Back to Journals » OncoTargets and Therapy » Volume 12
PNUTS mediates ionizing radiation-induced CNE-2 nasopharyngeal carcinoma cell migration, invasion, and epithelial–mesenchymal transition via the PI3K/AKT signaling pathway
Authors Yu D, An X, Fan W, Wang X, He Y, Li B
Received 9 October 2018
Accepted for publication 14 January 2019
Published 15 February 2019 Volume 2019:12 Pages 1205—1214
DOI https://doi.org/10.2147/OTT.S188571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Dan Yu, Xiang An, Wanlin Fan, Xin Wang, Yuxing He, Bing Li
Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
Purpose: Ionizing radiation (IR) is widely used for treating nasopharyngeal carcinoma (NPC). However, recent studies indicate that IR can also promote the migration and invasion of malignant tumors. Phosphatase 1 nuclear-targeting subunit (PNUTS), a novel interacting protein, was recently demonstrated to be involved in tumorigenesis and metastasis formation. This protein was hypothesized to take part in IR-induced migration and invasion in NPC cells in this study.
Materials and methods: Western blotting was used to detect how PNUTS was expressed in NPC cells with or without IR treatment. Wound-healing and Transwell assays were used to measure cell migration and invasion. Quantitative real-time PCR and Western blotting were used to determine the expression levels of PNUTS and epithelial–mesenchymal transition (EMT) proteins, respectively, after CNE-2 cells were infected with an adenovirus vector, ad-PNUTS, or transfected with PNUTS-specific siRNA. Finally, the expression levels of PI3K/AKT signaling-related proteins were detected by Western blotting.
Results: IR significantly promoted PNUTS expression and the migration and invasion in CNE-2 cells. Moreover, after exposure to IR, expression of the mesenchymal markers N-cadherin and vimentin increased, while that of the epithelial marker E-cadherin decreased. Silencing PNUTS remarkably attenuated IR-induced increases in cell migration and invasion and reversed the EMT process. Additionally, the overexpression of PNUTS restored the mobility and invasiveness of CNE-2 cells, which regained EMT characteristics. Furthermore, we found that PNUTS regulated IR-induced EMT via the PI3K/AKT signaling pathway.
Conclusion: Our research illustrates a relationship between PNUTS and IR-induced cell migration and invasion and provides a novel therapeutic target for preventing radiotherapy-induced metastasis in NPC patients.
Keywords: PNUTS, ionizing radiation, EMT, PI3K/AKT pathway, NPC
Introduction
As a common malignant tumor in the head and neck, nasopharyngeal carcinoma (NPC) has an obvious regional aggregation, especially in Guangdong, China.1–3 Radiotherapy, a medical method that utilizes ionizing radiation (IR) to achieve therapeutic goals, is the preferred treatment strategy for patients with NPC.4 In clinical practice, we typically use fractionated IR to reduce the side effects that generate the inevitable damage to normal tissues caused by radiotherapy.5,6 However, several recent studies suggest that IR contradictorily induces the malignant characteristics of tumor cells, resulting in local recurrence and distant metastasis in patients after radiotherapy.7,8 Therefore, it is essential to elucidate the effects of IR-induced cell metastasis and to identify the relevant molecular mechanisms involved.
Epithelial–mesenchymal transition (EMT) has been recognized as a key process in the invasion and metastasis of various malignancies,9 such as breast,10,11 prostate,12 and lung cancer.13,14 During this process, epithelial cells change their original morphology from a cobblestone phenotype to a spindle-like fibroblastic phenotype and obtain the properties of mesenchymal cells.15 This transition is characterized by the increased expression of mesenchymal marker proteins (for example, vimentin and N-cadherin) and the downregulation of epithelial marker proteins (for example, E-cadherin).16 Moreover, several lines of evidence indicate that exposure to IR causes tumor cells to undergo EMT, promoting the malignant characteristics of cancer cells.17,18 However, the potential mechanisms of IR-induced EMT and metastasis in cancer cells have not been fully elucidated.
