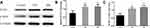Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
PM2.5 Induces Airway Remodeling in Chronic Obstructive Pulmonary Diseases via the Wnt5a/β-Catenin Pathway
Authors Zou W , Wang X, Sun R, Hu J, Ye D, Bai G , Liu S, Hong W, Guo M, Ran P
Received 20 August 2021
Accepted for publication 23 November 2021
Published 3 December 2021 Volume 2021:16 Pages 3285—3295
DOI https://doi.org/10.2147/COPD.S334439
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Weifeng Zou,1 Xiaoqian Wang,2 Ruiting Sun,3 Jinxing Hu,1 Dong Ye,4 Ge Bai,3 Sha Liu,5 Wei Hong,6 Meihua Guo,1 Pixin Ran3
1State Key Laboratory of Respiratory Disease, Guangzhou Chest Hospital, Guangzhou, Guangdong, People’s Republic of China; 2The Third Hospital of Mianyang, Mianyang, Sichuan, People’s Republic of China; 3National Center for Respiratory Medicine, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Diseases, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 4State Key Laboratory of Respiratory Disease, Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 5The Second Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, People’s Republic of China; 6GMU-GIBH Joint School of Life Sciences, Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
Correspondence: Pixin Ran
National Center for Respiratory Medicine, State Key Laboratory of Respiratory Diseases, National Clinical Research Center for Respiratory Diseases, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, 151 Yanjiang Road, Yuexiu District, Guangzhou, Guangdong, 510120, People’s Republic of China
Tel +86 13922765811
Email [email protected]
Background: Fine-particulate matter ≤ 2.5 μm in diameter (PM2.5)-associated airway remodeling has recently been recognized as a central feature of COPD. Activation of the Wnt/β-catenin pathway is closely related to the occurrence of airway remodeling. Accordingly, the goal of this study was to determine whether the Wnt5a/β-Catenin pathway is involved in PM2.5-induced smooth muscle proliferation in vivo and in vitro, which promotes the development of airway remodeling in subjects with COPD.
Methods: The effect of Wnt5a on β-Catenin-mediated airway remodeling was assessed using an in vivo model of PM2.5-induced COPD and PM2.5-exposed human bronchial smooth muscle cells (HBSMCs) in vitro. Small animal spirometry was used to measure lung function in mice. H&E staining and immunohistochemistry were performed to inspect emphysema and airway remodeling indices. Real-time PCR was used to detect Wnt5a, β-Catenin, TGF-β 1, CyclinD1 and c-myc mRNA expression. The CCK8 assay was performed to detect cellular activity. Western blotting was performed to assess PCNA, α-SMA, Wnt5a, β-Catenin, PDGFRβ and TenascinC protein expression. β-Catenin expression was detected using cellular immunofluorescence.
Results: Exposure to PM2.5 led to emphysema, airway wall thickening, an increased smooth muscle layer thickness, decreased lung function and increased expression of the Wnt5a, β-Catenin, PDGFRβ and Tenascin C proteins in the mouse lung tissue. BOX5 (a Wnt5a antagonist) alleviated these PM2.5-induced outcomes in mice. Moreover, PM2.5 induced the expression of the Wnt5a, β-Catenin, TGF-β 1, CyclinD1 and c-myc mRNAs in HBSMCs. BOX5 also inhibited the PM2.5-induced increases in PCNA, α-SMA, Wnt5a, β-Catenin, PDGFRβ and Tenascin C protein expression in HBSMCs.
Conclusion: Our findings suggest that PM2.5 exposure induces HBSMC proliferation, contributing to airway remodeling via the Wnt5a/β-Catenin signaling pathway in vivo and in vitro, which might be a target for COPD treatment.
Keywords: COPD, airway remodeling, PM2.5, Wnt5a/β-Catenin
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic disease that can be prevented and treated. It is accompanied by persistent respiratory symptoms and airflow limitation, generally with airway and alveolar abnormalities related to significant exposure to harmful particles or gases.1–3 Airway remodeling is a distinctive pathological feature of COPD, and the airway wall thickness correlates with the severity of COPD. Smooth muscle cell proliferation plays an important role in airway remodeling, and the smooth muscle layer of the small airways is thickened and negatively correlated with the severity of the disease in patients with COPD.4–6 Although advances in our understanding of the inflammatory features of COPD have been achieved, the mechanisms underlying the remodeling process are still poorly understood.
