan/prednisone protocol. Her clinical condition deteriorated soon after initiating treatment. She presented respiratory distress probably caused by impaired neuromuscular respiratory function and progressive neurological deterioration. Unfortunately, she died soon after the deterioration occurred, due to pulmonary sepsis.
We mention that written informed consent was provided by the patient and the patient’s next of kin to have the case details published.
Discussion
Diagnosis of early POEMS syndrome represents a challenge in clinical practice. It includes presence of polyradiculoneuropathy (typically demyelinating) and monoclonal protein plasma cell disorder (almost always λ), at least 1 of the 3 major criteria (CD, sclerotic bone lesions, and elevated VEGF) and at least 1 minor criterion: organomegaly, extravascular volume overload, endocrinopathy, skin changes, papilledema, or thrombocytosis/polycythemia.9 Our patient presented demyelinating polyradiculoneuropathy, IgA λ monoclonal protein, 2 major criteria (CD and sclerotic bone lesions), and several minor criteria: organomegaly, extravascular volume overload (pleural effusion in evolution), hypoparathyroidism, skin changes, papilledema, and thrombocytosis.
CD, also a rare systemic disorder, includes 2 types: localized and multicentric. Three histological forms are described: hyaline vascular, plasma cell, and mixed type. Multicentric CD (MCD) is generally plasma cell type, but the hyaline vascular type has also been described in some patients. Localized CD usually presents in young adults (20–30 years old), compared with MCD (40–50 years old). Patients present systemic symptoms, multiple enlarged lymph nodes, and organomegaly.19 Lymph node biopsy is mandatory for diagnosis. The histological findings in our case were consistent with CD mixed type. POEMS associated with CD was the diagnosis of our patient. The association between CD and POEMS is not fully understood, though it is well recognized. An association with MCD was reported in approximately half of POEMS patients.1,20,21
Pathogenesis of POEMS is not entirely known. Elevated levels of proangiogenic and proinflammatory cytokines like VEGF, IL-6, TNF-α are believed to be the hallmark of pathogenesis. VEGF is considered the driving cytokine in the regulation of endothelial cell proliferation, migration, and permeability and it is responsible for features as extra-vascular volume overload and papilledema. It was also suggested that increased VEGF levels induce higher endoneuronal pressure because of edema and increased microvascular permeability, possibly explaining the nerve damage.22 However, other mechanisms may be involved, as results of studies using anti-VEGF monoclonal antibody (bevacizumab) treatment are variable.23,24 The impact of these cytokines on the vascular system in POEMS is proven by the frequently associated thrombotic accidents. Most of the previous reports refer to lower limb and coronary artery thrombosis, and only a few described POEMS being associated with CD and cerebrovascular involvement.18,25,26 The pathophysiological mechanism of vascular events remains unclear. Kang et al suggested a causal relationship between vascular events and raised IL-6 that induces hyperfibrinogenemia.18 Other authors discussed that IL-1β, TNF-α, and histological abnormalities of intracranial vessels (scleroderma-like skin changes and non-inflammatory vasculopathy) could be triggers for thrombosis.10,11 Results of a retrospective study from Mayo Clinic stated thrombocytosis and evidence of plasma cell proliferation on bone marrow as independent thrombotic risk factors. The 5-year risk of ischemic stroke was 13.4%.27 Abnormalities of the coagulation system have also been reported. One study showed that patients present elevated levels of circulating coagulation factors (fibrinopeptide A and thrombin–antithrombin complex) during the active phase of illness, but normal values of factors related to fibrinolysis (plasminogen, α2plasmin inhibitor plasmin complex, and fibrin degradation product).28
Considering that this patient was a young female without any known cardiovascular risk factors, we suspected that the recurrent cerebrovascular ischemic events, associated with another extra-cerebrovascular event (chronic splenic infarction), were most likely the result of POEMS syndrome associated with CD. Our patient also presented hyperfibrinogenemia on both ischemic events, higher the second time; thrombocytosis; and evidence of plasma cell proliferation on bone marrow aspirate.
The median survival of POEMS syndrome is ~14 years.1,29 Until now, no standard risk stratification has been published. There are only few reports on clinical features associated with poor outcome: fingernail clubbing, extravascular volume overload, respiratory symptoms, and pulmonary hypertension.1,29 Our patient also presented respiratory manifestations related to impaired neuromuscular respiratory function within 2 years of the onset of her symptoms. In fact, the major cause of morbidity was the respiratory distress, which led to pulmonary sepsis and demise. Recent reports suggest that POEMS patients with coexisting CD have an inferior overall survival compared with those without, and this is related to infection, cardiopulmonary failure, and renal failure.30
Respiratory dysfunction and subsequent failure were also enhanced by the recurring cerebrovascular events. It is interesting to highlight the presence of thrombocytosis and bone marrow plasmacytosis in our case, both reported as increased risk factors for cerebrovascular thrombosis.27
Since there are no clinical trials with POEMS patients, therapeutic decisions are based on reported cases, and usually include treatment protocols used in classic plasma cell disorders. The treatment decision is based on the extent of the plasma cell infiltration. For patients with bone marrow involvement, systemic therapy is preferred. Corticosteroids provide symptomatic improvement, but with limited response duration.31–33 Promising results were reported with alkylator agents.34 High-dose chemotherapy with melphalan and peripheral blood stem cell transplant can also be effective, according to case series reported in literature with neurologic improvement for all patients, some of them with durable responses.35–38 Other promising treatments include lenalidomide and thalidomide, drugs with anti-VEGF and anti-TNF activity.39,40 Activity of proteosome inhibitors in POEMS was demonstrated in a few reports.41,42 In our patient, bortezomib was administered subcutaneously with cyclophosphamide and dexamethasone for 4 cycles at first with clinical improvement, but response duration was limited. She later presented rapidly progressing disease; therefore, another therapeutic strategy was used. No response assessment was possible because the patient died shortly after the second stroke due to neurological deterioration and pulmonary infection.
