Back to Journals » Journal of Inflammation Research » Volume 16
Platelet/Lymphocyte Ratio Predicted Long-Term Prognosis for Acute Upper Extremity Deep Vein Thrombosis from a Retrospective Study
Authors Liu Y , Sun H, Jiang J
Received 24 November 2022
Accepted for publication 10 January 2023
Published 14 January 2023 Volume 2023:16 Pages 225—234
DOI https://doi.org/10.2147/JIR.S399000
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Yang Liu,* Hongze Sun,* Jianjun Jiang
Department of General Surgery, Vascular Surgery, Qilu Hospital of Shandong University, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianjun Jiang, Department of General Surgery, Vascular Surgery, Qilu Hospital of Shandong University, Jinan, Shandong, 250012, People’s Republic of China, Tel +86 18560085133, Email [email protected]
Background: In this study, we aimed to determine the mortality risk factors and whether placement of a vena cava filter improves the prognosis of acute upper extremity deep vein thrombosis (UEDVT).
Methods: Clinical data and follow-up results were retrospectively analyzed. Cox regression analysis was conducted to identify the risk factors associated with all-cause mortality in all patients and subgroups of patients. Results are expressed as hazard ratio (HR) with 95% confidence intervals (95% CI). Receiver operating characteristic curves (ROC) were used to determine the optimal cut-off value. Kaplan–Meier survival curves were constructed and compared by the Log rank test.
Results: The study cohort comprised 109 patients of median age 56 years (47.5, 64.5). The median follow-up time was 25 months (8, 47): 39 patients (35.8%) had died by 12 months, 55 (50.5%) by 36 months, and 60 (55%) by the end of follow-up. Presence of malignancy (HR: 5.882, 95% CI: 2.128– 16.667), D-dimer (HR: 1.56, 95% CI: 1.09– 1.94), platelet/lymphocyte ratio (PLR; HR: 2.02, 95% CI: 1.15– 3.54), and the systemic immune/inflammatory index (SII; HR: 1.471, 95% CI: 1.062– 1.991) were identified as independent risk factors for mortality. Subgroup analysis of patients with malignancy determined gender (HR: 2.936, 95% CI: 1.599– 5.393) and PLR (HR: 1.427,95% CI: 1.023– 1.989) as independent risk factors. Kaplan–Meier analysis showed that the mortality rate was much higher in patients with malignancy, high D-dimer (≥ 0.92ug/mL), high PLR (≥ 291) and high SII (≥ 1487). However, there was no significant difference between patients with and without vena cava filters.
Conclusion: In this study, we identified PLR as an new independent predictor of mortality in patients with acute UEDVT. Emergency placement of a vena cava filter did not improve long-term prognosis.
Keywords: upper extremity deep vein thrombosis, mortality risk, vena cava filter, malignancy, platelet/lymphocyte ratio
Introduction
Upper extremity deep vein thrombosis (UEDVT), which is defined as thrombosis involving the ulnar, radial, brachial, axillary, subclavian and cephalic veins, and sometimes the jugular vein, accounts for 2%-14% of all deep vein thrombosis (DVT).1–3 Thus far, various risk factors associated with deep vein thrombosis have been identified, including malignancy, central venous placement, advanced age, male gender, obesity, history of deep vein thrombosis or pulmonary embolism (PE), physical inactivity, smoking, and fracture.4–6 However, there are relatively few studies on the independent risk factors associated with mortality in patients with UEDVT, and few large studies. Therefore, investigation of risk factors affecting the prognosis of patients with UEDVT is a critical issue in clinical practice. Such data could help guide risk stratification of UEDVT and individualization of therapeutic strategies.
Previous studies have shown that the immune/inflammatory response plays an important role in cardiovascular and peripheral vascular diseases, representative indicators including the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR) and systemic immune/inflammatory index (SII).7–9 NLR and PLR both predict the onset of DVT and also correlate strongly with in-hospital, 30-day, and all-cause mortality from DVT.10–13 The SII, a new indicator of inflammation, has been shown by two recent studies to be an independent risk predictor of risk of acute PE and acute venous thromboembolism (VTE).14,15 However, few studies have investigated the association between these immune inflammatory indicators and the prognosis of patients with UEDVT. To reduce the mortality associated with UEDVT and PE, anticoagulation therapy should be administered for at least 3 months after diagnosis of UEDVT. Emergency placement of a vena cava filter is a viable and effective option for preventing PE when anticoagulation is contraindicated or when thromboembolism occurs despite adequate anticoagulation.16–18 However, using permanent filters in younger patients is associated with an increased incidence of filter-related complications.19,20 Therefore, although vena cava filters do provide prophylaxis against PE, it is unclear whether they improve the long-term prognosis of patients with UEDVT.
