Back to Journals » Drug Design, Development and Therapy » Volume 16
Pleiotropic Effects of Ticagrelor: Influence on CYP4F2 Gene and Protein Expression in HUVEC and HepG2, and Escherichia coli Bacterial Survival
Authors Meskauskaite U, Andruskeviciute S, Ciapiene I, Giedraitiene A, Lesauskaite V , Tatarunas V
Received 11 January 2022
Accepted for publication 5 July 2022
Published 4 August 2022 Volume 2022:16 Pages 2559—2568
DOI https://doi.org/10.2147/DDDT.S357985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Ugne Meskauskaite,1 Silvija Andruskeviciute,1 Ieva Ciapiene,1 Agne Giedraitiene,2 Vaiva Lesauskaite,1 Vacis Tatarunas1
1Institute of Cardiology, Lithuanian University of Health Sciences, Kaunas, Lithuania; 2Institute of Microbiology and Virology, Lithuanian University of Health Sciences, Kaunas, Lithuania
Correspondence: Vacis Tatarunas, Institute of Cardiology, Lithuanian University of Health Sciences, Kaunas, Lithuania, Tel +370 37302874, Email [email protected]
Background: Antiplatelet drugs, such as ticagrelor, which target platelet P2Y12 receptors, are used for prevention of ischemic heart disease. Ticagrelor is also known to have pleiotropic effects of unknown mechanisms. Ticagrelor could influence the expression of molecules involved in resolution of inflammation. This study aimed to investigate if ticagrelor could change the expression of CYP4F2 and its encoded protein concentration and, additionally, to determine ticagrelor possible antibacterial activity against gram-negative bacteria.
Methods: CYP4F2 expression was determined in HUVEC and HepG2 cell lines by qPCR. CYP4F2 protein concentration was determined by ELISA. Antibiotic susceptibility testing was performed using a disc diffusion method.
Results: Ticagrelor was observed to reduce the expression of CYP4F2 in HUVEC and HepG2 cell lines. It also reduced CYP4F2 protein levels in HUVEC cells. Ticagrelor had no bactericidal activity against gram-negative third generation cephalosporin resistant E. coli.
Conclusion: Ticagrelor reduced CYP4F2 protein concentration in HUVEC, and CYP4F2 expression in HUVEC and HepG2 cells, but had no effect on third-generation cephalosporin-resistant E. coli strains.
Keywords: ticagrelor, HUVEC, HepG2, CYP4F2 gene, CYP4F2 protein, antimicrobial effect, ischemic heart disease
Introduction
According to the World Health Organization, ischemic heart disease is one of the most common causes of illness and death in the world. More than 9 million deaths in 2016 were attributed to this disease.1 Although mortality rates from ischemic heart disease are decreasing globally due to prevention programs and medical progress in improving diagnostic tools and treatment strategies, heart disease risk factors are becoming more prevalent. “Western lifestyle”, overweight and obesity, physical inactivity, stress, and an unhealthy diet led to higher prevalence of cardiovascular risk factors.2 Recently, COVID-19 has also been observed to promote the development of ischemic cardiovascular disease.3
Atherosclerosis is the main cause of ischemic heart disease. It is characterized by atherosclerotic plaque formation in the arterial walls, narrowing the lumen of the blood vessel and resulting in reduced oxygen supply to the heart muscle which can develop into ischemic heart disease. The atherosclerotic plaque might rupture then cause arterial thrombosis and occlusion of the vessel, dramatically reducing the blood supply to the myocardium followed by unstable angina.4 Endothelial damage and dysfunction were observed in COVID-19 patients additionally to microthrombus formation which could result in ischemia and end-organ damage.5