Originally isolated as a nuclear protein, protein phosphatase 1 nuclear-targeting subunit (PNUTS), also known as PPP1R10 or p99, combines with protein phosphatase 1 (PP1) to form a stable complex in mammalian cells and is involved in transcriptional regulation, cell cycle control, apoptosis, and DNA damage responses.19,20 PNUTS is known to be a potent modulator of PP1 catalytic activity toward exogenous substrates, such as retinoblastoma (Rb) protein.21 When cells suffer from exposure to external stimuli, such as chemotherapeutic drugs or hypoxia, PNUTS detaches from PP1 and causes the dephosphorylation of Rb, resulting in reduced cell viability due to the activation of apoptosis.21,22 Recently, increasing evidence demonstrated that PNUTS is involved in cancer development. PNUTS is ubiquitously expressed in multiple cancers and closely linked to tumorigenesis and metastasis formation.23,24 However, whether PNUTS participates in IR-induced metastasis and EMT in cancer cells is still unknown.
In this study, we demonstrate that PNUTS is a critical protein that regulates IR-induced cell migration and invasion and EMT in human NPC CNE-2 cells through the PI3K/AKT signaling pathway, suggesting that PNUTS can serve as a potential target for intervention in IR-induced NPC metastasis.
Materials and methods
Cell lines and reagents
Gibco (Waltham, MA, USA) provided us with FBS and RPMI-1640 medium. The Radiology and Oncology Laboratory of Chongqing Medical University provided us with the human NPC cell lines CNE-1 (highly differentiated), CNE-2 (poorly differentiated), and HONE-1 (poorly differentiated ). The Institutional Review Boards of The First Affiliated Hospital of Chongqing Medical University approved the use of the three cell lines. BiocolorBio Science & Technology Co. (Shanghai, China) provided us with goat anti-mouse IgG antibodies labeled with horseradish peroxidase (HRP; catalog no. A0216), tris buffered saline, radioimmunoprecipitation (RIPA) lysis buffer, PBS, the BCA protein assay kit, polyvinylidene fluoride (PVDF) membranes, the enhanced chemiluminescence (ECL) kit, and mouse anti-DAPDH (catalog no. AF0006). Santa Cruz Biotechnology (CA, USA) provided us with goat anti-rabbit IgG antibodies labeled with HRP (catalog no. sc-2040), mouse anti-PNUTS (catalog no. sc-271681), anti-N-cadherin (catalog no. sc-8424), and anti-vimentin antibodies (catalog no. sc-66001). Cell Signaling Technology (Beverly, MA, USA) provided us with mouse anti-E-cadherin (catalog no. 14472), anti-AKT (catalog no. 2920), rabbit anti-p-AKT antibodies (catalog no. 4060), and the PI3K inhibitor LY294002 (catalog no. 9901).
Cell culture and irradiation
A humidified incubator containing RPMI 1640 medium with 10% FBS was used to culture the three human NPC cells at 37°C under 5% CO2. When 70% confluence was reached in the cells, they were irradiated at a dose rate of 2 Gy/min with a linear 6 MV X-ray accelerator (CD2300; Varian, Palo Alto, CA, USA). The source–skin distance was set as previously described.25 When the radiation procedure was completed, the cells were returned to the incubator and then harvested at the specified times for subsequent experiments. For pathway inhibition experiments, the PI3K inhibitor LY294002 was added to the cells for 1 hour before IR treatment.
Protein extraction and Western blotting analysis
After IR treatment for 24 hours, we harvested and lysed the cells in RIPA buffer to extract all proteins. A BCA protein assay kit was used to measure the protein concentrations. Total proteins were then electrotransferred to PVDF membranes after separation by 10% SDS-PAGE. The membranes were then incubated overnight using primary antibodies at 4°C after being blocked for 2 hours with 5% nonfat dry milk. The membranes were incubated for 1 hour using HRP-conjugated secondary antibodies after being washed twice with PBS. Finally, an ECL kit was used to detect the target proteins. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used for the loading control.
Wound-healing assay
Cells were seeded in 60 mm dishes and incubated until 80% confluence was achieved. Then, a 20 μL pipette tip was used to create scratch wounds in the cell layers, and PBS was used to wash the wells twice. Finally, an inverted microscope was used to observe scratch closure at the beginning and after 24 and 48 hours.
Transwell migration and invasion assay
A Transwell system equipped with 8-μm-pore polycarbonate filters (Corning, Acton, MA, USA) was applied to detect cell migration and invasion. The upper chamber, which was coated with or without Matrigel (BD Biosciences, Bedford, MA, USA), contained 1×105 cells incubated in serum-free medium. The lower chamber contained RPMI 1640 with 10% FBS as a chemoattractant. The nonmigratory cells were removed after incubation for 24 hours, and the migrated or invaded cells were fixed, stained, and counted under a light microscope.