Fine-particulate matter ≤ 2.5 μm in diameter (PM2.5) is characterized by a small particle size, large surface area, and the ability to absorb toxins.7 Recently, PM2.5 exposure was reported to be associated not only with an increased risk of developing COPD but also with the exacerbated deterioration of lung function and symptoms experienced by patients with COPD.8,9 As shown in our previous study, PM2.5 induced Wnt5a expression in human bronchial epithelial cells.10 Wnt5a is a member of the Wnt glucoprotein family, which activates a variety of downstream signaling pathways to regulate cell migration, polarity, proliferation and survival.11 Previous studies have shown that increased expression of Wnt5a activates nonclassical Wnt signaling and contributes to the pathogenesis of COPD.12 Specifically, Wnt5a increases the TGF-β1-induced upregulation of α-Smooth muscle actin (α-SMA) expression to enhance smooth muscle cell contractility.13 TGF-β1 is involved in β-catenin-independent nonclassical Wnt5a signaling, which induces extracellular matrix (ECM) expression in airway smooth muscle cells and promotes tissue fibrosis.14 Wnt5a also regulates the proliferation of smooth muscle cells, but its role in the regulation of airway remodeling in response to PM2.5 remains unknown.
Platelet-derived growth factor receptor (PDGFRβ) is a cell surface tyrosine kinase receptor that is involved in cell proliferation, survival and migration.15 Emerging evidence suggests that Wnt signaling contributes to smooth muscle cell precursor proliferation and adult lung disease development by directly regulating Tenascin C transcription and participating in the regulation of PDGFRβ, which defines the Wnt/Tnc/PDGFR signaling axis.16 However, researchers have not clearly determined whether this axis promotes airway remodeling and accelerates disease progression in subjects with COPD.
Based on this information, we hypothesized that the Wnt5a/β-Catenin signaling pathway participates in PM2.5-induced airway smooth muscle cell proliferation through the upregulation of PDGFRβ expression and leads to airway remodeling. Hence, using HBSMCs and an experimental model of COPD, this study was designed to elucidate the effects of the Wnt/Tnc/PDGFRβ axis on PM2.5-induced airway remodeling in a COPD model.
Methods
Animal Experiments
C57BL/6 mice were purchased from the Experimental Animal Center of Guangzhou University of Chinese Medicine. Animal experiments were performed according to the Chinese Association for Laboratory Animal Science Policy, and were approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University.
Collection and Extraction of PM2.5
Traffic ambient PM2.5 was collected by aerodynamic impactors equipped with a glass fiber filter, quartz filter, or Teflon membrane, and DMSO was used to extract soluble parts of PM2.5. Sufficiently specific samples for biological experiments were collected with high-volume impactors equipped with glass fiber filters from arterial traffic roads. Gravimetric analysis was used to analyze specific physicochemical characteristics of PM2.5. The procedure of PM2.5 sampling and analysis methods were described in our previous study.10
PM2.5 Exposure in Mice
C57BL/6 mice (6–8w) were randomly divided into 4 groups of 12 mice each. PBS (20 μL), PM2.5 (100 μg/20 μL), BOX5 (0.5 μg/mL 10 μL), and PM2.5+BOX5 (100 μg/20 μL+0.5 μg/mL 10 μL) were injected via tracheal drip twice a week for 4 weeks of continuous exposure. Lung tissue was collected for subsequent experiments.
Lung Function Tests
After four weeks of treatment, mice were anaesthetized intraperitoneally with 1.25% avertin (2, 2, 2-tribromoethanol) 24 h after the last exposure to PM2.5. Tracheotomized mice were placed in a whole-body manometer (Buxco-Force Pulmonary Maneuvers) to determine the peak expiratory flow rate (PEF) and peak inspiratory flow rate (PIF) using Boyle’s law maneuvers.
Histology and Immunohistochemistry
Mouse lung tissues were collected, and small airways were analyzed using histochemistry and immunohistochemistry as described in our previous studies.17,18 Histology was performed on 4.0 μm thick paraffin sections of lung tissues. Sections were incubated with an anti-α-SMA antibody (ab5694, Abcam). Immunochemical staining to determine the α-SMA-positive stained area was evaluated with a confocal microscope at 200× magnification.
