Back to Journals » International Medical Case Reports Journal » Volume 16
Platypnea-Orthodeoxia Syndrome in Coronavirus Disease 2019 Pneumonia: A Case Report and Literature Review
Authors Tanimoto T , Eriguchi Y , Sato T, Yonekawa A, Miyake N, Akashi K, Shimono N
Received 21 January 2023
Accepted for publication 9 March 2023
Published 27 March 2023 Volume 2023:16 Pages 201—207
DOI https://doi.org/10.2147/IMCRJ.S402537
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Takahiko Tanimoto,1,2 Yoshihiro Eriguchi,1 Tomonori Sato,1 Akiko Yonekawa,1 Noriko Miyake,1 Koichi Akashi,1 Nobuyuki Shimono3
1Department of Clinical Immunology and Rheumatology / Infectious Disease, Kyushu University Hospital, Fukuoka, Japan; 2Department of Infectious Diseases, Kagoshima Seikyo Hospital, Kagoshima, Japan; 3Center for the Study of Global Infection, Kyushu University Hospital, Fukuoka, Japan
Correspondence: Yoshihiro Eriguchi, Department of Clinical Immunology and Rheumatology / Infectious Disease, Kyushu University Hospital, 3-1-1, Maidashi, Higashi-ku, Fukuoka, 812-8582, Japan, Tel +81 92-801-1011, Fax +81 92-862-8200, Email [email protected]
Abstract: Platypnea-orthodeoxia syndrome (POS) is a rare disorder associated with coronavirus disease 2019 (COVID-19) pneumonia. However, POS may be underdiagnosed. We report the case of a 59-year-old female patient with POS complicated by pulmonary embolism in COVID-19. Imaging revealed ground-glass opacities predominantly in the lower lobes and a pulmonary embolus in the right upper lobe. She was diagnosed with POS due to marked postural discrepancies between supine and upright oxygen saturations and blood oxygenation. Intracardiac shunt, one of the etiologies of POS, was not detected by bubble contrast echocardiography, and postural de-saturation gradually improved with methylprednisolone and edoxaban administration. In our literature review, only 3 of the 16 patients with POS associated with COVID-19 had cardiac shunting, suggesting that moderate to severe COVID-19 causes POS without cardiac shunts. COVID-19-associated vasculopathy and lower lung lesion predominance in COVID-19 pneumonia may cause ventilation-perfusion mismatch due to gravitational shunting of blood into the poorly ventilated lower lungs in the upright position, which may ultimately cause POS. Hypoxemia impedes rehabilitation, whereas early initiation of supine positioning in bed, with knowledge of the pathophysiology of POS, may have a positive effect.
Keywords: platypnea, orthodeoxia, COVID-19, ventilation-perfusion mismatch
Introduction
Platypnea-orthodeoxia syndrome (POS) is a rare clinical condition characterized by positional dyspnea (platypnea) and arterial de-saturation (orthodeoxia) while in the upright position. The hypoxia in POS has been attributed to the mixing of arterial and venous blood through a shunt. The most prevalent etiology of POS is cardiac disease,1 whereas pulmonary disease etiology is relatively rare.2
The clinical conditions of hypoxia in patients with coronavirus disease 2019 (COVID-19) have been previously elucidated. One characteristic is significant arterial hypoxemia without signs of respiratory distress, which is referred to as “Happy hypoxemia”.3 However, this may hinder the diagnosis of POS. In this report, we presented a case of POS associated with COVID-19 pneumonia and reviewed the previously reported cases in the literature.