This study included patients with UEDVT admitted to our hospital in the past 5 years. We identified independent risk factors associated with all-cause mortality that we expect will provide useful guidance for clinical practice.
Methods
Study Design and Setting
This was a single-center retrospective study. All data were obtained from the electronic medical record system of Qilu Hospital of Shandong University. Included patients with UEDVT were admitted to our institution between 1 January 2015 and 31 December 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Qilu Hospital of Shandong University. The need for written informed consent was waived because this was a retrospective study based on previous medical record data and did not involve human specimens. The personal identifiers of all patients were removed. All the data were anonymized and confidentiality was maintained.
Participants
Inclusion criteria were as follows: (1) definite diagnosis of UEDVT by ultrasonography and PE by pulmonary artery computed tomography angiography (CTA); (2) fewer than 15 days from onset to admission; (3) first episode of UEDVT; (4) blood samples and laboratory tests within 24 hours of admission; and (5) complete follow-up information. Exclusion criteria were: (1) more than 15 days from onset to hospital admission; (2) recurrent episode of UEDVT; and (3) unavailable follow-up data.
Data Collection and Endpoint
We collected basic clinical data of all study patients, including age, sex, comorbidities, malignancies, presence of central venous catheter, history of smoking and alcohol, days of hospitalization, blood test results, PE, and locations of venous thrombi. Biomarkers and derived immune/inflammatory indicators were obtained from hematological tests performed within 24 hours of admission. These included white blood cell, neutrophil, lymphocyte, and platelet counts, D-dimer, NLR, PLR, and SII. NLR was defined as neutrophil count/lymphocyte count. PLR was defined as platelet count/lymphocyte count. SII was defined as platelet count × neutrophil count/lymphocyte count. We also recorded the treatment strategies, including whether a vena cava filter was placed and whether it was retrieved, type of filter, and duration of anticoagulation therapy and type of anticoagulant administered. The follow-up period ended on 30 June 2022. We obtained follow-up results by telephone or via outpatient interviews. All-cause mortality at 12 months and 36 months and total mortality were recorded.
Statistical Analysis
Statistical analysis was performed using SPSS statistical software (version 20.0). Continuous factors data are expressed as median (p25, p75) and were analyzed using an independent Student’s t-test or Mann–Whitney test, as appropriate. Categorical factors are expressed as number of cases and percentages (%), and the χ2 test or Fisher exact test was used. Multivariate Cox regression analysis using a backward stepwise method was conducted to identify risk factors related to all-cause mortality in all patients and subgroups of patients. Results are expressed as hazard ratio (HR) with 95% confidence intervals (95% CI). Factors with HR>1 were considered risk factors, whereas factors with HR<1 were considered protective factors. Receiver operating characteristic curves (ROCs) were used to determine the optimal cut-off values. Survival curves were constructed by the Kaplan-Meier (KM) method, based on cut-off values and compared by Log rank tests. p<0.05 was considered to denote statistical significance.
Results
A search of the electronic medical record system yielded 139 patients with UEDVT during the study period. Of these, we excluded 21 who had been lost to follow-up, eight who had been admitted to hospital more than 15 days from onset, and one with recurrent venous thrombosis. The remaining 109 patients were included in this study. They comprised 56 men and 53 women of median age 56 years (47.5, 64.5), of whom four were diagnosed with UEDVT combined with PE. The median duration of hospitalization was 15 days (9, 21). Malignancy was present in 87 cases (79.8%) and 65 patients (59.6%) had central venous catheters. All patients received anticoagulation therapy for 3–6 months. Patients with malignancy received anticoagulation therapy for at least 12 months. Patients whose filters were not removed were recommended lifelong anticoagulation therapy. Anticoagulation therapy comprised low-molecular heparin bridged to warfarin therapy in 92 cases (84%) and oral rivaroxaban in 17 cases (16%). Thirteen of these patients (11.9%) underwent emergency placement of superior vena cava filters, including five Aegisy filters (Lifetech Science, China; Figure 1A) and eight Celect filters (Cook Corp, USA; Figure 1B), four of which were retrieved.