Platelets are the key elements in arterial thrombosis, thus, drugs, affecting platelet aggregation are used to prevent arterial thrombosis. Dual antiplatelet therapy with aspirin and platelet P2Y12 receptor blocker (ticagrelor, prasugrel, clopidogrel) is usually recommended to prevent arterial thrombus formation in clinical practice.6,7 P2Y12 receptors are located on the surface of platelets which can be activated by aggregation agonist adenosine diphosphate (ADP). P2Y12 is a key component in platelet activation and may play a significant role in the development of arterial thrombosis. Adenosine diphosphate (P2Y12) receptor blockers (such as ticagrelor, clopidogrel, and prasugrel) are usually used in combination with aspirin. However, most recent data showed that ticagrelor could be recommended and as a single antiplatelet agent.8 Ticagrelor binds directly and reversibly to P2Y12 receptors on platelet membranes, thereby blocking ADP binding and preventing the induction of platelet aggregation.9 Metabolic activation of ticagrelor is not necessary for its antiplatelet function. Ticagrelor is extensively metabolized by enzymes of the hepatic cytochrome P450 (CYP450) family, and AR-C124910XX is its only known active metabolite.10
Ticagrelor may affect not only platelets, but also endothelial cells on which surface P2Y12 receptors are present, eg, vascular smooth muscle cells, leukocytes, macrophages, osteoblasts, microglial, and dendritic cells. However, there is a lack of information about possible effects of ticagrelor on other cell types apart from platelets.11–13 Ticagrelor, to its antiplatelet properties, has also shown to have pleiotropic effects, such as erythrocyte adenosine reabsorption inhibition, stimulation of ATP secretion from erythrocytes, and atherosclerotic plaque stabilization.12,14 A recent study observed a new pleiotropic effect of ticagrelor. Lancellotti et al15 determined that ticagrelor and its active metabolite (AR-C124910XX) have an antibacterial effect on antibiotic-resistant gram-positive bacteria. However, the antibacterial properties of ticagrelor and its metabolite have not been extensively studied, thus the mechanisms of most ticagrelor pleiotropic effects including its possible antibacterial activity are unknown.
The influence of ticagrelor on humans may vary depending on the activity of cytochrome P450 (CYP450) enzymes. Cytochrome P450 is involved in metabolism of ticagrelor but also might metabolize arachidonic acid (AA). AA is a polyunsaturated fatty acid (PUFA) which is consumed in small amounts from a regular human diet, and it is found in cell membrane’s phospholipids.16 Arachidonic acid is vital for the synthesis of eicosanoids influencing numerous metabolic activities including platelet aggregation, inflammation, hemorrhages, vasoconstriction and vasodilation, blood pressure, and immune function. AA is known to be metabolized in various organs, such as the liver, kidneys, lungs, brain, heart, and blood vessels.17 AA metabolite 20-hydroxyeicosatetraenoic acid (20-HETE) plays a crucial role in the development of atherosclerosis, cardiovascular diseases, thrombosis, and hypertension due to its vasoconstrictive and pro-inflammatory properties.18 20-HETE is synthesized by CYP4A (CYP4A11, CYP4A22) and CYP4F (CYP4F2, CYP4F3) enzyme subfamilies in the human liver.19,20 CYP4F2 is the main enzyme acting in 20-HETE biosynthesis of the kidney’s and blood vessels’s smooth muscle cells.21,22 Another significant 20-HETE-synthesizing enzyme, CYP4A11, is highly expressed in the liver and kidneys. Elevated concentration of 20-HETE promotes tissue damage, endothelial dysfunction, inflammation, atherosclerosis, and hypertension, and increases risk of circulatory system diseases and arterial thrombosis.14,23,24 Inhibition of CYP4F2 and, by extension, 20-HETE biosynthesis could reduce the risk of developing heart disease, hypertension, stroke, and some types of cancer.25
Both CYP4F2 and CYP4A11 enzymes are responsible for the biosynthesis of the pro-inflammatory 20-HETE which contributes to the development and progression of ischemic heart disease. For this reason, we aimed to investigate the influence of ticagrelor on CYP4F2 enzyme concentration and CYP4F2 gene expression. The experiment was performed with the human umbilical vein endothelial cell (HUVEC) line as these endothelial cells express the P2Y12 receptor, the target of ticagrelor. To confirm ticagrelor influence on CYP4F2 enzyme, we determined CYP4F2 gene expression in hepatocellular carcinoma, HepG2, cell line. For the pleotropic role of ticagrelor confirmation besides CYP4F2 gene we decided to determine CYP4A11 gene expression. Previous studies showed that ticagrelor might have antibacterial activity,15 thus we investigated the effect of ticagrelor on Escherichia coli.