Case Report
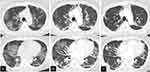
In the middle of the first Omicron variant pandemic wave, a 59-year-old female patient presented with fatigue, fever, and oliguria with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) test. She was admitted to a local hospital 6 days after symptom onset. Her past medical history included type 2 diabetes treated since the age of 43, hypertension, kidney transplant at the age of 57 due to end-stage renal failure, myocardial infarction, peripheral artery disease, and brain infarction. She had been prescribed 1500 mg of mycophenolate mofetil, 4 mg of tacrolimus, and 2 mg of methylprednisolone daily as immunosuppressants post-kidney transplant. On the day of initial admission, a chest radiograph revealed pneumonia. However, due to serum creatinine levels of 2.94 mg/dL, sotrovimab was administered instead of remdesivir. Oxygen therapy at 1 L/min and a 10-day course of dexamethasone 6.6 mg intravenous infusion were initiated the next day. Simultaneously, oxygen saturation improved with treatment, and renal function worsened, with serum creatinine levels increasing to 7.33 mg/dL and blood urea nitrogen levels increasing to 138.3 mg/dL. Subsequently, the patient was transferred to our hospital on day 12 after symptom onset. At the time of transfer, the patient was afebrile, but tachypneic at 28 breaths/min with an oxygen saturation of 95% on ambient air. Physical examination revealed late inspiratory crackles in the bilateral lower lungs, and arterial blood gas analyses showed metabolic acidosis of pH 7.32, partial pressure of carbon dioxide 19 mmHg, bicarbonate 9.5 mmol/L, base excess −14.7 mmol/L with normal lactate, and mild hypoxemia with partial pressure of oxygen (PO2) 70.5 mmHg. Her white blood cell count was 5580 cells/µL, and laboratory analyses were remarkable for renal dysfunction, with serum creatinine levels of 6.52 mg/dL and BUN levels of 129 mg/dL. Liver function test showed no remarkable abnormality with total bilirubin 0.5 mg/dL, aspartate aminotransferase 16 IU/L, alanine aminotransferase 8 IU/L, alkaline phosphatase 55 IU/L, gamma-glutamyl transferase 13 IU/L, albumin 3.3 mg/dL, and prothrombin time 81%. Inflammatory markers were slightly elevated, including C-reactive protein of 0.31 mg/dL, ferritin of 537.4 ng/dL, and procalcitonin of 0.56 ng/mL. Additionally, the D-dimer level was elevated at 4.1 μg/mL. Pyuria was detected in the urine analysis, and a urine smear revealed gram-negative rods, which was consistent with a history of recurrent urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Subsequently, the patient was given meropenem intravenously. Renal function gradually improved over the next days with adequate rehydration. However, on the second day after transfer, dyspnea and desaturation occurred while the patient was sitting on a portable toilet, and high-flow oxygen therapy was initiated. Computed tomography (CT) revealed deteriorating bilateral peripheral and patchy ground-glass opacities (Figure 1). Methylprednisolone 125 mg was administered for 5 days, and mycophenolate mofetil was discontinued until the viral infection could be controlled. Eighteen days after symptom onset, we observed that her oxygen saturation was considerably lower in an upright position in bed compared with a supine position (95% and 85%, respectively). Due to an elevated plasma D-dimer level of 22.0 μg/mL and a pulmonary embolus in the right upper lobe on contrast CT (Figure 2), edoxaban 30 mg was started on day 19 after symptom onset. CT also showed new ground-glass opacities in the lung fields. Positional de-saturation continued even when the patient was asymptomatic or complained only of mild dyspnea. On day 30, a comparison of arterial blood gas analyses in supine and seated positions, with O2 at 3 L/min by Oxymizer P-244 (Nihon Rufuto, Tokyo, Japan), showed PO2 of 90.6 mmHg and 67.2 mmHg, respectively, which was consistent with the POS diagnosis. Orthodeoxia and oxygen dependence gradually improved with methylprednisolone dose reduction every 3 days. Chest CT showed diminished opacification and augmentation of consolidation, particularly predominant at the lung bases (Figure 1). Additionally, the pulmonary embolism resolved on day 37. The patient’s SARS-CoV-2 PCR examination resulted in a negative on day 47, and dyspnea resolved without oxygen administration. Moreover, postural de-saturation in the supine and sitting positions improved to 97% and 94%, respectively, and oxygen therapy was subsequently discontinued. However, PO2 in the supine and sitting positions remained abnormal at 77.5 mmHg and 61.7 mmHg, respectively. We performed an agitated saline contrast bubble echocardiography to evaluate intracardiac shunting as the possible etiology of POS; however, no shunt was noted. The patient was ultimately discharged home on day 56 after symptom onset.
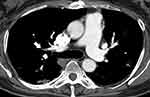 |
Figure 2 Computed tomography. Day 19 after symptom onset. The arrow shows a pulmonary embolus in the right upper lobe on contrast CT. |
Discussion
POS is a rare clinical condition characterized by positional dyspnea and arterial de-saturation while in the upright position, which has been attributed to arteriovenous shunting. A meta-analysis by Agrawal et al reported that 87% of POS was of cardiac origin, with a patent ovale foramen being the most common cause.1 POS may also be less frequently caused by extracardiac shunts, related to a ventilation-perfusion mismatch, and has been reported in interstitial lung disease, interstitial Pneumocystis jirovecii pneumonia, cytomegalovirus pneumonia, and drug-induced lung injury.2 Hepatopulmonary syndrome can also cause POS by dilatation of pulmonary capillaries leading to ventilation-perfusion mismatch, reduced alveolar-arterial oxygen diffusion, and arteriovenous shunting.1 Recently, cases of POS associated with COVID-19 pneumonia have been reported. Therefore, to review the characteristics of patients with COVID-19 with concomitant POS, including our case, we reviewed the literature in PubMed using following keywords: “Platypnea-orthodeoxia” and “COVID-19”. We excluded literature in languages other than English. A retrospective analysis conducted in India4 was also excluded, as the profiles of each case were not elaborated.
