Back to Journals » Clinical Ophthalmology » Volume 14
Plume Sign in the Deturgescence of Macular Cysts – A Novel OCT Finding
Authors Nair U , Mohan A , Soman M
Received 11 December 2019
Accepted for publication 7 February 2020
Published 10 March 2020 Volume 2020:14 Pages 759—765
DOI https://doi.org/10.2147/OPTH.S241796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Unnikrishnan Nair, 1, 2 Ashwin Mohan, 1, 2 Manoj Soman 1, 2
1Department of Vitreo-Retina, Chaithanya Eye Hospital and Research Institute, Trivandrum, India; 2CITE, Trivandrum, India
Correspondence: Ashwin Mohan
Email [email protected]
Aim: To describe a novel OCT finding called “Plume sign” in macular cysts.
Methods: Case records of five cases were retrospectively reviewed. Their case history and imaging findings on spectral domain optical coherence tomography (SD-OCT, Spectralis, Heidelberg, Germany) have been described.
Results: We observed five cases which had a solitary cyst foveal or parafoveal in location, was unique due to the presence of a plume-shaped internal substance, was treated with topical NSAIDs and was associated with good visual outcomes. We observed three cardinal events; firstly, retinal thickening followed by the formation of a foveal or parafoveal solitary cyst. Secondly, a vertical expansion of the solitary cysts in an inverted flask-shaped configuration associated with splitting of the retinal layers suggested by a hyper-reflective line and/or a hyporeflective cleft in the outer nuclear layer. Thirdly and finally, a breach of the outer retina with involvement of the external limiting membrane (ELM) and ellipsoid zone (EZ) with an exit trail of proteinaceous material through the defect in the shape of a plume of smoke hyper-reflective on OCT followed by deturgescence of the cyst.
Conclusion: In conclusion, we describe the “plume” sign – a novel OCT finding is cysts and provide a possible pathogenic hypothesis.
Keywords: plume, CME, fibrin, vertical, expansion
Introduction
The pathology of cystoid macular oedema has been previously described and retinal vascular changes consisting mainly of endothelial cell abnormalities,1 the breakdown of the blood–retinal barrier and microinfarction2 and intracytoplasmic swelling (oedema) of the Müller (glial) cells3 have been assumed to be the primary causative events. Intercellular (extracellular) collections of fluid probably are late, end-stage results of the process that result from prolonged, excessive, intracellular oedema, cell death and disruption.3 While the central pathology would remain the same, it can be associated with a number of conditions like diabetic retinopathy,4 retinal vein occlusions5 and even choroidal melanomas.1 It has been treated with laser photocoagulation,6 intravitreal injection of triamcinolone acetonide,7 topical non-steroidal anti-inflammatory drugs8 and anti-VEGF agents like ranibizumab and aflibercept.4 In one study in eyes with diabetic retinopathy, they had a mean presenting visual acuity of 56 ETDRS letters (20/80 Snellen equivalent) which improved to 80 ETDRS letters (Snellen equivalent of 6/7.5) with multiple anti-VEGF injections,4 suggesting favourable visual outcomes; however, the visual outcomes differ for different associated conditions, a summary of which is beyond the scope of this article.
We observed a series of cases which had a solitary cyst subfoveal or parafoveal in location, was unique due to the presence of a plume-shaped internal substance, was treated with topical NSAIDs and was associated with good visual outcomes. In this case series, we aim to describe each case independently and attempt to describe a previously unreported pathological entity that we termed as the “plume” sign in cystoid macular oedema.
Methods
This was a retrospective chart review of five cases presenting to our outpatient department with the “Plume sign” which is defined as an irregular hyper-reflective substance present within a foveal or parafoveal cyst. The study followed the tenets of the Declaration of Helsinki and was approved by the institutional review board (Chaithanya Eye Hospital & Research institute – Institutional Ethics Committee). Written informed consent has been provided by the patients to have the case details and any accompanying images published. Each case is described below.