Supportive care plays an important role in the treatment of POEMS patients (control of extravascular volume overload and hormone replacement therapy when necessary). The question of using cytoreductive therapy in POEMS patients with coexisting CD remains a problem that is open for debate. More and more data are favoring the use of hydroxyurea in addition to anticoagulation and/or antiplatelet therapy to reduce the risk of vascular events recurrence, especially when risk factors like thrombocytosis/bone marrow plasmacytosis are identified.27 An argument in favor of this theory might be the case presented before – the second stroke occurred at the time of progressive disease with thrombocytosis, after systemic treatment for the underlying disorder associated with supportive care, which included antiplatelet therapy and prophylactic anticoagulation. Whether cytoreductive treatment should be included in POEMS patients with coexisting CD remains to be seen, but a careful follow-up and interdisciplinary approach (hematologist/neurologist) are crucial for a better outcome of these patients.
Conclusion
Patients diagnosed with POEMS present heterogeneous clinical and laboratory features. For this reason, often the diagnosis, treatment, and follow-up represent a major challenge. A careful examination of medical history and a thorough physical examination can contribute to a rapid diagnosis. A prompt multidisciplinary approach reduces the risk of irreversible complications, which are also related to shorter survival. Thus, we hope that this review and report of a patient with POEMS syndrome accompanied by CD will emphasize the recognition of thrombotic risk associated with this disorder.
Disclosure
The authors report no conflicts of interest in this work.
References
Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496–2506. | ||
Dispenzieri A. POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86(7):591–601. | ||
Takatsuki K, Sanada I. Plasma cell dyscrasia with polyneuropathy and endocrine disorder: clinical and laboratory features of 109 reported cases. Jpn J Clin Oncol. 1983;13(3):543–555. | ||
Fukase M, Kakimatsu T, Nishitani H. Report of a case of solitary plasmacytoma in the abdomen presenting with polyneuropathy and endocrinological disorders. Clin Neurol. 1969;9:657. | ||
Scheinker I. Myelom und Nervensystem [Myeloma and the nervous system]. Deutsche Zeitschrift für Nervenheilkunde. 1938;147(5–6):247–273. German. | ||
Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine. 1980;59(4):311–322. | ||
Dispenzieri A. POEMS syndrome. Blood Rev. 2007;21(6):285–299. | ||
Li J, Zhou DB. New advances in the diagnosis and treatment of POEMS syndrome. Br J Haematol. 2013;161(3):303–315. | ||
Dispenzieri A. POEMS syndrome: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(2):213–223. | ||
Lee MR, Choi HJ, Lee EB, Baek HJ. POEMS syndrome complicated by extensive arterial thromboses. Clin Rheumatol. 2007;26(11):1989–1992. | ||
Lesprit P, Authier FJ, Gherardi R, et al. Acute arterial obliteration: a new feature of the POEMS syndrome? Medicine. 1996;75(4):226–232. | ||
Yu H, Yao F, Li Y, Li J, Cui QC. Castleman disease variant of POEMS syndrome complicated with multiple cerebral infarction: a rare case report and review of literature. Int J Clin Exp Pathol. 2015;8(10):13578–13583. | ||
Dacci P, Lessi F, Dalla Bella E, Morbin M, Briani C, Lauria G. Ischemic stroke as clinical onset of POEMS syndrome. J Neurol. 2013;260(12):3178–3181. | ||
Sommer B, Schaumberg J. Therapeutic challenges in a patient with POEMS syndrome and recurrent stroke: presentation of a case and review of the literature. Acta Neurol Belg. 2012;112(1):9–13. | ||
Wu SF, Zhou GQ, Zhu ZF. [A case of POEMS syndrome patient with main clinical manifestation of multiple acute cerebral infarction]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25(12):753. Chinese. | ||
Erro ME, Lacruz F, Aymerich N, et al. Acute carotid obliteration: a new vascular manifestation in POEMS syndrome. Eur J Neurol. 2003;10(4):383–384. | ||
Huang J, Wang L, Zhou W, Jin J. Hyaline vascular Castleman disease associated with POEMS syndrome and cerebral infarction. Ann Hematol. 2007;86(1):59–61. | ||