Method for Analyzing Airway Remodeling
The thickness of the small airway wall was calculated as the total area of the small airway wall (WAt) after normalization of the perimeter of the basement membrane (Pbm), ie, WAt/Pbm (μm2/μm), which was used as an index for small airway remodeling. The airways were selected based on the following criteria: 1. basement membrane perimeter <2000 μm; 2. minimum internal diameter of airway/maximum internal diameter of airway >0.5; Pbm, area of basement membrane (Abm) and airway wall outer membrane area (Ao) measured using Image-Pro Plus 6.0 (IPP6.0) software. The percentage of smooth muscle layer area (smooth muscle-specific marker protein α-SMA positive area) to airway wall area, ie, Area/(Ao-Abm)% (WA%), was used as an indicator of the smooth muscle layer in airway remodeling.
Method for Analyzing Emphysema
The mean linear intercept (MLI) was used to evaluate the size of the alveolar cavity, which was used as an index of emphysema. In the same field of view, “十” intersecting lines were drawn, the length of each line (L) and the number of alveoli passing through each line (NS) were calculated, and the MLI was calculated as MLI=L/NS.
Cell Culture and Treatment
The human bronchial smooth muscle cell line (HBSMC; American Type Culture Collection, Manassas, VA, USA) was cultured in SMCM (ScienCell, USA). Cells were cultured at 37°C with 5% CO2 in a humidified chamber and then exposed (or not) to PM2.5 (3 μg/mL). HBSMCs were pretreated with the Wnt5a antagonist BOX5 (Merck Millipore, Burlington, MA, USA) for 1 h prior to the addition of 3 μg/mL PM2.5.
Cell Viability Assay
The viability of HBSMCs was detected using a WST8 assay kit (CCK8; Promoter Biotechnology, Wuhan, China), according to the manufacturer’s instructions. Cells were exposed to BOX5 (100, 200, or 300 μM) in SMCM culture medium (100 μL). The optical density (OD) at 450 nm was detected with a microplate reader (Multiskan MK3; Thermo Fisher Scientific, Waltham, MA, USA).
Immunofluorescence Staining
HBSMCs were cultured for 48 h to reach a 60% density. Then, immunofluorescence staining was performed using standard procedures. Cells were incubated with an anti-β-catenin antibody (ab32572, Abcam, 1:500) for 12 h at 4°C, followed by a goat anti-rabbit IgG antibody (ab150077, Abcam, 1:400) for 1 h at room temperature. Finally, cell nuclei were stained with 4′-6-diamidino-2-phenylindole (DAPI; Beyotime, Shanghai, China) for 5 min. Images were captured using an inverted fluorescence microscope (Olympus-FL 500; Olympus Corporation, Tokyo, Japan).
Isolation of mRNA and Real-Time PCR Analysis
Total RNA was extracted using the Universal RNA Extraction Kit (B0004D, EZB, Shanghai, China) according to the manufacturer’s instructions. RNA was quantified using a NanoDrop spectrophotometer (NanoDrop Tech, Rockland, DE, USA). Gene-specific primers (listed in Table 1) were provided by Sangon Biotech (Shanghai, China). Quantitative real-time PCR was performed using SYBR Premix Ex Taq II (Novozymes) and a Bio–Rad instrument II (Roche Diagnostics, Basel, Switzerland). GAPDH served as the housekeeping control.
 |
Table 1 Primer Sequence |
Protein Extraction and Western Blot Analysis
Protein extraction and Western blotting were conducted according to the manufacturer’s instructions. The membranes were blocked with 5% BSA (G5001, Servicebio) and then incubated with antibodies (anti-PCNA antibody, ab18197, Abcam; anti-α-SMA antibody, ab5694; anti-Wnt5a antibody, ab179824; anti-β-catenin antibody, ab32572; anti-PDGFRβ antibody, ab69506; anti-TenascinC antibody, ab108930; anti-α-tubulin, ab7291; anti-β-actin, ab8226, Abcam) at 4°C for 12 h. Subsequently, the blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 2 h at room temperature. The proteins of interest were detected using enhanced chemiluminescence reagents (FDbio science), and the band intensities were quantified using ImageJ software (National Institute of Health, Bethesda, MD, USA). The expression of the target proteins was normalized to the loading control β-actin or α-tubulin.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism software version 7.04 for Windows (GraphPad Software, San Diego, USA) and are presented as the medians and IQRs. Data were analyzed with one-way ANOVA followed by the Bonferroni post hoc test or Kruskal-Wallis with Dunn’s test for multiple comparisons, depending on whether datasets had a parametric or nonparametric distribution. P < 0.05 was considered statistically significant.