Materials and Methods
HUVEC Cell Culture
The HUVEC cell line was obtained from Gibco (Gibco by Life Technologies, USA). HUVEC cells were grown in 6-well cell culture plates (0.3x106 cells/well, Nunclon Delta Surface, Thermo Fisher Scientific, USA) in Medium 200 basal culture media (Gibco by Life Technologies, USA) supplemented with Large Vessel Endothelial Supplement (LVES 50X, Gibco by Life Technologies, USA). Cells were kept in a cell incubator at 37°C, 5% CO2, and under maintaining humidity conditions. Cell viability and concentration were determined using an automated cell counter (Countess II Automated Cell Counter, Invitrogen, USA) and 0.4% trypan blue (Gibco by Life Technologies, USA) dye.
Commercially purchased HepG2 cell line (ATCC, USA), obtained from the Institute for Digestive Research (Kaunas, Lithuania), was used. Cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco by Life Technologies, USA) supplemented with Fetal Bovine Serum (FBS) (Gibco by Life Technologies, USA). Environmental growth conditions for HepG2 were the same as for HUVECs.
Treatment with Ticagrelor
Ticagrelor stock solution was prepared by dissolving 90 mg ticagrelor tablets (AstraZeneca, UK) in ddH2O. Quantitative analysis of dissolved ticagrelor was determined by using UPLC-QTOF-MS method. Ticagrelor solutions of different concentrations (0.5 μM, 1 μM, 2 μM, 3 μM, 5 μM) were prepared by diluting the calculated amount of ticagrelor stock solution with saline (0.9% NaCl). HUVECs were incubated with 10 μL of each ticagrelor solution (0.5 μM, 1 μM, 2 μM, 3 μM, 5 μM) for 24 hours (Figure 1), thus the final concentration of ticagrelor in HUVECs medium was obtained, respectively, 2.5 nM, 5 nM, 10 nM, 15 nM, and 25 nM. Cells from passages 3 to 7 were actively proliferating when samples were harvested and analyzed.
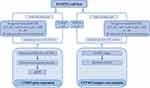 |
Figure 1 Experimental set-up. |
RNA Extraction, Reverse Transcription Reaction, and Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was extracted from HUVEC cells after treatment with ticagrelor solution using mirVana miRNA Isolation Kit (Invitrogen, USA), and reverse transcription was carried out with a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. The expression of human CYP4F2 and CYP4A11 genes were quantified by quantitative PCR (qPCR) using Maxima SYBR Green/ROX qPCR Master Mix 2X (Thermo Fisher Scientific, USA) on real time thermal cycler ABI 7900HT (Applied Biosystems, USA) (Figure 1). qPCR reactions were performed in triplicate. Sequences of the primers and thermal cycling program parameters used in the qPCR are shown in Tables 1 and 2, respectively. The expression of CYP4F2 and CYP4A11 genes was normalized to human TFRC gene using the ΔCt method. The results were evaluated using the 2−∆∆Ct method.
 |
Table 1 qPCR Primers Used for Gene Expression Evaluation |
 |
Table 2 qPCR Program |
ELISA
Sandwich enzyme immunoassay (Enzyme-linked Immunosorbent Assay Kit for Cytochrome P450 4F2 (CYP4F2), Cloud-Clone Corp., USA) was used to quantify cytochrome P450 4F2 (CYP4F2) in HUVEC cell lysates (Figure 1). The assay was carried out in accordance with the instructions of the manufacturer. The concentration of CYP4F2 was determined by measuring optical density at 450 nm using a microplate reader (Stat Fax 4200, Awareness Technologies, USA) and performing the calculations by using a standard calibration curve.