Results
Case 1 (Figure 1)
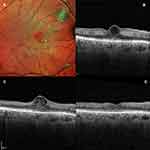
A 65-year-old diabetic male presented with a 1-week history of gradual painless decrease in vision in his right eye. He has been previously diagnosed with moderate non-proliferative diabetic retinopathy (NPDR) in both eyes. His corrected distance visual acuity (CDVA) was 20/200 in the right eye and 20/20 in the left eye on Snellen’s chart; anterior segment evaluation and intraocular pressure were unremarkable in both eyes. His retinal condition was stable bilaterally with moderate NPDR. The foveal reflex was altered in the right eye with the presence of cystoid macular oedema and surrounding retinal greying; the foveal reflex was normal in the left eye. An optical coherence tomography (SD-OCT, Heidelberg, Spectralis) showed the presence of a centre involving cyst that extended till the ILM anteriorly and stopped short of the ELM posteriorly (Figure 1). Inner retinal thinning was also noted and a provisional diagnosis of paracentral acute middle maculopathy (PAMM) was made. The patient was started on topical non-steroidal anti–inflammatory drugs (NSAIDs, Nepalact, Nepafenac 0.1% one drop three times daily) and a thorough systemic workup was ordered which was unremarkable. A follow-up SD-OCT 1 month later showed the cyst enlarging to an inverted flask-shaped vertical cyst involving the foveal centre that spanned the vertical extent of the retina. Importantly, the external limiting membrane (ELM) and the ellipsoid zone (EZ) seemed to be breached and there was a “plume”-shaped hyperreflective material present inside the cyst. The CDVA remained unchanged at this visit. No change was advised in management and a repeat SD-OCT 1 month later showed disappearance of the cyst with foveal flattening, inner retinal layer thinning and defective reformation of the ELM and the EZ; the CDVA had improved to 20/20.
Case 2 (Figure 2)
A 50-year-old female presented with complaints of eye strain for the last 3 days. Her past medical history included dyslipidaemia and thyroid disease for the last 10 years well controlled with treatment. Her CDVA was 20/20 in both eyes and her anterior segment exam and intraocular pressures were unremarkable. The fundus examination of her right eye was normal, her left eye had superficial flame-shaped haemorrhages with macular oedema suggestive of a macular superotemporal tributary retinal vein occlusion. Her SD-OCT showed a similar balloon-shaped cyst close to the foveal centre that traversed the vertical length of the retina, breaching the ELM and EZ and filled with plume-shaped hyper-reflective material. Since her visual acuity was 20/20 she was started on topical NSAIDs (Nepalact, Nepafenac 0.1% one drop three times daily). She was followed up with serial SD-OCT scans which showed a decrease in the size of the cyst and disappearance of the plume 10 days later, disappearance of the cyst with residual parafoveal macular oedema 2 months later and complete resolution with normal foveal contour and no residual signs of the cyst or the ELM/EZ breach 8 months later.
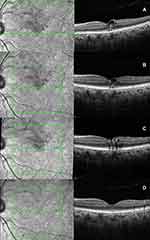 |
Figure 2 (A) SD-OCT of Case 2 at presentation showing the balloon-shaped foveal cyst with the plume sign; (B–D) follow-up SD-OCTs demonstrating complete resolution. |
Case 3 (Figure 3)
A 58-year-old female presented with painless gradual decrease in vision in the right eye over the last 2 weeks. She is a known diabetic with a 10-year history on oral hypoglycaemic agents and insulin. Her previous visit 10 months back was remarkable for bilateral moderate NPDR with no macular oedema and an SD-OCT of her right eye showed minimal retinal thickening with early alteration of the foveal contour. On examination, her CDVA was 20/40 and 20/20 in the right and left eyes, respectively, anterior segment evaluation and intraocular pressures were unremarkable. Her fundus examination showed moderate NPDR in both eyes, the foveal reflex was altered in the right eye. On SD-OCT, a balloon-shaped cyst was noted temporal to the foveal centre that corresponded to the area of retinal thickening observed 10 months earlier, a plume-shaped hyper-reflective material was seen that almost completely filled the cyst and a fine vertical hyper-reflective line was seen that extended from the cyst through the ELM and the EZ was may be suggestive of a narrow occult defect. Similar to the previous two cases, she was also started on topical NSAIDs (Nepalact, Nepafenac 0.1% one drop three times daily); however, she did not return for a review.