Cell transfection
Genechem (Shanghai, China) synthesized the specific siRNA against human PNUTS and its negative control. The PNUTS target and control sequences were as follows: 5′-GCAGACCCGUUCACCAGAA-3′ and 5′-UUCUCCGAACGUGUCACGUTT-3′. In brief, we seeded 2×105 cells/well in six-well plates and then divided the plates into three groups: si-PNUTS, si-NC, and control. PBS was only used to treat the control group cells. Then, Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used to transfect the cells with si-PNUTS or si-NC after the cells reached 50% confluence. The transfection efficiency was verified with RT-qPCR and Western blotting.
RNA extraction and quantitative real-time PCR (qRT-PCR)
A HiPure Total RNA Mini Kit (Magen Biotechnology Co., Guangzhou, China) was used to isolate total RNA. Then, the quality of the total RNA was determined by absorbance readings at 260 nm using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). One microgram of RNA was reverse-transcribed into cDNA with a gDNA Eraser (TaKaRa, Japan) using a PrimerScript™ RT Reagent Kit. The primers for PNUTS and GAPDH were as follows: PNUTS (forward 5′-CCCATAGACCCCAAAGAACTTC-3′, reverse 5′-ATCGACTCACCATCTTTCGTG-3′) and GAPDH (forward 5′-CGGATTTGGTCGTATTGGGC-3′, reverse 5′-AGCATCGCCCCACTTGATTT-3′). Next, an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and a SYBR Premix Ex Tag™ Kit (TaKaRa, Shiga, Japan) were used for amplification process according to the user instructions. The PCR amplification conditions were 40 cycles at 95°C for 30 seconds, 95°C for 5 seconds, and 60°C for 1 minute. GAPDH was screened as an internal control by the geNorm software. PNUTS expression was normalized against GAPDH, and the results were analyzed using the 2−ΔΔCt method and presented in the form of relative expression. The experiments were repeated in triplicate to confirm the findings.
Adenovirus infection
An adenovirus expressing PNUTS and its control vector GFP were packaged and synthesized by Genechem (Shanghai, China). We seeded 1×105 CNE-2 cells/well in six-well plates and divided them into two groups: ad-PNUTS and ad-GFP. The cells were infected with adenovirus encoding PNUTS and negative control ad-GFP at a total titer of 3×109 PFU/mL after incubation for 24 hours. The infection was stopped after another 24 hours of incubation, and the cells were used for subsequent experiments.
Statistical analyses
All data were analyzed with SPSS 21.0 software (IBM SPSS Statistics) and presented as the mean ± SD. One-way ANOVA or Student’s t-test was used to analyze the differences between groups. All statistical analyses used P<0.05 as an indicator of statistical significance.
Results
IR increases PNUTS expression in CNE-2 cells
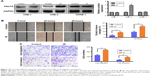
We performed a Western blotting analysis using the HONE-1, CNE-1, and CNE-2 human NPC cell lines to determine whether IR affects the expression level of PNUTS in different human NPC cells. A single dose of 4 Gy of X-rays was administered to these cells. Among the three cell lines, PNUTS expression was significantly increased only in CNE-2 cells (Figure 1A). Wound-healing and Transwell assays were carried out to investigate whether IR promotes the invasive and metastatic capability of CNE-2 cells. The wound-healing assay detected markedly enhanced migration of CNE-2 cells, as shown in Figure 1B, and the same result was obtained in the Transwell assay (Figure 1C). Based on these findings, CNE-2 cells were used for subsequent experiments.