All patients were followed up for a median of 25 months (8, 47) after discharge. By the 12 -month follow-up, 70 patients (64.2%) had survived and 39 (35.8%) had died. By the 36- month follow-up, 54 patients (49.5%) had survived and 55 (50.5%) had died. And by the end of follow-up, 49 patients (45%) had survived and 60 (55%) had died. There were no thrombosis recurrences, PEs, or filter-related complications during follow up. Detailed clinical baseline characteristics are presented in Table 1. There were significant differences between survivors and deceased patients in D-dimer, PLR and SII. (Table 1) Additionally, there were markedly more malignancies in the patients who had died. However, there was no difference between survivors and deceased patients regarding vena cava filter placement. Multivariate Cox regression analysis identified malignancy (HR: 5.882, 95% CI: 2.128–16.667, p=0.001), D-dimer (HR: 1.56, 95% CI: 1.09–1.94, p=0.045), PLR (HR: 2.02, 95% CI: 1.15–3.54, p=0.015), and SII (HR: 1.471, 95% CI: 1.062–1.991, p=0.005) as independent risk factors for mortality. KM analysis (Log rank test) based on the cutoff values showed significant differences in all-cause mortality between patients with and without malignancy (Figure 2A), D-dimer≥0.92 µg/mL and <1.45 µmol/L (Figure 2B), PLR≥291 and <291 (Figure 2C), and SII≥1487 and <1487 (Figure 2D). The mortality rate was much higher in patients with malignancy, high D-dimer (≥0.92 µg/mL), high PLR (≥291) and high SII (≥1487).
 |
Table 1 Clinical Baseline Characteristics |
In the subgroup of 87 patients with malignancy, 37 (42.5%) had died by the 12-month follow-up, 52 (59.8%) by 36 months, and 56 (64.4%) by the end of follow-up. Detailed comparisons are presented in Table 2. Subgroup analysis of patients with malignancy revealed gender (HR: 2.936, 95% CI: 1.599–5.393, p=0.001) and PLR (HR: 1.427, 95% CI:1.023–1.989, p=0.007) as independent risk factors for mortality.
 |
Table 2 Subgroup Analysis of Patients with Malignancy |
Patients with vena cava filters had more numerous thrombosed veins than did those without filters (p<0.001). There were no significant differences in other blood test findings. Detailed comparison results are presented in Table 3. There was no statistically significant differences in 12-month/36-months/total mortality risk between these two subgroups. KM analysis also revealed no significant difference in survival probability between these two subgroups (log-rank p=0.68), indicating that placement of a vena cava filter did not improve the long-term prognosis (Figure 1C).
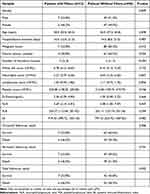 |
Table 3 Comparison Between the Patients with and without Vena Cava Filters |
Discussion
Primary UEDVT, which accounts for only 20% of all cases of UEDVT, is also known as Paget-Schroetter syndrome and is characteristically induced in young adults by excessive activity of the upper extremity.21 Secondary UEDVT is mostly associated with central venous catheters, malignancy, radiotherapy and trauma.2 PE occurs in approximately 9% of patients with UEDVT, sometimes resulting in death. Further, patients with secondary UEDVT have a higher mortality than do patients with primary UEDV,(16% and 34% mortality rates at 1 and 3 months, respectively).1,22,23 However, the causes of death in patients with secondary UEDVT are usually related to their underlying disease rather than to PE. Malignancy is an important risk factor for UEDVT, accounting for 38% of such cases according to the RIETE study.24 In the present study, we also identified malignancy as an independent risk factor for overall mortality in patients with UEDVT; these patients had a significantly higher mortality rate. A previous study has also shown that a central venous catheter is the strongest independent risk factor for UEDVT, resulting in a seven-fold greater incidence of UEDVT than in patients without a central venous catheter.24 In the present cohort, although the proportion of patients with central venous catheters was relatively high (59.6%), Cox regression analysis showed that their placement had no impact on the prognosis of UEDVT.
Previous studies have shown that immune/inflammatory indicators are closely related to the onset and prognosis of VTE, PLR and SII having been identified as independent predictors.10,14,15,25–27 In the present study, we demonstrate, to the best of our knowledge for the first time that the PLR and SII index are significantly associated with all-cause mortality in patients with acute UEDVT, and that patients with high PLR and SII are at higher risk of death. Therefore, PLR and SII can serve as valuable predictors of prognosis of acute UEDVT in clinical practice. PLR was also identified as an independent risk factor in the subgroup of patients with malignancy. Previous studies have revealed that venous thromboembolism is associated with an increase in circulating platelet-monocyte aggregates, platelet-neutrophil aggregates and platelet-lymphocyte aggregates.28 Enhanced platelet activation is critical for the recruitment of innate leukocytes, neutrophil extracellular trap formation and the expression of cytokines and tissue factors. These platelet-mediated effects contribute further to a hypercoagulable state and endothelial dysfunction, which are crucial factors in deep vein thrombosis and subsequent PE. Based on the physiological processes described above, we believe that PLR can also be used as a predictor of mortality in UEDVT. D-dimer, which has been one of the most clinically used indicators, has a high specificity for VTE, but an average sensitivity. In the present study, we found that D-dimer is also significantly associated with mortality risk from acute UEDVT. A prediction model using a combination of identified indicators may be a worth investigating.