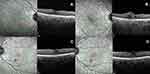 |
Figure 3 (A,B) SD-OCTs of Case 3 and (C,D) SD-OCTs of Case 4 showing the precursor OCTs (A, C) and the development of the plume (B,D). |
Case 4 (Figure 3)
A 52-year-old male presented with gradual painless blurring of vision in both eyes over the last few months. He was a known diabetic on treatment. On examination, his CDVA was 20/20 in both eyes and anterior segment exam and intraocular pressures were within normal limits. The right eye showed the presence of a retinal artery microaneurysm along the inferior arcade, mild NPDR and a normal foveal reflex; the left eye showed moderate NPDR with cystoid macular oedema. SD-OCT confirmed with findings with the presence of a solitary optically empty foveal cyst that extended vertically from the internal limiting membrane (ILM) to the outer nuclear layer. The patient was started on topical NSAIDs (Nepalact, Nepafenac 0.1% one drop three times daily). A follow-up visit 6 weeks later his CDVA remained stable at 20/20, while the SD-OCT showed vertical enlargement of the cyst with a fine vertical hyper-reflective line which extended from the cyst through the ELM and the EZ which may be suggestive of a narrow occult defect and the presence of a plume-shaped hyper-reflective material that partially filled the cyst. The patient was asked to continue the same treatment and has not yet returned for a review.
Case 5 (Figure 4)
A 59-year-old male, a known diabetic for the last 15 years on insulin, hypertensive and dyslipidaemic for the last 5 years on treatment presented for a change of glasses. On examination, his CDVA was 20/20 in both eyes, his anterior examination and intraocular pressures were within normal limits. He had moderate NPDR bilaterally with an altered foveal reflex in his left eye. An SD-OCT showed the presence of retinal thickening, a balloon-shaped cyst involving the foveal centre, bounded superiorly by the ganglion cell layer and extending inferiorly through a breach in the ELM and the EZ into a small subfoveal neurosensory retinal detachment (NSD). There was the presence of a plume-shaped hyper-reflective material that partially filled the cystic cavity. He was also started on topical NSAIDs (Nepalact, Nepafenac 0.1% one drop three times daily); however, he did not return for a review.
 |
Figure 4 SD-OCT of Case 5 showing the cyst with plume communicating with the neurosensory detachment. |
Discussion
In summary, we saw five cases with a common pathological finding, namely, a foveal or parafoveal inverted flask-shaped vertically oriented cyst that was associated with a narrow breach in the ELM and the EZ with possible communication with the subretinal space and the presence of a plume-shaped hyper-reflective material partially or completely filling the cyst.
In 4 of the 5 cases, there is a documentation of the SD-OCT before the appearance of the plume sign. In case 3, there is only thickening without the presence of the vertical split. In cases 1, 2 and 4, a cyst is present and is associated with a fine vertical hyper-reflective line that extends from the cyst to the RPE suggestive of a breach in ELM and EZ. We presume that this is a vertical split or is the precursor to a vertical split in the retina that allows communication of the cyst to the subretinal space. In cases 1 and 2, we can further see the hyper-reflective line progress to a hyporeflective cleft filled with hyper-reflective substance which is possibly the exit trail of proteinaceous material. It may thus be hypothesised that the initial event is possibly intracellular oedema (case 3) which is followed by extracellular oedema that results in the formation of a cyst followed by a vertical split and enlargement of the cyst (cases 1, 2 & 4) with subsequent communication to the subretinal space via a hypo-reflective cleft filled with hyper-reflective substance (cases 1 & 2).
