Back to Journals » International Journal of Women's Health » Volume 12
Platelet Indices and CXCL12 Levels in Patients with Intrauterine Growth Restriction
Authors Shahgheibi S, Mardani R, Babaei E, Mardani P, Rezaie M , Farhadifar F, Roshani D , Naqshbandi M, Jalili A
Received 9 October 2019
Accepted for publication 30 March 2020
Published 21 April 2020 Volume 2020:12 Pages 307—312
DOI https://doi.org/10.2147/IJWH.S233860
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Shole Shahgheibi,1,* Roya Mardani,1,* Erfan Babaei,2 Parastoo Mardani,3 Masomeh Rezaie,1 Fariba Farhadifar,1 Daem Roshani,2 Mobin Naqshbandi,2 Ali Jalili2
1Deparment of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran; 2Cancer & Immunology Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran; 3Department of Biology, Faculty of Sciences, Payame Noor University, Sanandaj, Iran
*These authors contributed equally to this work
Correspondence: Ali Jalili
Cancer & Immunology Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran
Tel +98 9 183771862
Email [email protected]
Background: Intrauterine growth restriction (IUGR) is a multifactorial condition, and the precise mechanism is still unknown. In the current study, we aimed to determine the relationship between the platelet (PLT) indices and CXC12 levels in patients with IUGR.
Patients and Materials: In this study, 36 patients with IUGR and 36 healthy pregnant mothers were enrolled as the case and control groups, respectively. Gestational age for both groups was between 24 and 40 years. Blood samples were taken, and platelet indices were examined by a full-diff cell counter. Serum levels of CXCL12 were measured by ELISA, and the data were analyzed using an independent Student’s t-test.
Results: In this study, we observed that the mean value of PLT count (154.3 ± 50 vs 236 ± 36) and plateletcrit (0.124 ± 0.038 vs 0.178 ± 0.021) were significantly lower in the case than the control group. In contrast, the mean platelet volume (7.94 ± 0.55 vs 7.62 ± 0.53) and platelet distribution width (17.57 ± 0.7 vs 16.96 ± 0.59) were significantly higher in the case than the control group. More importantly, we found that the serum levels of CXCL12 were significantly higher (5.3 ng/mL± 3.1 vs 2.8 ± 1.6) in the patients compared to the pregnancy controls.
Conclusion: Our data show that platelet indices are changed in IUGR, and the levels of circulating CXCL12 are increased in patients with IUGR. These findings provide a base for further studies to better defining the pathophysiology of IUGR.
Keywords: platelets, SDF-1, intrauterine growth restriction, CXCL12
Introduction
Intrauterine growth restriction (IUGR) is a multifactorial obstetrical condition and is characterized as uteroplacental ischemia, resulting in disturbing the balance of nutrition between the mother and the fetus.1,2 In a normal baby, birth weight should be between the 10th and 90th percentiles, in terms of gestational age, sex, and race. Generally, if the baby’s birth weight is less than the 10th percentile and the abdominal circumference is less than 2.5th percentile, then IUGR is considered to be diagnosed. IUGR is, in fact, a clinical definition, and is used for infants showing clinical manifestations of malnutrition and impairment of intrauterine growth and is not necessarily dependent on growth-related issues. Thus, even for an infant with appropriate weight for gestational age, IUGR can be used if evidence shows intrauterine retardation at birth.3,4 The prevalence of IUGR in developing countries is 6 times higher than in developed countries. The prevalence of this disorder is higher in Asia than in other continents, accounting for approximately 75% of all affected infants. In Asia, the prevalence is highest in Bangladesh, India, Pakistan, Sri Lanka, Cambodia, Vietnam, Philippines, Indonesia, Malaysia, Thailand, and China.5
There are many causes for the etiology of IUGR, but in general, any factor that interferes with the balance between the transfer of food supplies through the placenta and the demand of the fetus can lead to IUGR. It should be noted that in a few cases, embryonic disorders such as fetal malformations, metabolic disorders, and chromosomal abnormalities are involved in the pathogenesis of this disease.6 Therefore, in general, IUGR can occur due to fetal, maternal, and placental causes. Growing of evidence indicate that maternal weight deficiency, malnutrition, pregnancy-induced hypertension, gestational diabetes, smoking cigarettes, alcohol consumption, and some medications are the risk factor for IUGR.7,8
Moreover, a few previous studies demonstrated that platelets contribute to the pathogenesis of IURG. Abnormal platelets morphology and function, as well as increased mean platelet volume (PMV), have been proven as a feature of IUGR.9 The association between IUGR and platelet function is so strong that even aspirin consumption is recommended for preventing recurrence of IUGR.10 Platelet activation is associated with arterial thrombosis, which in addition to IUGR may lead to numerous clinical conditions such as hypertension, myocardial infarction, and stroke.11 Moreover, a decreased in platelet aggregation in patients with IUGR, particularly with hypertension, have been reported. Considering the major role of platelets in hemostasis regulation and thrombosis and its function in the coagulation process, which can lead to disorders in placental circulation,9,12,13 implying that studying platelets indices in IURG could lead to better understanding the pathophysiology of the disease.