Inhibition of PNUTS reverses IR-induced EMT and abolishes CNE-2 cell migration and invasion ability
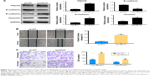
EMT has a pivotal role during tumor cell invasion and metastasis.9 Given that IR induces cancer cells to undergo EMT,18 and PNUTS mediates the metastatic potentiality of malignancies,24 we hypothesized that PNUTS participates in IR-induced EMT. First, the expression levels of EMT marker proteins in CNE-2 cells were examined after 24 hours of exposure to IR. As seen in Figure 2C, comparison of lanes 1 and 2 shows that IR upregulated the expression levels of N-cadherin and vimentin while downregulating that of E-cadherin. To clarify the function of PNUTS in IR-induced EMT, we transfected CNE-2 cells with si-PNUTS or si-NC to inhibit PNUTS expression, and we validated the efficiency by performing Western blotting assays and qRT-PCR. Compared with the control group, PNUTS expression was significantly reduced at both the mRNA and protein levels in CNE-2 cells (Figure 2A and B). Furthermore, Western blotting analysis demonstrated that PNUTS inhibition increased the expression levels of vimentin and N-cadherin while suppressing E-cadherin expression in CNE-2 cells after IR relative to the group treated with si-PNUTS alone (lane 3 vs lane 4 in Figure 2C). Moreover, IR-induced cell migration and invasiveness were remarkably abolished by PNUTS inhibition in CNE-2 cells (Figure 2D and E). All of these findings suggest that IR-induced EMT was reversed by inhibiting PNUTS expression, and PNUTS plays an important part in regulating the migration and invasion of CNE-2 cells.
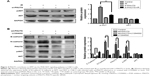
Overexpression of PNUTS induces EMT and significantly increases the mobility and invasiveness of CNE-2 cells
To clarify how PNUTS functions in the EMT process and in cell mobility and metastasis, we performed rescue experiments by infecting CNE-2 cells with adenovirus (ad-PNUTS). PNUTS expression was markedly increased in PNUTS-overexpressing cells relative to cells infected with ad-GFP (Figure 3A). Additionally, E-cadherin expression was upregulated in PNUTS-overexpressing cells, whereas vimentin and N-cadherin levels were downregulated (Figure 3A). Meanwhile, the exogenous overexpression of PNUTS promoted migration and invasion, similar to IR, in CNE-2 cells, according to wound-healing and Transwell assays (Figure 3B and C). Collectively, these results indicate that PNUTS is a key regulator of the EMT process and mediates the mobility and invasiveness of CNE-2 cells.
PNUTS contributes to EMT via the PI3K/AKT signaling pathway in CNE-2 cells
To clarify the molecular mechanism by which IR induces mobility and invasiveness, we attempted to investigate PNUTS-mediated EMT in CNE-2 cells to identify the involved signaling pathway. Multiple signaling pathways, for example, PI3K/AKT, nuclear factor kappa B, and TGF-β/Smad, can be activated during the EMT process.26 In this study, we focused on the PI3K/AKT signaling pathway because it can induce EMT to accelerate tumor progression. Western blotting results revealed that IR significantly increased phosphor-AKT levels, whereas the level of total AKT was not obviously changed relative to control cells (lane 1 vs lane 2 in Figure 4A). Next, a comparison of lane 2 and lane 4 indicated that the increased levels of phosphor-AKT were significantly downregulated by PNUTS inhibition, which suggested that PNUTS knockdown could override the effect of IR on PI3K/AKT signaling activation (Figure 4A). Additionally, PNUTS overexpression obviously enhanced phosphor-AKT levels relative to those in the control group (Figure 4B). To clarify how PI3K/AKT signaling regulates IR-induced EMT via PNUTS, we treated CNE-2 cells with a PI3K inhibitor, LY294002, which previously has been shown to inhibit the PI3K/AKT signaling pathway. Western blotting analysis showed that AKT phosphorylation was markedly reduced (lane 1 vs lane 4 in Figure 4A). Intriguingly, after LY294002 treatment, PNUTS overexpression decreased the expression of N-cadherin while increasing that of E-cadherin (lane 2 vs lane 3 in Figure 4B). These results demonstrate that PNUTS activates the PI3K/AKT signaling pathway in CNE-2 cells and consequently induces EMT.
Discussion
Radiotherapy is an effective and common treatment for NPC. However, accumulating evidence indicates that IR not only kills cancer cells but also promotes cell migration and invasion by initiating the EMT.17,18 Our results showed that IR induced EMT and promoted the invasion and migration of CNE-2 cells, whereas the inhibition of PNUTS expression reversed IR-induced EMT and abolished the migration and invasion capacity of CNE-2 cells. Interestingly, although PNUTS was expressed in all three NPC cell lines tested, increased PNUTS expression was only detected in CNE-2 cells after IR treatment, which may be related to the tumor cell heterogeneity and cancer stem cell-like characteristics of CNE-2 cells.27 To further clarify how PNUTS functions in the EMT process and the mobility and invasiveness of CNE-2 cells, we overexpressed PNUTS using adenovirus infection to conduct rescue experiments. Our results showed the recovery of PNUTS and EMT characteristics. Moreover, the migration and invasion capacity of CNE-2 cells also recovered. Therefore, PNUTS may be a promising target for the treatment of radiation-induced metastasis.