Turbid cysts or reflectivity within the cysts have been observed in DME and RVO.9 Horii et al suggested that reflective grey signal within cysts could represent high molecular weight proteins, fibrin, hyaline material, macrophages or erythrocytes.10 Spaide et al observed that as RBCs have Brownian movement, these intracystic structures may be picked up even on OCTA.11 We believe that the plume may be proteinaceous material originating from the retinal vessels. In eyes with altered inner retinal barrier functions, proteins are released through transcytosis and caveola-based mechanisms.12 The glymphatic pathway is driven by aquaporin 4 located in Z Muller fibres and is seen in the retinal layers supplied by the deep capillary plexus; the outer nuclear layer cysts too predominate in these retinal layers. In contrast, turbid cysts are rarely seen in eyes with Irvin Gass syndrome which predominantly involves the inner nuclear layers.13 None of our eyes in our series were primarily Irvine Gass CME eyes.
Presence of outer retinal communications in eyes with CME communicating to NSD in DME has been reported with demonstrated communications ranging from ≤190 um to >225 um which included ELM and photoreceptor layer in some cases.14 These defects might occur because of the rupture of ELM in outer walls of cysts. The size of communication also correlated positively with the presence of subretinal hyper-reflective dots; such hyper-reflective dots are seen in Case 5 (Figure 4), Case 2 (Figure 2) and Case 3 (Figure 3). The authors speculated that these defects may represent a path for the flow of fluid and proteins from intraretinal cysts or the outer layers of oedematous retina into the subretinal space. We also found such defects in these plume eyes at the level of ELM. As observed in our cases, the proteinaceous plume flows into these defects. Ota et al had reported that hyper-reflective dots represent hard exudates and may flow into the subretinal space in a larger quantity if the size of the communication is larger.15 Case 5 with SRF in our series probably thus represents a larger breach.
Also it is known that proteins exit the retina through ELM breaches.13,16 ELM breach is believed to favour large stoke radius protein movement from neural retina to subretinal space. Moreover, the osmotic gradient also propels these proteinacous materials towards the choroid. The concept of expanding cyst as seen in our cases is very critical to the plume trail. Retinal cysts though a misnomer (as no lining wall) could just Muller cell swelling or necrosis especially in the Henles layer and is still intracellular at this stage. As the cystic swelling of the Muller cells predominantly involves the central and Z fibres,17 these vertically expanding cysts are located centrally (cases 1 & 3) or parafoveally (cases 2 & 4). With expansion of the cyst, it is no longer intracellular but becomes extracellular and osmotic gradient works better in extracellular fluid. Once the communication is established, the pump of the RPE also possibly results in efficient absorption of the cystic fluid as seen by dramatic resolution of the cyst in our cases. As the drainage of this fluid happens through the ELM breach and the RPE pump favouring it, the protein plume disappears in this trail.
Though ELM breach is a bad visual prognostic sign18 especially in chronic cystoid retinal oedema, in this case series, it is an acute and temporary phenomenon which closes as soon as the fluid drains thereby not affecting the visual outcomes.
Of our 5 patients, 4 had a good presenting corrected distance visual acuity (CDVA). Cases 2, 4 & 5 had 20/20, Case 3 had 20/40. One case (Case 1) had a CDVA of 20/200; however, it was diagnosed as PAMM. Hence, none of the five cases were ideal candidates for intravitreal anti-VEGF therapy. Topical NSAIDs have a favourable risk to benefit ratio and a possible placebo effect. Hence, all the patients were started on topical NSAIDs. It must be stated that the role of NSAIDs is questionable in the resorption process and our observations could well be the natural history of some cysts and NSAIDs could be acting as a placebo. This study is not designed to answer questions on the possible mechanisms of action of topical NSAIDs and this report does not claim that NSAIDs should be used in the management of eyes with the plume sign.