Result
PM2.5 Stimulation Induced Airway Remodeling and Emphysema in Mice
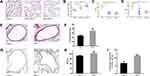
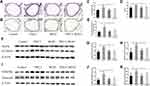
We performed H&E staining to observe the changes in lung histology and immunohistochemistry to detect the expression of α-SMA. Compared with the control group, the lung tissue destruction in mice exposed to different concentrations of PM2.5 was obvious and characterized by emphysematous features, such as enlarged and fused alveolar cavities and thinning, breaking and widening of alveolar septa (Figure 1A and B), indicating the occurrence of emphysema-like lesions. Lung function tests showed that exposure to different concentrations of PM2.5 (50μg/20μL and 100μg/20μL) reduced PIF and PEF in mice (Figure 1C and D). Based on the structural changes in alveoli, lung function and survival rate of mouse, the mouse in the group exposed to PM2.5 (100μg/20μL) were selected for follow-up experiments with reference to the relevant literature.19 Compared with the control group, the airway wall thickness of mice exposed to PM2.5 for 1 month was significantly thickened (Figure 1E and F). Furthermore, the PM2.5 group showed increased α-SMA expression, thickening of the smooth muscle layer, and a significantly increased percentage of the smooth muscle layer in the airway wall (Figure 1G–I), suggesting that PM2.5 induced small airway remodeling in vivo.
PM2.5 Induced HBSMC Proliferation
We detected the expression of α-SMA and proliferating cell nuclear antigen (PCNA) in airway smooth muscle cells after PM2.5 stimulation to investigate its effect on the airway smooth muscle. PCNA is an intranuclear protein that is involved in DNA replication and is mainly produced in proliferating and transformed cells.20 After PM2.5 stimulation of airway smooth muscle cells, PCNA and α-SMA expression were upregulated (Figure 2A–C), indicating that PM2.5 promoted airway smooth muscle cell proliferation.
PM2.5 Increased Wnt5a/β-Catenin Pathway Activation in the Mice Lung Tissue
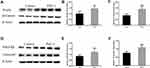
We measured the expression of proteins in the Wnt5a/β-Catenin signaling pathway in PM2.5-exposed lung tissues of mice using Western blotting to investigate the effect of PM2.5 on the expression of proteins in the Wnt5a/β-Catenin signaling pathway and their potential roles in airway remodeling. Compared with the control group, increased expression of the Wnt5a and β-Catenin proteins was detected in the PM2.5-exposed group (Figure 3A–C), and PM2.5 exposure also significantly upregulated PDGFRβ and Tenascin C protein expression in mouse lung tissue (Figure 3D–F), indicating that PM2.5 exposure induced the expression of proteins involved in the Wnt5a/β-Catenin signaling pathway in vivo.
PM2.5 Increased Wnt5a/β-Catenin Pathway Activation in HBSMCs
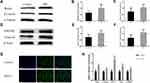
We measured the expression of proteins in the Wnt5a/β-Catenin signaling pathway in PM2.5-exposed HBSMCs using Western blotting to further investigate the effect of PM2.5 on the expression of proteins in the Wnt5a/β-Catenin signaling pathway in vitro. Compared with the control group, increased expression of the Wnt5a and β-Catenin proteins was detected in PM2.5-exposed HBSMCs (Figure 4A–C), and PM2.5 exposure significantly upregulated PDGFRβ and Tenascin C protein expression in HBSMCs (Figure 4D–F). We also detected increased expression of Wnt5a and β-Catenin, as well as their downstream target genes TGF-β1, CyclinD1, and c-myc, at the transcript level (Figure 4H). Cellular immunofluorescence staining of HBSMCs revealed increased expression of β-Catenin after PM2.5 stimulation and increased intranuclear staining compared to the control group (Figure 4G), suggesting that PM2.5 induced the expression of Wnt5a and activated β-Catenin signaling in HBSMCs.