Antibiotic Susceptibility Testing
The antibiotic susceptibility testing of three third generation cephalosporins, cefotaxime, ceftazidime, and cefepime, resistant Escherichia coli strains (12–7010, 12–12,159, and N-11044.1) was performed using the standard disc diffusion method on Mueller-Hinton agar (MH) with or without ticagrelor (final ticagrelor concentration in MH agar was 0.5 μM) (Figure 2). 0.5 McFarland standard bacterial suspension was prepared from an overnight culture of E. coli according to EUCAST recommendations.26 Using a sterile swab, standardized E. coli inoculum were spread on both plain MH agar plates and plates containing ticagrelor (0.5 μM). Three antibiotic discs of third generation cephalosporins (Oxoid, UK) were placed equidistant from each other on the agar plates which were incubated at 35±2°C for 24 hours. The antibiotics used in the study are listed in Table 3. Inhibition zone diameters were measured, and the results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines.27 A reference strain of Escherichia coli ATCC 25922 was used as a quality control for antibiotic susceptibility testing.
 |
Table 3 Antibiotics Used in the Antibiotic Susceptibility Testing |
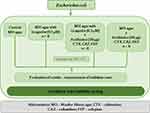 |
Figure 2 Experimental design of antibiotic susceptibility test. |
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics V26 (IBM Corp., USA) and GraphPad Prism V8 (La Jolla, USA) software. Differences between the two independent groups were evaluated using a nonparametric Mann Whitney U-test, and p<0.05 was considered to indicate a statistically significant difference.
Results
CYP4F2 Gene Expression
CYP4F2 expression was determined to be reduced in HUVEC cells treated with ticagrelor (1 μM concentration and higher) in comparison to untreated control cells, as shown in Table 4. Ticagrelor influence on CYP4F2 checked using HepG2 cell line, the results showed a tendency of CYP4F2 expression reduction (1 μM concentration and higher) presented in Table 5.
 |
Table 4 CYP4F2 Expression in HUVEC Cells After Treatment with Ticagrelor |
 |
Table 5 CYP4F2 Expression in HepG2 Cells After Treatment with Ticagrelor |
Quantification of CYP4F2 Protein
Significantly lower concentration of CYP4F2 was determined in ticagrelor treated HUVEC cells in comparison to untreated cells (Table 6).
 |
Table 6 CYP4F2 Protein Concentration in HUVEC Cells After Treatment with Ticagrelor |
CYP4A11 Gene Expression
CYP4A11 gene was not expressed in HUVEC cells. HepG2 cells treated with ticagrelor showed a tendency of CYP4F2 expression reduction (1 μM concentration and higher) in comparison to untreated control cells (Table 7).
 |
Table 7 CYP4A11 Expression in HepG2 Cells After Treatment with Ticagrelor |
Antibiotic Susceptibility Test
Ticagrelor treatment had no effect on third-generation cephalosporin-resistant E. coli strains.
Discussion
The results of this study demonstrated that ticagrelor reduced CYP4F2 expression in HUVEC and HepG2 cells, additionally CYP4F2 protein concentration in HUVEC cell cultures. Possibly, ticagrelor reduced CYP4A11 expression in HepG2, but HUVEC cells did not show any CYP4A11 expression. The antimicrobial effect of 0.5 μM ticagrelor on E. coli was not detected.