CXCL12 is a chemokine that through binding with its G-coupled receptor plays an important role in the migration, reproduction, and differentiation of hematopoietic stem cells and leukocytes.14,15 CXCL12 is secreted by trophoblastic cells and decreases gradually with increasing gestational age, and induces proliferation and differentiation of trophoblast.16 It also binds to CXCR4 and attracts decidua natural killer cells (dNK) cells into the decidua. The CXCL12/CXCR4 signal plays a very important role in the cross-talk between different functional cell types in the human maternal/fetal interface.16,17 dNK cells regulate angiogenesis in the placenta and regulate extravillous trophoblast invasion by secreting many cytokines.18 CXCL12 deficiency can lead to developmental defects, bone marrow myelopoiesis, congenital anomalies such as ventricular septal defect and even perinatal mortality.19 Reduced number of dNK cells were seen in patients with IUGR, which was associated with a decrease in trophoblastic invasion in decidua.18 Although a recent study has shown that the elevated levels of CXCL12 in the amniotic fluid could predict pregnancy outcome,20 the levels of this chemokine in IUGR has been yet studied. Herein we examined the levels of CXCL12 in the serum of patients with IUGR in comparison with normal pregnant women and observed that demonstrated that the level of this cytokine was significantly reduced in the patients that the normal counterpart group.
Patients and Materials
Patients
In this study, all pregnant mothers with the gestational age between 24 and 40 weeks who were referred to the Pregnant Mothers Clinic, Besat Hospital, Sanandaj, were screened according to the risk factors of intrauterine growth restriction., IUGR was diagnosed according to the criteria of Figueras and Gratacos21 on the basis of an EFW less than the 10th centile with abnormal blood flow defined by pulsatility index (PI; peak systolic flow velocity − end-diastolic flow velocity)/time averaged flow velocity in UA (PI > 95th percentile) and/or in MCA (PI < 5th percentile) or EFW less than the third centile without Doppler blood flow abnormalities. Patients with platelet dysfunction, aneuploidy detection or embryonic structural malformations, multiple pregnancies, and taking low molecular weight heparin or aspirin within 7 days prior to blood sampling were excluded. Cigarette smoking was not considered as an exclusion criterion because one of the participants in the two groups were smokers. Finally, 36 samples were selected as the case group and the same number of healthy pregnant mothers was considered as the control group. The protocol was approved by the Ethical Research Committee of Kurdistan University of Medical Sciences (KUMS) according to the criteria set by the declaration of Helsinki.
Detection of CXCL12 and Platelets Indices
After receiving written consent and explaining all the steps of the study, the patients were asked to fast from the night before blood sampling. Platelet count and indices were examined by employing blood cell analyzer (Nihon Koden, Tokyo, Japan) and for detection of CXCL12, 2 mL peripheral blood samples were then drawn and then centrifuged at 3000 rpm for 10 minutes within 1 hour after phlebotomy. Serums were separated and kept in a new microtube, labeled and kept at −80 °C until the test. The level of CXCL12 was measured by employing an Enzyme-Linked Immunosorbent Assay (ELISA) kit according to the manufacturer’s instruction (R& D system, Minneapolis, MN). The optical absorbance (OD) was analyzed by ELISA Reader (Tazhir Azema, Tehran, Iran) at 450 nm. Finally, the data were analyzed using independent T-test by SPSS (version 22, Armonk, NY, USA).