The Wnt5a-Specific Antagonist BOX5 Alleviated PM2.5-Induced Airway Remodeling in Mice
BOX5, a Wnt5a-derived antagonistic peptide, was used to block endogenous Wnt5a signaling and investigate the role of Wnt5a in PM2.5 exposure-induced airway remodeling in mice. Then, morphological changes in lung tissues were observed using H&E staining, and the expression of the α-SMA protein was detected using immunohistochemistry. As depicted in Figure 5A–E, compared with the PM2.5 group, the airway wall thickness of PM2.5+BOX5-exposed mice was thinner, the expression of α-SMA protein in the smooth muscle layer decreased, and the ratio of smooth muscle layer to airway thickness (WA%) decreased (Figure 5A–E), indicating that BOX5 inhibited PM2.5-induced airway smooth muscle layer thickening and airway remodeling. Moreover, the expression of proteins in the Wnt5a/β-Catenin signaling pathway in the mice lung tissues was observed using Western blotting. Compared with the PM2.5 group, the expression of Wnt5a, β-Catenin, PDGFRβ and Tenascin C proteins was downregulated in the BOX5 + PM2.5 group (Figure 5F–K), indicating that BOX5 inhibited PM2.5-induced expression of proteins in the Wnt5a/β-Catenin signaling pathway during the process of airway remodeling in the mice lung tissues.
The Wnt5a-Specific Antagonist BOX5 Alleviated PM2.5-Induced HBSMC Proliferation
First, the cytotoxicity of BOX5 in HBSMCs was examined. HBSMCs were stimulated with different doses of BOX5 (100–300 μM) for 48 h with reference to the relevant literature.21 No significant changes in cell activity were observed after the treatment with 100 μM BOX5 (Figure 6D); therefore, 100 μM BOX5 was subsequently selected as the experimental dose in combination with a literature review. As expected, compared with the PM2.5 group, the levels of PCNA and α-SMA were downregulated in the BOX5 + PM2.5 group (Figure 6A–C), indicating that BOX5 inhibited PM2.5-induced HBSMC proliferation. In addition, BOX5 treatment reduced the PM2.5-induced increase in Wnt5a, β-Catenin, PDGFRβ and Tenascin C levels compared to the PM2.5 group (Figure 6E–J). These results suggest that the Wnt5a/β-Catenin signaling pathway may participate in the regulation of PM2.5-induced HBSMC proliferation in vitro.
Discussion
With the continuous advancement of industrialization and urbanization, PM2.5 is playing an increasingly important role in the development of COPD.22 Recent epidemiological studies conducted both in China and abroad have found that PM2.5 in air pollution increases the incidence, risk of acute exacerbation, lung function decline and mortality of patients with COPD.23 The incidence of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and acute respiratory infections (ARIs) is significantly increased when PM2.5 pollution is severe.24
Our findings revealed that PM2.5 exposure caused aberrant upregulation of components of the Wnt5a/β-Catenin signaling pathway in both HBSMCs and mice lung tissue. Upregulated Wnt5a led to the activation of β-Catenin, increased expression of TenascinC and PDGFRβ, and the induction of HBSMC proliferation and airway remodeling. Approaches targeting the Wnt5a/β-Catenin signaling pathway may serve as an effective therapeutic strategy for PM2.5-induced airway remodeling in patients with COPD.
Epidemiological studies have shown that exposure to PM2.5 poses a serious threat and risk to human health and is significantly associated with increased mortality from respiratory disease, lung cancer and cardiovascular disease.25–27 We constructed a mice model by administering a tracheal drip injection of PM2.5. COPD-like models have been successfully constructed by a tracheal drip injection of PM2.5, which are mainly characterized by small bronchial mucosal damage, wall thickening, squamous cell hyperplasia, and fibrous tissue hyperplasia. In contrast to the cigarette smoke extract (CSE) model, PM2.5 mainly causes small airway lesions, such as bronchial dilatation and wall thickening, while CSE mainly induces severe emphysema lesions and poor diffusion function.28–30 Our study also found that PM2.5 exposure led to thickening of the airway wall in mice, especially the smooth muscle layer, and PM2.5 stimulation of HBSMCs in vitro led to proliferation, hypertrophy, and upregulation of intracellular proliferation-related protein PCNA expression. Based on our results, PM2.5 exposure induced emphysema and airway remodeling in mice and induced airway smooth muscle cell proliferation, leading to airway remodeling in vitro, which contributes to the development of COPD.