Previous studies have shown that using vena cava filters is an important means of preventing PE in patients with VTE, and that this procedure is reliable and feasible.16,18,29 Vena cava filters may be considered in patients with VTE who have contraindications to anticoagulation, active bleeding, or recurrent thrombosis.30 In the present study, there were no significant differences in the 12-month, 36-month, or overall mortality between patients with and without vena cava filters, impling that placement of a vena cava filter does not appear to improve the prognosis of patients with UEDVT. However, this is a preliminary conclusion. Because this was a small study, the results may be biased. Our findings need to be validated by further investigation. In addition, patients with vena cava filters had more numerous thrombosed veins, suggesting that the number of thrombosed veins should be considered when deciding on placement of a vena cava filters in a patient with UEDVT. This possibility also requires confirmation by a larger study.
This study had the following limitations. First, it was relatively small; thus, the study results may be biased. Second, it was a single-center retrospective study; these preliminary results need to be further investigated by a multi-center prospective study. Finally, in the present study we only identified prognosis-related high-risk factors. A clinical model using D-dimer and immune/inflammatory indicators for predicting the prognosis of patients with UEDVT may provide more valuable clinical guidance.
Conclusion
In this study, we identified PLR as a new independent indicator of long-term mortality risk from acute UEDVT. Additionally, emergency placement of a vena cava filter did not improve long-term prognosis. These preliminary findings remain to be further validated by large prospective studies.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). And it was approved by the Ethics Committee of Qilu Hospital of Shandong University and individual consent for this retrospective analysis was waived.
Acknowledgment
We thank Dr Trish Reynolds, MBBS, FRACP, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Funding
This report is supported by National Natural Science Foundation of China (Grant No.82000451).
Disclosure
Yang Liu and Hongze Sun are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Mai C, Hunt D. Upper-extremity deep venous thrombosis: a review. Am J Med. 2011;124(5):402–407. doi:10.1016/j.amjmed.2010.11.022
2. Klitfod L, Broholm R, Baekgaard N. Deep venous thrombosis of the upper extremity. A Review Int Angiol. 2013;32(5):447–452.
3. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I22–30. doi:10.1161/01.CIR.0000078464.82671.78
4. Wendelboe AM, Raskob GE. Global Burden of Thrombosis: epidemiologic Aspects. Circ Res. 2016;118(9):1340–1347. doi:10.1161/CIRCRESAHA.115.306841
5. McLendon K, Goyal A, Bansal P, et al. Deep Venous Thrombosis Risk Factors. Treasure Island (FL): StatPearls Publishing; 2021:29262230.
6. Bartlett MA, Mauck KF, Stephenson CR, et al. Perioperative Venous Thromboembolism Prophylaxis. Mayo Clin Proc. 2020;95(12):2775–2798. doi:10.1016/j.mayocp.2020.06.015
7. Mozos I, Malainer C, Horbańczuk J, et al. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front Immunol. 2017;8:1058. doi:10.3389/fimmu.2017.01058
8. Neupane R, Jin X, Sasaki T, et al. Immune Disorder in Atherosclerotic Cardiovascular Disease― Clinical Implications of Using Circulating T-Cell Subsets as Biomarkers ―. Circ J. 2019;83(7):1431–1438. doi:10.1253/circj.CJ-19-0114
9. Kremers B, Wübbeke L, Mees B, et al. Plasma Biomarkers to Predict Cardiovascular Outcome in Patients With Peripheral Artery Disease: a Systematic Review and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2020;40(9):2018–2032. doi:10.1161/ATVBAHA.120.314774
10. Ates H, Ates I, Kundi H, et al. Diagnostic validity of hematologic parameters in evaluation of massive pulmonary embolism. J Clin Lab Anal. 2017;31(5):e22072. doi:10.1002/jcla.22072