Results
In this case–control study, there were no significant relationships between age and gestational age in both the case and the control groups (P = 0.501 and P = 0.806, respectively). Also, there was no significant relationship between the history of abortion, diabetes, and BMI in both groups (P = 0.285, P = 0.735, and P= 0.824, respectively) (Table 1).
 |
Table 1 Demographic Characterization of the Patients with IUGR and Control Groups |
The results of this study showed that there was a significant difference between platelet count in patients with IUGR compared with the control group so that the mean platelet count in patients with IUGR was lower than the mean platelet count in the control group (P = 0.0001). Also, according to the results, the mean MPV in patients with IUGR was more than the control group, which was significant (P = 0.015). In the present study, there was a significant difference between the mean PDW in the two groups, so that the mean PDW was higher in the patients with the IUGR group (P = 0.0001). In contrast, our data show that the mean plateletcrit (PCT) in the control group was higher than the case group and the difference was significant (P = 0.0001) (Table 2).
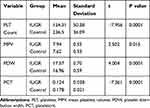 |
Table 2 Comparison of Platelet Indices Between the Patients with IUGR and Control Groups |
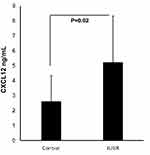
Interestingly, there was a significant difference between the mean serum level of CXCL12 in the case group and the control group, so that the mean serum level of this factor was higher in the case group compared to the control group (P = 0.02) (Figure 1).
Discussion
Reduced fetal growth is seen in approximately 10% of pregnancies but only a minority develop a pathological manifestation known as IUGR.22 IUGR is considered as the most common and complex problem in modern obstetrics by America Obstetrics and Gynecologist.23 In general, any factor that disturbs the balance of nutrition between the mother and the fetus could be considered as a cause for IUGR.1,4,24 Platelet activation is associated with arterial thrombosis which may involve in pathogenesis of several diseases including hypertension, myocardial infarction and stroke.25 Abnormal platelet function and alteration of platelet indices are known to be involved in the pathobiology of various pregnancy-associated diseases including IUGR.9,20 According to the results of this study, there was no significant difference between age, BMI, and gestational age in the two groups (P=0.501, P=0.824, and P=0.806, respectively). Also, there was no significant difference in the history of abortion and the history of diabetes mellitus (P=0.285 and P=0.735, respectively). Moreover, the levels of CXCL12 and the mean value of PDW and MPV were increased in the patients compared to the control group. Conversely, the platelet count and PCT were much higher in the control group than the test.
Norris et al previously showed that platelets aggregation was approximately 50% lower in hypertensive IUGR patients compared with normal pregnancy controls; however, there were no differences in platelet aggregations in normotensive pregnancies and normal pregnancy controls.26 Similarly, Piazze et al demonstrated that increased platelets count and MPV was associated with Doppler velocities in patients with IUGR or preeclampsia compared to normal pregnancy controls.27 In the current study, in contrast to the platelet count that was significantly decreased in IUGR pregnancies, MPV was increased in these patients, indicating that platelet activation leads to enhanced platelet production which directly causes an increase in MPV. Consistently, a recently study has shown that both MPV and PDW were elevated in patients with preeclampsia than normal controls.28 On the other hand, we observed that the number of platelets and the mean value of PCT were decreased in patients with IURG, indicating a continuous consumption and activation of platelets in the patients. Furthermore, previous studies reported that in patients with IUGR, indicating that increased platelet activation in the uteroplacental interface leads to the secretion of vasoactive substances into the maternal circulation, causing subsequent complications such as hypertension.26,29,30
It has been demonstrated that endothelial dysfunction and imbalance in vasoactive factors such as endothelin, nitric oxide, and prostacyclin are features of the placenta-mediated complications of pregnancy.31 Aspirin suppresses the production of prostaglandins and thromboxanes inhibiting platelet aggregation and was associated with reduction of IUGR incidence.32,33 The release of fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin into the maternal circulation causes endothelial dysfunction, which is the common feature of IUGR.34 sFlt-1 is an inhibitor of vascular endothelial growth factor (VEGF) and placental growth factor (PGF), and its upregulation in patients with IUGR may inhibit the functions of VEGF and PGF.35