Furthermore, the expression of Wnt5a and β-Catenin increased in HBSMCs after PM2.5 exposure, and the expression of the TGF-β1, CyclinD1 and C-myc mRNAs was upregulated in HBSMCs. TGF-β1 is secreted by airway epithelial cells, airway smooth muscle cells and eosinophils and promotes matrix protein production, contractile protein expression and proliferation of airway smooth muscle cells and airway fibroblasts.31 Cyclin D1 and c-myc are the core transcription factors and target genes of the Wnt/β-catenin signaling pathway.32 C-myc and CyclinD1 are widely involved in cell and tissue development and are key downstream effectors of cell proliferation.33,34 In addition, Wnt5a and β-catenin expression were upregulated in the lung tissues of mice exposed to PM2.5. Dysregulation of Wnt5a signaling has been observed in many lung diseases, including COPD, idiopathic pulmonary fibrosis (IPF) and asthma. Fibroblast-derived Wnt5a levels are increased in COPD models and specimens from patients with COPD.14 Very recently, a study indicated that smooth muscle-derived Wnt5a induced inflammatory cytokines and the α-SMA expression in the airway smooth muscle in asthma, resulting in increased airway wall inflammation and remodeling.35 Combined with previous reports, the relationship between the Wnt5a/β-catenin signaling pathway and PM2.5-associated airway remodeling was also established in our study.
PDGFRβ is a cell surface tyrosine kinase receptor that transduces extracellular signals into intercellular regions and promotes cell proliferation, survival and migration.15 Tenascin C is a large hexameric, multimodal extracellular matrix protein that binds to cell surface receptors, ECM proteins, soluble factors or pathogens to regulate cell adhesion, migration, proliferation and differentiation.36,37 In a chronic asthma model, blockade of the Wnt/β-catenin signaling pathway inhibited airway remodeling, such as subepithelial fibrosis and smooth muscle proliferation, by downregulating TGF-β1 and tenascin C/PDGFRβ expression.38 Consistent with previous studies, we also detected that PM2.5 induced the expression of the PDGFRβ and tenascin C proteins in the lung tissue and in HBSMCs. Therefore, we speculate that the Wnt5a/β-Catenin signaling pathway is probably involved in PM2.5-induced airway remodeling by regulating the tenascin C/PDGFRβ pathway.
We applied the Wnt5a-specific antagonist BOX5 to block endogenous Wnt5a signaling in mice and HBSMCs as a method to further investigate the mechanisms by which the Wnt5a/β-Catenin signaling pathway may be involved in PM2.5–associated airway remodeling in COPD. Our current data showed that BOX5 did not change basal Wnt5a expression, while BOX5 alleviated PM2.5-induced airway wall thickening and the smooth muscle layer thickness of mice, attenuated PM2.5-induced HBSMC proliferation. Wnt, tenascin C and PDGFR expression are increased in adults with smooth muscle-related lung disease.39 Administration of a β-Catenin siRNA led to a significant decrease in PDGFRβ expression. Moreover, Wnt signaling regulates PDGFRβ in part by directly regulating Tnc transcription to promote SMC precursor proliferation and adult lung disease development.16 This study defines the Wnt/Tnc/PDGFRβ signaling axis, which is essential for lung smooth muscle development and disease progression, and finds that this signaling axis is upregulated in both mice and human models of asthma and pulmonary arterial hypertension. Consistently, we also found that BOX5 decreased β-Catenin activation and downregulated Tenascin C and PDGFRβ protein expressions in both in vivo and in vitro, indicating that the Wnt5a/β-Catenin signaling pathway predominantly participates in airway remodeling caused by PM2.5 in COPD models.
Limitations
The limitations of our study could not be ignored, primary human bronchial smooth muscle cells should ideally be used for experimental in vitro studies; however, because of technical limitations for culturing primary cells, we used the human bronchial smooth muscle cell line, and cannot exclude the possibility of differences due to altered sensitivity.
Conclusions
In summary, we investigated the possible role of PM2.5 in COPD by focusing on the airway remodeling mechanism and showed that PM2.5 induced the upregulation of Wnt5a, which in turn promoted β-Catenin activation and increased the expression of Tenascin C and PDGFRβ to promote HBSMC proliferation, contributing to airway remodeling in vivo and in vitro. These results provide evidence that treatments targeting Wnt5a/β-Catenin signaling may be a new therapeutic approach for PM2.5-associated airway remodeling in individuals with COPD.