Ticagrelor Can Change CYP4F2 Expression and Concentration
Besides antiplatelet activity, ticagrelor is known to exhibit pleiotropic effects which could determine its clinical impact in decreasing mortality associated with cardiovascular events.28,29 There is ongoing research to determine other, currently unknown mechanisms of ticagrelor’s pleiotropic effects.14 One of the best-known pleiotropic effects of ticagrelor is vasodilatation, which is explained by prevention of cellular adenosine uptake which results in increased plasma levels of adenosine. Extracellular adenosine has many positive physiological effects, such as vasodilation, secretion of endothelial factors, also cardioprotection. Adenosine inhibits inflammation by reducing neutrophil adhesion in the vascular endothelium, increases coronary blood flow, and inhibits vascular smooth muscle contraction.12,14 Several clinical studies have suggested that ticagrelor has anti-atherosclerotic effects and might improve endothelial function.14,29–31 It could be determined by an increase of endothelial progenitor cells which are critical for vascular healing and endothelial regeneration.32 Additionally, anti-atherosclerotic effects were shown by ticagrelor mediated reduction of circulating epidermal growth factor which is associated with endothelial dysfunction, hypertension, restenosis, atherogenesis, and cardiac remodeling.33 Ticagrelor also reduces procoagulant tissue factor expression and activity in human aortic endothelial cells. This factor is essential for activation of the extrinsic pathway of the coagulation cascade that can lead to thrombus formation, thus, ticagrelor could reduce the risk of thrombosis by inhibiting the procoagulant tissue factor.34 In our study, ticagrelor was found to decrease CYP4F2 gene expression in both HUVEC and HepG2 cells and enzyme concentration in HUVEC cells. The results of the experiment performed with HUVEC cells, clearly demonstrated that ticagrelor reduces CYP4F2 expression in a dose-dependent manner. Moreover, ticagrelor potentially reduced CYP4A11 expression in HepG2 cells when expression of CYP4A11 in HUVEC cells was not determined. Some studies have shown that ticagrelor in vivo and in vitro could influence the expression of other genes which are involved in vascular inflammation.13,35 Ticagrelor was determined to inhibit the expression of certain inflammatory molecules (TNFα, IL-1, IL-6, IL-8, and IL-2) in the HUVEC cell line and reduce levels of proteins involved in the NF-κB pathway. It can influence the formation of atherosclerosis and is associated with acute coronary syndrome.36 Aquila et al37 study showed that patients with coronary artery and concomitant chronic obstructive pulmonary disease using ticagrelor had increased mRNA levels of SIRT1 and HES1. These proteins play a protective role in a process of inflammation and oxidative stress. Furthermore, treatment with ticagrelor was associated with elevated expression of the PON1 gene in mouse aorta and observed to increase serum activity of paraoxonase-1 (PON1), an anti-inflammatory and anti-atherosclerotic molecule.38 These studies suggest that ticagrelor could play a role in reducing vascular inflammation either by inhibiting pro-inflammatory molecule production or by increasing expression of anti-inflammatory factors. The results of our study showed that ticagrelor might reduce CYP4F2 and CYP4A11 activity. CYP4F2 and CYP4A11 are the main enzymes that participate in the production of 20-HETE.21 20-HETE have significant implications regarding the development of atherosclerosis and its cardiovascular complications. High concentrations of 20-HETE can result in endothelial dysfunction, it can also stimulate platelet aggregation and proinflammatory cytokine release.17,19,39,40 Previously it has been shown that a decrease of 20-HETE levels reduces vascular inflammation and hypertension.41 As CYP4F2 enzyme activity depends on inflammatory response, lower CYP4F2 activity might correspond to reduced inflammation during ticagrelor treatment.42
Ticagrelor Was Not Found to Possess Antibacterial Activity
Recent investigations have shown that ticagrelor possesses antibacterial activity and it can reduce the severity of bacterial infection and improve the survival of acute coronary syndrome and pneumonia patients.15,43,44 The study of Lancellotti et al15 revealed that it could act as a bactericidal agent against antibiotic resistant gram-positive bacteria, such as Staphylococcus aureus, S. epidermidis, and Enterococcus faecalis, but not against gram-negative Escherichia coli and Pseudomonas aeruginosa. We investigated a possible antibacterial activity of ticagrelor against gram-negative third generation cephalosporins resistant E. coli. The results from this study are consistent with previous research as ticagrelor was not effective against E. coli.15 Thus, it is uncertain if ticagrelor acts as an antibacterial agent against only certain bacteria which are gram-positive or if this property of the medication depends on other characteristics of bacteria. It is known that ticagrelor exerts bactericidal activity against gram-positive bacteria by disrupting their phospholipid membrane.45
Moreover, it has been reported that ticagrelor has in vitro dose-dependent bacterial activity against antibiotic gram-positive bacteria, but not against gram-negative E. coli and P. aeruginosa even when the ticagrelor concentrations were up to 80 µg/mL.15 Further experiments with more bacterial species are necessary to determine if the possible antibacterial activity of ticagrelor depends on the characteristics of the bacterial wall. It is also important to note that this study investigated the effects of ticagrelor only at one concentration (0.5 μM) which is not enough to conclusively state that ticagrelor had no antibacterial activity against E. coli.