Ultimately, we found 17 reports on 22 patients with POS associated with COVID-19 (Table 1). Regarding the presence of cardiac shunting, foramen ovale patency was found in three cases5–7 and absent in 13 cases,8–18 while no echocardiographic results were mentioned in the remaining three reports.19–21 Most cases required respiratory support with high-volume oxygen, noninvasive ventilation, or invasive mechanical ventilation. Many of the cases reported CT findings of ground-glass opacities and consolidations predominantly in bilateral lower lobes and lung bases. The lower lung predominant lesion in COVID-19 pneumonia causes ventilation-perfusion mismatch due to the occurrence of gravitational shunting of blood into the poorly ventilated lower lungs when in the upright position.21 Moreover, pulmonary embolism was found in 3 of the 23 cases,8,17 including our case. However, pulmonary micro-thrombosis and vasculopathy undetectable using CT may also be an etiology of POS in COVID-19.21 The time to POS diagnosis ranged from 4 to 28 days after COVID-19 onset or hospitalization (Table 1). Notably, POS was more likely determined after rehabilitation following acute-phase treatment.10,17,19 Furthermore, the prognosis for POS associated with COVID-19 was generally good, with most patients recovering within 4 to 65 days; however, three patients required long-term oxygen therapy (LTOT) after discharge.6,8,12 One patient was re-hospitalized for worsening respiratory status 2 months after discharge home on LTOT and subsequently died from pulmonary fibrosis, pneumonia, and pneumomediastinum.8,22
 |
Table 1 POS Caused by COVID-19 Pneumonia |
POS may impede the rehabilitation and lead to disuse related to prolonged bed rest. The resulting lower extremity muscle weakness may subsequently exacerbate POS by decreasing venous return and cardiac output in the upright position.10 Rehabilitation in bed with supplemental oxygen support may be beneficial to maintain muscle activity and joint range of motion until the patients can adapt to an upright position.10 In a retrospective analysis conducted in India, 15 of the 53 (28%) patients with moderate COVID-19 were diagnosed with POS,4 suggesting that POS associated with COVID-19 is more common than previously believed. Therefore, physicians and health-care providers should recognize POS as a cause of dyspnea and hypoxemia in patients with COVID-19, investigate possible etiologies, and avoid excessive bed rest, while providing appropriate treatments.
Conclusion
POS may be an under-recognized cause of dyspnea and hypoxemia in patients with moderate to severe COVID-19. Although most POS associated with COVID-19 were reversible and had good outcomes, hypoxemia may ultimately interfere with rehabilitation. The search for treatable arteriovenous shunting and early on-bed rehabilitation with appropriate treatments may lead to good outcomes.
Ethics Approval and Consent to Participate
No institutional approval was required to publish the case details.
Informed Consent
Informed consent for the publication of clinical details and clinical images was obtained from the patient.
Acknowledgments
The authors would like to thank Dr. Hiroki Yamakuchi, Department of Infectious Diseases, Kagoshima Seikyo Hospital, and Dr. Masanari Komatsu, Division of Generalist Medicine, Kagoshima Seikyo Hospital, for their support and practical advice in preparing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Agrawal A, Palkar A, Talwar A. The multiple dimensions of Platypnea-Orthodeoxia syndrome: a review. Respir Med. 2017;129:31–38. doi:10.1016/j.rmed.2017.05.016
2. Mathew U, Mittal A, Vyas S, Ray A. Interstitial pneumonia with autoimmune features and platypnea-orthopnea syndrome. BMJ Case Rep. 2019;12(9):e230948. doi:10.1136/bcr-2019-230948
3. Khan S, Talwar D, Kumar S, Acharya S. Happy hypoxia in COVID-19: the paradoxical killer. Med Sci. 2021;25(112):1295–1300.
4. Athavale T, Athavale A, Khatri V, et al. Platypnea-Orthodeoxia Syndrome (POS) in moderate COVID-19: an uncommonly common bedside sign? J Assoc Physicians India. 2021;69(6):11–12.
5. Dodson BK, Major CK, Grant M, Yoo BS, Goodman BM. Platypnea orthodeoxia due to a patent foramen ovale and intrapulmonary shunting after severe COVID-19 pneumonia. Am J Case Rep. 2021;22:e933975.