Acknowledgments
This study was supported by the Natural Science Foundation of Guangdong, China (2020A1515010264), Science and Technology Program of Guangzhou, China (202002030080 and 202102080045), National Natural Science Foundation of China (81900044, 82000044 and 82000045), and an independent project of the State Key Laboratory of Respiratory Diseases (CN)-SKLRD-QN-201913.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barnes PJ, Burney PG, Silverman EK, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076. doi:10.1038/nrdp.2015.76
2. Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. doi:10.1056/NEJMoa1411532
3. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/S0140-6736(17)31222-9
4. Barnes PJ. Small airway fibrosis in COPD. Int J Biochem Cell Biol. 2019;116:105598. doi:10.1016/j.biocel.2019.105598
5. Black PN, Ching PS, Beaumont B, Ranasinghe S, Taylor G, Merrilees MJ. Changes in elastic fibres in the small airways and alveoli in COPD. Eur Respir J. 2008;31(5):998–1004. doi:10.1183/09031936.00017207
6. Hollins F, Sutcliffe A, Gomez E, et al. Airway smooth muscle NOX4 is upregulated and modulates ROS generation in COPD. Respir Res. 2016;17(1):84. doi:10.1186/s12931-016-0403-y
7. Kim Y, Seo J, Kim JY, Lee JY, Kim H, Kim BM. Characterization of PM(2.5) and identification of transported secondary and biomass burning contribution in Seoul, Korea. Environ Sci Pollut Res Int. 2018;25(5):4330–4343. doi:10.1007/s11356-017-0772-x
8. Schultz ES, Litonjua AA, Melen E. Effects of long-term exposure to traffic-related air pollution on lung function in children. Curr Allergy Asthma Rep. 2017;17(6):41. doi:10.1007/s11882-017-0709-y
9. Zhao B, Zheng H, Wang S, et al. Change in household fuels dominates the decrease in PM2.5 exposure and premature mortality in China in 2005–2015. Proc Natl Acad Sci U S A. 2018;115(49):12401–12406. doi:10.1073/pnas.1812955115
10. Zou W, Wang X, Hong W, et al. PM2.5 induces the expression of inflammatory cytokines via the Wnt5a/Ror2 pathway in human bronchial epithelial cells. Int J Chron Obstruct Pulmon Dis. 2020;15:2653–2662. doi:10.2147/COPD.S270762
11. Oishi I, Suzuki H, Onishi N, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8(7):645–654. doi:10.1046/j.1365-2443.2003.00662.x
12. Mikels AJ, Nusse R, Arias AM. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi:10.1371/journal.pbio.0040115
13. Koopmans T, Kumawat K, Halayko AJ, Gosens R. Regulation of actin dynamics by WNT-5A: implications for human airway smooth muscle contraction. Sci Rep. 2016;6:30676. doi:10.1038/srep30676
14. Baarsma HA, Skronska-Wasek W, Mutze K, et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J Exp Med. 2017;214(1):143–163. doi:10.1084/jem.20160675
15. Mellgren AM, Smith CL, Olsen GS, et al. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ Res. 2008;103(12):1393–1401. doi:10.1161/CIRCRESAHA.108.176768
16. Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119(9):2538–2549. doi:10.1172/JCI38079
17. He F, Liao B, Pu J, et al. Exposure to ambient particulate matter induced COPD in a rat model and a description of the underlying mechanism. Sci Rep. 2017;7:45666. doi:10.1038/srep45666
18. Bracke KR, D’Hulst AI, Maes T, et al. Cigarette smoke-induced pulmonary inflammation, but not airway remodelling, is attenuated in chemokine receptor 5-deficient mice. Clin Exp Allerg. 2007;37(10):1467–1479. doi:10.1111/j.1365-2222.2007.02808.x
19. Xu Z, Li Z, Liao Z, et al. PM2.5 induced pulmonary fibrosis in vivo and in vitro. Ecotoxicol Environ Saf. 2019;171:112–121. doi:10.1016/j.ecoenv.2018.12.061
20. He S, Chen M, Lin X, Lv Z, Liang R, Huang L. Triptolide inhibits PDGF-induced proliferation of ASMCs through G0/G1 cell cycle arrest and suppression of the AKT/NF-κB/cyclinD1 signaling pathway. Eur J Pharmacol. 2020;867:172811. doi:10.1016/j.ejphar.2019.172811