We had also checked possible ticagrelor and third generation cephalosporins, cefotaxime, ceftazidime, and cefepime synergistic activity against E. coli as ticagrelor might also act as a sensitizing agent, by increasing the activity of other antibiotics.45 However, ticagrelor had no impact on the bactericidal activity of third generation cephalosporins, cefotaxime, ceftazidime, and cefepime. Therefore, additional experiments with varying concentrations of the medication should be carried out.
Limitations of the Experiment
The metabolite of AA breakdown, 20-HETE, is known to induce vascular inflammation and increase oxidative stress as well as induce vascular dysfunction.41,46 This study observed that ticagrelor could reduce CYP4F2 and CYP4A11 expression and CYP4F2 enzyme concentration which in turn could decrease the amount of pro-inflammatory 20-HETE. However, it is uncertain if the reduced CYP4F2 levels were also translated into lower 20-HETE concentration, as due to time limitations of the experiment, determination of metabolite using mass spectrometry was not performed. Detailed metabolite profiling will be performed in future studies.
Conclusion
This study showed that ticagrelor reduced CYP4F2 protein concentration in HUVEC, and CYP4F2 expression in HUVEC and HepG2 cells. Perhaps, ticagrelor reduced CYP4A11 expression in HepG2, but HUVEC cells did not show any CYP4A11 expression. The antimicrobial effect of ticagrelor (0.5 μM) on third-generation cephalosporin-resistant E. coli strains was not detected.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Research Council of Lithuania under Grant 09.3.3.-LMT-K-712-16-0187.
Disclosure
The authors declare no conflict of interest.
References
1. World Health Organization. The top 10 causes of death. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12:1–11. doi:10.1161/CIRCOUTCOMES.118.005375
3. Nishiga M, Wang DW, Han Y, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi:10.1038/s41569-020-0413-9
4. Turpie AGG, Esmon C. Venous and arterial thrombosis – pathogenesis and the rationale for anticoagulation. Thromb Haemost. 2011;105:586–596. doi:10.1160/TH10-10-0683
5. Evans PC, Rainger GE, Mason JC, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC council of basic cardiovascular science. Cardiovasc Res. 2020;116:2177–2184. doi:10.1093/cvr/cvaa230
6. Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113:340–345. doi:10.1172/JCI20986
7. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi:10.1093/eurheartj/ehx393
8. Valgimigli M, Mehran R, Franzone A, et al. Ticagrelor monotherapy versus dual-antiplatelet therapy after PCI: an individual patient-level meta-analysis. JACC Cardiovasc Interv. 2021;14:444–456. doi:10.1016/j.jcin.2020.11.046
9. Vidal SGM, Ruland S. Platelet antiaggregants in stroke prevention. Neurol Clin. 2013;31:633–657. doi:10.1016/j.ncl.2013.03.004
10. Adamski P, Buszko K, Sikora J, et al. Metabolism of ticagrelor in patients with acute coronary syndromes. Sci Rep. 2018;8:1–8. doi:10.1038/s41598-018-29619-9