6. Vanhomwegen C, Taton O, Selvais N, Vanhove O, Leduc D. Patent foramen ovale revealed by COVID-19 pneumonia. BMC Pulm Med. 2021;21(1):126.
7. Jenab Y, Hosseinsabet A, Vaskelyte L, Hosseini K. Platypnoea-orthodeoxia syndrome after percutaneous treatment of ruptured sinus Valsalva complicated by SARS-Cov-2 pneumonia: a case report. Eur Heart J Case Rep. 2021;5(5):ytab176.
8. Longo C, Ruffini L, Zanoni N, et al. Platypnea-orthodeoxia after fibrotic evolution of SARS-CoV-2 interstitial pneumonia. A case report. Acta Biomed. 2020;91(3):ahead of print.
9. Singh K, Kadnur H, Ray A, et al. Platypnea-orthodeoxia in a patient with severe COVID-19 pneumonia. Monaldi Arch Chest Dis. 2020;90(4):154.
10. Tham SL, Ong PL, Lee AJY, Tay MRJ. Rehabilitation of patients with Platypnea-Orthodeoxia Syndrome in COVID-19 pneumonia: two case reports. J Rehabil Med Clin Commun. 2020;3:1000044. doi:10.2340/20030711-1000044
11. Ismail MA, Trevest K. Platypnoea-orthodeoxia syndrome in an older patient with COVID-19 pneumonia and orthostatic hypotension-a case of prolonged hospital admission. Age Ageing. 2021;50(6):1886–1887. doi:10.1093/ageing/afab166
12. Hoshi T, Tadokoro Y, Nemoto M, Honda J, Matsukura S. Platypnea-orthodeoxia syndrome associated with COVID-19 pneumonia: a case report. JA Clin Rep. 2021;7(1):67. doi:10.1186/s40981-021-00471-7
13. Salvotti F, Poiatti F, Bressa S, Montani G, Nardin M, Rizzoni D. Platypnoea-Orthodeoxia Syndrome in COVID-19. Eur J Case Rep Intern Med. 2021;8(10):002849. doi:10.12890/2021_002849
14. Aprea C, Imbriani S, Cirigliano G, et al. Platypnea-orthodeoxia syndrome in SARS-CoV-2 related ARDS: a case report. Acta Biomed. 2022;93(S1):e2022102. doi:10.23750/abm.v93iS1.12824
15. Asami-Noyama M, Harada M, Hisamoto Y, et al. Platypnea-orthodeoxia syndrome in a patient with ongoing COVID-19. Respirol Case Rep. 2022;10(8):e01009. doi:10.1002/rcr2.1009
16. Bhushan D, Kumar V, Sahoo BH, Hegde A. Platypnea-orthodeoxia syndrome: an important cause of morbidity in post coronavirus disease patients. Indian J Crit Care Med. 2022;26(3):401–402. doi:10.5005/jp-journals-10071-24126
17. Abreu Fernandes J, Santos Faria J, Fernandes A, Ramalho AR, Girao A, Petrova M. Severe pneumonia caused by SARS-CoV-2: a novel cause of Platypnoea-Orthodeoxia Syndrome. Eur J Case Rep Intern Med. 2022;9(8):003385. doi:10.12890/2022_003385
18. Talwar D, Kumar S, Acharya S, Madaan S, Hulkoti V. COVID-19 associated Platypnea Orthodexia syndrome in a young male. Med Sci. 2021;25(112):1326–1330.
19. Tan GP, Ho S, Fan BE, et al. Reversible platypnea-orthodeoxia in COVID-19 acute respiratory distress syndrome survivors. Respir Physiol Neurobiol. 2020;282:103515. doi:10.1016/j.resp.2020.103515
20. Oldani S, Ravaglia C, Bensai S, et al. Pathophysiology of light phenotype SARS-CoV-2 interstitial pneumonia: from histopathological features to clinical presentations. Pulmonology. 2022;28(5):333–344. doi:10.1016/j.pulmoe.2021.03.003
21. Aayilliath KA, Singh K, Ray A, Wig N. Platypnoea-orthodeoxia syndrome in COVID-19. BMJ Case Rep. 2021;14(5):e243016. doi:10.1136/bcr-2021-243016
22. Zanoni N, Longo C, Frizzelli A, Longo F, Accogli R, Chetta AA. Platypnea-Orthodeoxia Syndrome after SARS-CoV-2 interstitial pneumonia: an overview and an update on our patient. Acta Biomed. 2022;93(1):e2022015. doi:10.23750/abm.v93i1.11814
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.