11. Bi W, Liang S, He Z, et al. The Prognostic Value of the Serum Levels of Brain Natriuretic Peptide, Troponin I, and D-Dimer, in Addition to the Neutrophil-to-Lymphocyte Ratio, for the Disease Evaluation of Patients with Acute Pulmonary Embolism. Int J Gen Med. 2021;14:303–308. doi:10.2147/IJGM.S288975
12. Liu C, Zhan HL, Huang ZH, et al. Prognostic role of the preoperative neutrophil-to-lymphocyte ratio and albumin for 30-day mortality in patients with postoperative acute pulmonary embolism. BMC Pulm Med. 2020;20(1):180. doi:10.1186/s12890-020-01216-5
13. Karataş MB, Ipek G, Onuk T, et al. Assessment of Prognostic Value of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Patients with Pulmonary Embolism. Acta Cardiol Sin. 2016;32(3):313–320. doi:10.6515/acs20151013a
14. Gok M, Kurtul A. A novel marker for predicting severity of acute pulmonary embolism: systemic immune-inflammation index. Scand Cardiovasc J. 2021;55(2):91–96. doi:10.1080/14017431.2020.1846774
15. Peng J, Wang H, Zhang L, et al. Construction and efficiency analysis of prediction model for venous thromboembolism risk in the elderly after Hip fracture. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46(2):142–148. doi:10.11817/j.issn.1672-7347.2021.190722
16. Lopera JE, Barnes L. A single center 10-year clinical experience with superior vena cava retrievable filters. Catheter Cardiovasc Interv. 2020;95(1):1–6. doi:10.1002/ccd.28533
17. Spence LD, Gironta MG, Malde HM, et al. Acute upper extremity deep venous thrombosis: safety and effectiveness of superior vena caval filters. Radiology. 1999;210(1):53–58. doi:10.1148/radiology.210.1.r99ja1353
18. Owens CA, Bui JT, Knuttinen MG, et al. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21(6):779–787. doi:10.1016/j.jvir.2010.02.021
19. Van Ha TG, Chien AS, Funaki BS, et al. Use of retrievable compared to permanent inferior vena cava filters: a single-institution experience. Cardiovasc Intervent Radiol. 2008;31(2):308–315. doi:10.1007/s00270-007-9184-5
20. Kinney TB. Update on inferior vena cava filters. J Vasc Interv Radiol. 2003;14(4):425–440. doi:10.1097/01.RVI.0000064860.87207.77
21. Keller RE, Croswell DP, Medina GIS, et al. Paget-Schroetter syndrome in athletes: a comprehensive and systematic review. J Shoulder Elbow Surg. 2020;29(11):2417–2425. doi:10.1016/j.jse.2020.05.015
22. Bosch FTM, Nisio MD, Büller HR, et al. Diagnostic and Therapeutic Management of Upper Extremity Deep Vein Thrombosis. J Clin Med. 2020;9(7):2069. doi:10.3390/jcm9072069
23. Mustafa J, Asher I, Sthoeger Z. Upper Extremity Deep Vein Thrombosis: symptoms, Diagnosis, and Treatment. Isr Med Assoc J. 2018;20(1):53–57.
24. Cote LP, Greenberg S, Caprini JA, et al. Comparisons Between Upper and Lower Extremity Deep Vein Thrombosis: a Review of the RIETE Registry. Clin Appl Thromb Hemost. 2017;23(7):748–754. doi:10.1177/1076029616663847
25. Köse N, Yıldırım T, Akın F, et al. Prognostic role of NLR, PLR, and LMR in patients with pulmonary embolism. Bosn J Basic Med Sci. 2020;20(2):248–253. doi:10.17305/bjbms.2019.4445
26. Yao C, Zhang Z, Yao Y, et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for acute deep vein thrombosis after total joint arthroplasty: a retrospective study. J Orthop Surg Res. 2018;13(1):40. doi:10.1186/s13018-018-0745-x
27. Grilz E, Posch F, Königsbrügge O, et al. Association of Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio with the Risk of Thromboembolism and Mortality in Patients with Cancer. Thromb Haemost. 2018;118(11):1875–1884. doi:10.1055/s-0038-1673401
28. Schrottmaier WC, Mussbacher M, Salzmann M, et al. Platelet-leukocyte interplay during vascular disease. Atherosclerosis. 2020;307:109–120. doi:10.1016/j.atherosclerosis.2020.04.018
29. Koury JP, Burke CT. Endovascular management of acute upper extremity deep venous thrombosis and the use of superior vena cava filters. Semin Intervent Radiol. 2011;28(1):3–9. doi:10.1055/s-0031-1272975
30. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315–352. doi:10.1016/j.chest.2015.11.026
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