PNUTS, a novel interacting protein, has been defined as a nuclear conditioner of PP1 that combines with PP1 and sharply reduces the catalytic activity of PP1 toward exogenous substrates.20 PNUTS is involved in various biologic functions, such as apoptosis, cell cycle control, transcriptional regulation, and telomere maintenance.19,28,29 Emerging evidence suggests a role for PNUTS in tumorigenesis and metastasis formation.23,30,31 PNUTS depletion leads to Rb dephosphorylation and the formation of a complex with the transcription factor ZEB1, thus inhibiting the ZEB1 transcriptional repressor activity of E-cadherin, and increasing E-cadherin expression, which impedes the EMT process.32 Moreover, PNUTS can modulate TGFβ and AKT signaling and promote the migration and metastasis of cancer cells, which allow it to serve as a potential oncogene.23,33 However, the mechanism involving PNUTS is still unknown.
To further our research into the underlying mechanism by which PNUTS mediates IR-induced EMT and metastasis, we explored how IR-induced PNUTS expression activates the PI3K/AKT signaling pathway, which significantly contributes to the regulation of cancer cell and EMT progression.34,35 Recent studies have revealed that PI3K/AKT signaling significantly mediates the responsiveness of tumor cells to radiation.36 Therefore, IR-induced AKT phosphorylation stimulates multiple transcription factors, for example, by increasing the expression of ZEB1 and thus promoting the EMT process, which correlates with recurrence and distant metastasis in NPC patients.37 This study showed that IR increased the phosphorylation of AKT and that silencing PNUTS obviously attenuated this effect, suggesting that PI3K/AKT signaling pathway activation was mediated PNUTS. Furthermore, pretreating CNE-2 cells with a PI3K inhibitor (LY294002) facilitated the downregulation of N-cadherin and the upregulation of E-cadherin. When cells overexpressing PNUTS were treated with LY294002, the EMT process was reversed. In this study, it is not clear how PNUTS interacted with the PI3K/AKT signaling pathway under our experimental conditions. However, molecules such as PTEN have been reported to mediate the expression of PNUTS via the PI3K/AKT signaling pathway.38,39 In addition, because PTEN, PI3K, and AKT are closely related, one possible mechanism is that PTEN mediates the effects of PNUTS on IR-induced EMT and metastasis of CNE-2 cells and consequently plays a role in the PI3K/AKT signaling pathway, but this hypothesis requires further confirmation in future studies. According to our findings, the tumor-promoting effects of PNUTS on the IR-induced EMT and metastasis of CNE-2 cells are activated at least in part by the PI3K/AKT signaling pathway.
Conclusion
PNUTS regulates IR-induced cell invasion and migration via EMT through activation of the PI3K/AKT signaling pathway, which provides a new target to understand how tumor invasion and metastasis are affected by IR.
Acknowledgment
This project was supported by the Key Project of the Chongqing Natural Science Foundation (cstc2013jcyjA10059).