We did not have fluorescein angiographic (FFA) documentation of any of the cases. The theories about the course of events that lead to the plume and its drainage and the cyst absorption need to be further confirmed by a larger case series, FFA documentation and a regular follow-up.
In conclusion, we describe three cardinal events; firstly, retinal thickening followed by the formation of a foveal or parafoveal solitary cyst. Secondly, a vertical expansion of the solitary cysts in an inverted flask-shaped configuration associated with splitting of the retinal layers suggested by a hyper-reflective line and/or a hyporeflective cleft in the outer nuclear layer. Thirdly and finally, a breach of the outer retina with involvement of the external limiting membrane (ELM) and ellipsoid zone (EZ) with an exit trail of proteinaceous material through the defect in the shape of a plume of smoke hyper-reflective on OCT followed by deturgescence of the cyst.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol. 1981;92:466–481. doi:10.1016/0002-9394(81)90638-3
2. Tso M. Pathology of cystoid macular edema. Ophthalmology. 1982;89:902–915. doi:10.1016/S0161-6420(82)34698-9
3. Yanoff M, Fine BS, Brucker AJ, et al. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984;28:505–511. doi:10.1016/0039-6257(84)90233-9
4. Diabetic Retinopathy Clinical Research N, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. doi:10.1056/NEJMoa1414264
5. Clarkson JG. Central vein occlusion study: photographic protocol and early natural history. Trans Am Ophthalmol Soc. 1994;92:203–205.
6. Group ETDRSR. Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Arch Ophthalmol. 1985;103:1796–1806. doi:10.1001/archopht.1985.01050120030015
7. Elman MJ, Aiello LP, Beck RW, Diabetic Retinopathy Clinical Research N, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 117;2010:1064–1077e1035. doi:10.1016/j.ophtha.2010.02.031
8. Hariprasad SM, Akduman L, Clever JA, et al. Treatment of cystoid macular edema with the new-generation NSAID nepafenac 0.1%. Clin Ophthalmol. 2009;3:147–154. doi:10.2147/OPTH
9. Liang MC, Vora RA, Duker JS, et al. Solid-appearing retinal cysts in diabetic macular edema: a novel optical coherence tomography finding. Retin Cases Brief Rep. 2013;7:255–258. doi:10.1097/ICB.0b013e31828eef49
10. Horii T, Murakami T, Nishijima K, et al. Relationship between fluorescein pooling and optical coherence tomographic reflectivity of cystoid spaces in diabetic macular edema. Ophthalmology. 2012;119:1047–1055. doi:10.1016/j.ophtha.2011.10.030
11. Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35:2163–2180. doi:10.1097/IAE.0000000000000765
12. Anderson RG. Transendothelial movement and caveolae. Nat Biotechnol. 2008;26:380–382. doi:10.1038/nbt0408-380
13. Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi:10.1016/j.preteyeres.2015.05.003
14. Gupta A, Raman R, Mohana KP, et al. Communications between intraretinal and subretinal space on optical coherence tomography of neurosensory retinal detachment in diabetic macular edema. Oman J Ophthalmol. 2013;6:183–188. doi:10.4103/0974-620X.122275
15. Ota M, Nishijima K, Sakamoto A, et al. Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology. 2010;117:1996–2002. doi:10.1016/j.ophtha.2010.06.019
16. Bunt-Milam AH, Saari JC, Klock IB, et al. Zonulae adherentes pore size in the external limiting membrane of the rabbit retina. Invest Ophthalmol Vis Sci. 1985;26:1377–1380.
17. Spaide RF, Lee JK, Klancnik JM, et al. Optical coherence tomography of branch retinal vein occlusion. Retina. 2003;23:343–347. doi:10.1097/00006982-200306000-00009
18. Noma H, Funatsu H, Mimura T, et al. Visual function and serous retinal detachment in patients with branch retinal vein occlusion and macular edema: a case series. BMC Ophthalmol. 2011;11:29. doi:10.1186/1471-2415-11-29
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.