Kang K, Chu K, Kim DE, Jeong SW, Lee JW, Roh JK. POEMS syndrome associated with ischemic stroke. Arch Neurol. 2003;60(5):745–749. | ||
Kang J, Yang F, Zhang HY, et al. POEMS syndrome associated with Castleman disease: a case report and literature review. Neuroimmunol Neuroinflammation. 2014;1:40–43. | ||
Peterson BA, Frizzera G. Multicentric Castleman’s. Multicentric Castleman’s disease. Semin Oncol. 1993;20(6):636–647. | ||
Perdaens C, de Raeve H, Goossens A, Sennesael J. POEMS syndrome characterized by glomeruloid angioma, osteosclerosis and multicentric Castleman disease. J Eur Acad Dermatol Venereol. 2006;20(4):480–481. | ||
Scarlato M, Previtali SC, Carpo M, et al. Polyneuropathy in POEMS syndrome: role of angiogenic factors in the pathogenesis. Brain. 2005;128(Pt 8):1911–1920. | ||
Watanabe O, Maruyama I, Arimura K, et al. Overproduction of vascular endothelial growth factor/vascular permeability factor is causative in Crow-Fukase (POEMS) syndrome. Muscle Nerve. 1998;21(11):1390–1397. | ||
Straume O, Bergheim J, Ernst P. Bevacizumab therapy for POEMS syndrome. Blood. 2006;107(12):4972–4974. | ||
Forster A, Müri R. [Recurrent cerebrovascular insult – manifestation of POEMS syndrome?]. Schweiz Med Wochenschr. 1998;128(26):1059–1064. German. | ||
Rössler M, Kiessling B, Klotz JM, Langohr HD. Rezidivierende zerebrale Ischämien bei zerebraler Vaskulitis im Rahmen eines inkompletten POEMS-Syndroms mit M. Castleman [Recurrent cerebral ischemias due to cerebral vasculitis within the framework of incomplete POEMS syndrome with Castleman disease]. Nervenarzt. 2004;75(8):790–794. German. | ||
Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308–1312. | ||
Saida K, Kawakami H, Ohta M, Iwamura K. Coagulation and vascular abnormalities in Crow-Fukase syndrome. Muscle Nerve. 1997;20(4):486–492. | ||
Allam JS, Kennedy CC, Aksamit TR, Dispenzieri A. Pulmonary manifestations in patients with POEMS syndrome: a retrospective review of 137 patients. Chest. 2008;133(4):969–974. | ||
Li J, Zhou DB, Huang Z, et al. Clinical characteristics and long-term outcome of patients with POEMS syndrome in China. Ann Hematol. 2011;90(7):819–826. | ||
Orefice G, Morra VB, de Michele G, et al. POEMS syndrome: clinical, pathological and immunological study of a case. Neurol Res. 1994;16(6):477–480. | ||
Sano M, Terasaki T, Koyama A, Narita M, Tojo S. Glomerular lesions associated with the Crow-Fukase syndrome. Virchows Arch A Pathol Anat Histopathol. 1986;409(1):3–9. | ||
Arima F, Dohmen K, Yamano Y, et al. [Five cases of Crow-Fukase syndrome]. Fukuoka Igaku Zasshi. 1992;83(2):112–120. Japanese. | ||
Li J, Zhang W, Jiao L, et al. Combination of melphalan and dexamethasone for patients with newly diagnosed POEMS syndrome. Blood. 2011;117(24):6445–6449. | ||
Dispenzieri A, Moreno-Aspitia A, Suarez GA, et al. Peripheral blood stem cell transplantation in 16 patients with POEMS syndrome, and a review of the literature. Blood. 2004;104(10):3400–3407. | ||
Schliamser LM, Hardan I, Sharif D, et al. Significant Improvement of POEMS syndrome with pulmonary hypertension following autologous peripheral blood stem cell transplant. Blood. 2003;102:5664 [abstract]. | ||
Soubrier M, Ruivard M, Dubost JJ, Sauvezie B, Philippe P. Successful use of autologous bone marrow transplantation in treating a patient with POEMS syndrome. Bone Marrow Transplant. 2002;30(1):61–62. | ||
Peggs KS, Paneesha S, Kottaridis PD, et al. Peripheral blood stem cell transplantation for POEMS syndrome. Bone Marrow Transplant. 2002;30(6):401–404. | ||
Dispenzieri A, Klein CJ, Mauermann ML. Lenalidomide therapy in a patient with POEMS syndrome. Blood. 2007;110(3):1075–1076. | ||
Royer B, Merlusca L, Abraham J, et al. Efficacy of lenalidomide in POEMS syndrome: a retrospective study of 20 patients. Am J Hematol. 2013;88(3):207–212. | ||
Tang X, Shi X, Sun A, et al. Successful bortezomib-based treatment in POEMS syndrome. Eur J Haematol. 2009;83(6):609–610. | ||
Kaygusuz I, Tezcan H, Cetiner M, Kocakaya O, Uzay A, Bayik M. Bortezomib: a new therapeutic option for POEMS syndrome. Eur J Haematol. 2010;84(2):175–177. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.