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Wei WI, Sham JST. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–2054. | ||
Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. | ||
Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53(1):12–22. | ||
Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79(2):420–428. | ||
Kwong DL, Pow EH, Sham JS. Intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma: a prospective study on disease control and preservation of salivary function. Cancer. 2010;101(7):1584–1593. | ||
Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661–668. | ||
Guo R, Tang LL, Mao YP, et al. Clinical outcomes of volume-modulated arc therapy in 205 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. PLoS One. 2015;10(7):e0129679. | ||
Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35(4):645–654. | ||
Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13(3):R59. | ||
Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–620. | ||
Byles V, Zhu L, Lovaas JD, et al. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31(43):4619–4629. | ||
Shi H, Ji Y, Zhang D, Liu Y, Fang P. MiR-135a inhibits migration and invasion and regulates EMT-related marker genes by targeting KLF8 in lung cancer cells. Biochem Biophys Res Commun. 2015;465(1):125–130. | ||
Lin LC, Hsu SL, Wu CL, Hsueh CM. TGFβ can stimulate the p(38)/β-catenin/PPARγ signaling pathway to promote the EMT, invasion and migration of non-small cell lung cancer (H460 cells). Clin Exp Metastasis. 2014;31(8):881–895. | ||
Zavadil J, Haley J, Kalluri R, Muthuswamy SK, Thompson E. Epithelial-mesenchymal transition. Cancer Res. 2008;68(23):9574–9577. | ||
Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21–45. | ||
Zhou YC, Liu JY, Li J, et al. Ionizing radiation promotes migration and invasion of cancer cells through transforming growth factor-beta-mediated epithelial-mesenchymal transition. Int J Radiat Oncol Biol Phys. 2011;81(5):1530–1537. | ||
Lee SY, Jeong EK, Ju MK, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16(1):10. | ||
Allen PB, Kwon YG, Nairn AC, Greengard P. Isolation and characterization of PNUTS, a putative protein phosphatase 1 nuclear targeting subunit. J Biol Chem. 1998;273(7):4089–4095. | ||
Kim YM, Watanabe T, Allen PB, et al. PNUTS, a protein phosphatase 1 (PP1) nuclear targeting subunit. Characterization of its PP1- and RNA-binding domains and regulation by phosphorylation. J Biol Chem. 2003;278(16):13819–13828. | ||
De Leon G, Sherry TC, Krucher NA. Reduced expression of PNUTS leads to activation of Rb-phosphatase and caspase-mediated apoptosis. Cancer Biol Ther. 2008;7(6):833–841. | ||
Graña X. Downregulation of the phosphatase nuclear targeting subunit (PNUTS) triggers pRb dephosphorylation and apoptosis in pRB positive tumor cell lines. Cancer Biol Ther. 2008;7(6):842–844. | ||
Kavela S, Shinde SR, Ratheesh R, et al. PNUTS functions as a proto-oncogene by sequestering PTEN. Cancer Res. 2013;73(1):205–214. | ||
Kiweler N, Krämer OH. PNUTS at the crossroads of tumorigenesis and metastasis formation. J Thorac Dis. 2018;10(2):560–563. | ||
Zang C, Liu X, Li B, et al. IL-6/STAT3/TWIST inhibition reverses ionizing radiation-induced EMT and radioresistance in esophageal squamous carcinoma. Oncotarget. 2017;8(7):11228–11238. | ||
Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8. | ||
Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67(8):3716–3724. | ||
Udho E, Tedesco VC, Zygmunt A, Krucher NA. PNUTS (phosphatase nuclear targeting subunit) inhibits retinoblastoma-directed PP1 activity. Biochem Biophys Res Commun. 2002;297(3):463–467. | ||
Choy MS, Hieke M, Kumar GS, et al. Understanding the antagonism of retinoblastoma protein dephosphorylation by PNUTS provides insights into the PP1 regulatory code. Proc Natl Acad Sci U S A. 2014;111(11):4097–4102. | ||
Kiweler N, Krämer OH. PNUTS at the crossroads of tumorigenesis and metastasis formation. J Thorac Dis. 2018;10(2):560–563. | ||
Grelet S, Link LA, Howley B, et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol. 2017;19(9):1105–1115. | ||
Egger JV, Lane MV, Antonucci LA, Dedi B, Krucher NA. Dephosphorylation of the retinoblastoma protein (Rb) inhibits cancer cell EMT via ZEB. Cancer Biol Ther. 2016;17(11):1197–1205. | ||
Krucher NA, Perry B, Lentine B, Dzaferovic V, Cardoso F. Abstract 5058: phosphatase nuclear targeting subunit (PNUTS) is phosphorylated by Akt kinase. Cancer Res. 2010;70(8 Suppl):5058. | ||
Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. | ||
Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9(4):317–324. | ||
Toulany M, Rodemann HP. Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin Cancer Biol. 2015;35(3):180–190. | ||
Viniegra JG, Martínez N, Modirassari P, et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280(6):4029–4036. | ||
He E, Pan F, Li G, Li J. Fractionated ionizing radiation promotes epithelial-mesenchymal transition in human esophageal cancer cells through PTEN deficiency-mediated Akt activation. PLoS One. 2015;10(5):e0126149. | ||
Zhang HY, Wang ZQ, Li YY, et al. Transforming growth factor-β1-induced epithelial-mesenchymal transition in human esophageal squamous cell carcinoma via the PTEN/PI3K signaling pathway. Oncol Rep. 2014;32(5):2134–2142. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.