21. Wang Z, Zhao J, Wang T, Du X, Xie J. Fine-particulate matter aggravates cigarette smoke extract-induced airway inflammation via Wnt5a-ERK pathway in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:979–994. doi:10.2147/COPD.S195794
22. Osornio-Vargas AR, Bonner JC, Alfaro-Moreno E, et al. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect. 2003;111(10):1289–1293. doi:10.1289/ehp.5913
23. Churg A, Brauer M, Del Carmen Avila-Casado M, Fortoul TI, Wright JL. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ Health Perspect. 2003;111(5):714–718. doi:10.1289/ehp.6042
24. Horne BD, Joy EA, Hofmann MG, et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med. 2018;198(6):759–766. doi:10.1164/rccm.201709-1883OC
25. Guo W, Tan Y, Yin X, Sun Z. Impact of PM(2.5) on second birth intentions of China’s floating population in a low fertility context. Int J Environ Res Public Health. 2019;16(21):4293. doi:10.3390/ijerph16214293
26. Mirabelli MC, Boehmer TK, Damon SA, et al. Air quality awareness among U.S. adults with respiratory and heart disease. Am J Prev Med. 2018;54(5):679–687. doi:10.1016/j.amepre.2018.01.037
27. Li P, Xin J, Wang Y, et al. The acute effects of fine particles on respiratory mortality and morbidity in Beijing, 2004–2009. Environ Sci Pollut Res Int. 2013;20(9):6433–6444. doi:10.1007/s11356-013-1688-8
28. Zhao D, Zhou Y, Jiang C, Zhao Z, He F, Ran P. Small airway disease: a different phenotype of early stage COPD associated with biomass smoke exposure. Respirology (Carlton, Vic). 2018;23(2):198–205. doi:10.1111/resp.13176
29. Rivera RM, Cosio MG, Ghezzo H, Salazar M, Pérez-Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12(8):972–977.
30. Camp PG, Ramirez-Venegas A, Sansores RH, et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43(3):725–734. doi:10.1183/09031936.00206112
31. Januskevicius A, Vaitkiene S, Gosens R, et al. Eosinophils enhance WNT-5a and TGF-β1 genes expression in airway smooth muscle cells and promote their proliferation by increased extracellular matrix proteins production in asthma. BMC Pulm Med. 2016;16(1):94. doi:10.1186/s12890-016-0254-9
32. Jia XX, Zhu TT, Huang Y, Zeng XX, Zhang H, Zhang WX. Wnt/β-catenin signaling pathway regulates asthma airway remodeling by influencing the expression of c-Myc and cyclin D1 via the p38 MAPK-dependent pathway. Exp Ther Med. 2019;18(5):3431–3438. doi:10.3892/etm.2019.7991
33. Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast (Edinburgh, Scotland). 2006;15(6):718–727. doi:10.1016/j.breast.2006.02.005
34. Poli V, Fagnocchi L, Fasciani A, et al. MYC-driven epigenetic reprogramming favors the onset of tumorigenesis by inducing a stem cell-like state. Nat Commun. 2018;9(1):1024. doi:10.1038/s41467-018-03264-2
35. Koopmans T, Hesse L, Nawijn MC, et al. Smooth-muscle-derived WNT5A augments allergen-induced airway remodelling and Th2 type inflammation. Sci Rep. 2020;10(1):6754. doi:10.1038/s41598-020-63741-x
36. Chiquet-Ehrismann R. Tenascins. Int J Biochem Cell Biol. 2004;36(6):986–990. doi:10.1016/j.biocel.2003.12.002
37. Midwood KS, Chiquet M, Tucker RP, Orend G. Tenascin-C at a glance. J Cell Sci. 2016;129(23):4321–4327. doi:10.1242/jcs.190546
38. Kwak HJ, Park DW, Seo JY, et al. The Wnt/β-catenin signaling pathway regulates the development of airway remodeling in patients with asthma. Exp Mol Med. 2015;47(12):e198. doi:10.1038/emm.2015.91
39. Haczku A, Atochina EN, Tomer Y, et al. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol. 2001;25(1):45–50. doi:10.1165/ajrcmb.25.1.4391
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.