11. Liverani E, Kilpatrick LE, Tsygankov AY, Kunapuli SP. The role of P2Y12 receptor and activated platelets during inflammation. Curr Drug Targets. 2014;15:720–728. doi:10.2174/1389450115666140519162133
12. Adamski P, Kozinski M, Ostrowska M, et al. Overview of pleiotropic effects of platelet P2Y12 receptor inhibitors. Thromb Haemost. 2014;112:224–242. doi:10.1160/TH13-11-0915
13. Ganbaatar B, Fukuda D, Salim HM, et al. Ticagrelor, a P2Y12 antagonist, attenuates vascular dysfunction and inhibits atherogenesis in apolipoprotein-E-deficient mice. Atherosclerosis. 2018;275:124–132. doi:10.1016/j.atherosclerosis.2018.05.053
14. Kubisa MJ, Jezewski MP, Gasecka A, et al. Ticagrelor – toward more efficient platelet inhibition and beyond. Ther Clin Risk Manag. 2018;14:129–140. doi:10.2147/TCRM.S152369
15. Lancellotti P, Musumeci L, Jasques N, et al. Antibacterial activity of ticagrelor in conventional antiplatelet dosages against antibiotic-resistant Gram-positive bacteria. JAMA Cardiol. 2019;4:596–599. doi:10.1001/jamacardio.2019.1189
16. Roberts MD, Iosia M, Kerksick C, et al. Effects of arachidonic acid supplementation on training adaptations in resistance-trained males. J Int Soc Sports Nutr. 2007;4:85–93. doi:10.1186/1550-2783-4-21
17. Wu CC, Gupta T, Garcia V, et al. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev. 2014;22:1–12. doi:10.1097/CRD.0b013e3182961659
18. Roman RJ, Fan F. 20-HETE: hypertension and beyond. Am Heart J. 2018;72:12–18. doi:10.1161/HYPERTENSIONAHA.118.10269
19. Hoopes SL, Garcia V, Edin ML, et al. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat. 2016;120:9–16. doi:10.1016/j.prostaglandins.2015.03.002
20. Fan F, Roman RJ. Effect of cytochrome P450 metabolites of arachidonic acid in nephrology. J Am Soc Nephrol. 2017;28:2845–2855. doi:10.1681/ASN.2017030252
21. Lasker JM, Chen WB, Wolf I, et al. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of CYP4F2 and CYP4A11. J Biol Chem. 2000;275:4118–4126. doi:10.1074/jbc.275.6.4118
22. Chen L, Joseph G, Zhang FF, et al. 20-HETE contributes to ischemia-induced angiogenesis. Vascul Pharmacol. 2016;83:57–65. doi:10.1016/j.vph.2016.04.002
23. Christmas P. Role of cytochrome P450s in inflammation. Adv Pharmacol. 2015;74:163–192. doi:10.1016/bs.apha.2015.03.005
24. Imig JD. Epoxyeicosatrienoic acids and 20-hydroxyeicosatetraenoic acid on endothelial and vascular function. Adv Pharmacol. 2017;77:105–141. doi:10.1016/bs.apha.2016.04.003
25. Edson KZ, Rettie AE. CYP4 enzymes as potential drug targets: focus on enzyme multiplicity, inducers and inhibitors, and therapeutic modulation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthase and fatty acid ω-hydroxylase activities. Curr Top Med Chem. 2014;13:1429–1440. doi:10.2174/15680266113139990110
26. Antimicrobial susceptibility testing. EUCAST disk diffusion method. European Committee on Antimicrobial Susceptibility Testing. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/Manual_v_5.0_EUCAST_Disk_Test.pdf.
27. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 11.0. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf.
28. Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67:2719–2728. doi:10.1016/j.jacc.2016.03.524
29. Guerbaai RA, Mahata I, Marechaux S, et al. Is ticagrelor worth its high cost and side-effects? Acta Cardiol. 2019;74:93–98. doi:10.1080/00015385.2018.1469371
30. Campo G, Sega FVD, Pavasini R, et al. Biological effects of ticagrelor over clopidogrel in patients with stable coronary artery disease and chronic obstructive pulmonary disease. Thromb Haemost. 2017;117:1208–1216. doi:10.1160/TH16-12-0973
31. Alemayehu M, Kim RB, Lavi R, et al. Effect of ticagrelor versus clopidogrel on vascular reactivity. J Am Coll Cardiol. 2017;69:2246–2248. doi:10.1016/j.jacc.2017.02.048
32. Bonello L, Frere C, Cointe S, et al. Ticagrelor increases endothelial progenitor cell level compared to clopidogrel in acute coronary syndromes: a prospective randomized study. Int J Cardiol. 2015;187:502–507. doi:10.1016/j.ijcard.2015.03.414
33. Sega FVD, Fortini F, Aquila G, et al. Ticagrelor improves endothelial function by decreasing circulating epidermal growth factor (EGF). Front Physiol. 2018;9:337. doi:10.3389/fphys.2018.00337
34. Reiner MF, Akhmedov A, Stivala S, et al. Ticagrelor, but not clopidogrel, reduces arterial thrombosis via endothelial tissue factor suppression. Cardiovasc Res. 2017;113:61–69. doi:10.1093/cvr/cvw233
35. Jeong HS, Hong SJ, Cho S, et al. Comparison of ticagrelor versus prasugrel for inflammation, vascular function, and circulating endothelial progenitor cells in diabetic patients with non-ST-segment elevation acute coronary syndrome requiring coronary stenting: a prospective, randomized, crossover trial. Cardiovasc Interven. 2017;10:1646–1658. doi:10.1016/j.jcin.2017.05.064
36. Jia Z, Huang Y, Ji X, et al. Ticagrelor and clopidogrel suppress NF-κB signaling pathway to alleviate LPS-induced dysfunction in vein endothelial cells. BMC Cardiovasc Disord. 2019;19:318. doi:10.1186/s12872-019-01287-1
37. Aquila G, Sega FVD, Marracino L, et al. Ticagrelor increases SIRT1 and HES1 mRNA levels in peripheral blood cells from patients with stable coronary artery disease and chronic obstructive pulmonary disease. Int J Mol Sci. 2020;21:1576. doi:10.3390/ijms21051576
38. Halim H, Pinkaew D, Chunhacha P, et al. Ticagrelor induces paraoxonase-1 (PON1) and better protects hypercholesterolemic mice against atherosclerosis compared to clopidogrel. PLoS One. 2019;14:1–26. doi:10.1371/journal.pone.0218934
39. Westphal C, Konkel A, Schunck WH. Cytochrome p450 enzymes in the bioactivation of polyunsaturated fatty acids and their role in cardiovascular disease. Adv Exp Med Biol. 2015;854:151–187. doi:10.1007/978-3-319-16009-2_6
40. Kim WY, Lee SJ, Min J, et al. Identification of novel CYP4F2 genetic variants exhibiting decreased catalytic activity in the conversion of arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE). Prostaglandins Leukot Essent Fatty Acids. 2018;131:6–13. doi:10.1016/j.plefa.2018.02.003
41. Waldman M, Peterson SJ, Arad M, Hochhauser E. The role of 20-HETE in cardiovascular diseases and its risk factors. Prostaglandins Other Lipid Mediat. 2016;125:108–117. doi:10.1016/j.prostaglandins.2016.05.007
42. Johnson AL, Edson KZ, Totah RA, et al. Cytochrome P450 ω-hydroxylases in inflammation and cancer. Adv Pharmacol. 2015;74:223–262. doi:10.1016/bs.apha.2015.05.002
43. Storey RF, James SK, Siegbahn A, et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets. 2014;25:517–525. doi:10.3109/09537104.2013.842965
44. Sexton TR, Zhang G, Macaulay TE, et al. Ticagrelor reduces thromboinflammatory markers in patients with pneumonia. Basic Transl Sci. 2018;3:435–449. doi:10.1016/j.jacbts.2018.05.005
45. Musumeci L, Jacques N, Penoy N, et al. Ticagrelor mechanism of action on Gram-positive bacteria. Res Pract Thromb Haemost. 2020;4:56.
46. Fan F, Ge Y, Lv W, et al. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci. 2016;21:1427–1463. doi:10.2741/4465
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