In the present study, we observed that the levels of CXCL12 were significantly higher in patients with IUGR than the normal pregnancy controls. CXCL12 plays an important role in the formation of blood vessels, which is a key element in the development of the placenta. Dysregulation of placental angiogenesis in pregnancy can cause pregnancy outcomes.36 Following the remodeling of the placental arteries, CXCL12 is responsible for the utero-placental circulation, apoptosis suppression and increase in the survival of trophoblast cells during pregnancy.37 Hwang et al reported in their study that CXCL12 levels were increased in people with preeclampsia in comparison to healthy people, which could be a compensatory mechanism in preeclampsia.38 In healthy subjects, this factor is found in the syncytiotrophoblast layer, especially in the second and third trimester.39 In patients with preeclampsia, the CXCL12 at mRNA levels is expressed in syncytiotrophoblasts, and extravillous cytotrophoblasts. Although the pathophysiology of preeclampsia and IUGR are different, our data show that the levels of CXCL12 are much higher in the serum of the patients with IUGR implying that this chemokine could play a role in the pathogenesis of this disease. Even though the mechanism that regulates the expression of CXCL12 during the cytotrophoblast differentiation has not been completely elucidated, but it seems that this chemokine display many biological activities during fetus development and utero-placental circulation.40 We postulate that elevated levels of CXCL12 could be a compensatory mechanism for either preventing more damage to endothelial cells or chemoattracting endothelial cells to the fetal–maternal interface. Moreover, peripheral blood mononuclear cells of preeclamptic patients express a high amount of hypoxia induced factor-1 (HIF-1a) when exposed to hypoxia.41 Since HIF-1a mediates upregulation of CXCL12 in a hypoxia state,41 we could hypothesis that elevated levels of CXCL12 is somehow a response to hypoxia in patients with IUGR. In addition to the syncytium of placental villi, platelet activation or endothelial damage can also be considered as an alternative source for increasing CXCL-12 in both IUGR and preeclampsia.42 Moreover, circulating endothelial progenitor cells can be mobilized by CXCL12 and play an important role in angiogenesis associated with tissue repairmen after ischemia and endothelium regeneration.43,44 Herein, we envision that the elevated circulating levels of CXCL12 observed in IUGR may function as a rescue mechanism in the repair of maternal endothelium by facilitating the recruitment and homing of endothelial progenitor cell. Taken together, detection of serum CXCL12 and platelets induces might have some benefits for diagnosis of IUGR.
The present study showed that platelet indices are changed in IUGR, and the levels of circulating CXCL12 increased in maternal blood. The results of this study support the hypothesis that imbalance of angiogenic factors/chemokines and platelet dysfunction may play important roles in the development of IUGR, and these findings provide a base for further studies to better defining the pathophysiology of IUGR.
Acknowledgments
This work was supported by grants from the Kurdistan University of Medical Sciences (KUMS) to AJ and SS for supporting of RM’s thesis as a resident in Obstetrics and Gynecology at KUMS.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Walker D, Marlow N. Neurocognitive outcome following fetal growth restriction. Arch Dis Childhood Fetal Neonatal Ed. 2008;93(4):F322–F325. doi:10.1136/adc.2007.120485
2. Murray E, Fernandes M, Fazel M, Kennedy S, Villar J, Stein A. Differential effect of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG. 2015;122(8):1062–1072. doi:10.1111/1471-0528.13435
3. Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6(Suppl 3):332–336.
4. Sharma D, Shastri S, Farahbakhsh N, Sharma P. Intrauterine growth restriction - part 1. J Mater Fetal Neonatal Med. 2016;29(24):3977–3987. doi:10.3109/14767058.2016.1152249
5. Blössner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52:S5–15.
6. Sharma D, Sharma P, Shastri S. Genetic, metabolic and endocrine aspect of intrauterine growth restriction: an update. J Mater Fetal Neonatal Med. 2017;30(19):2263–2275. doi:10.1080/14767058.2016.1245285
7. Wen SW, Goldenberg RL, Cutter GR, Hoffman HJ, Cliver SP. Intrauterine growth retardation and preterm delivery: prenatal risk factors in an indigent population. Am J Obstet Gynecol. 1990;162(1):213–218. doi:10.1016/0002-9378(90)90853-Y
8. Kleijer ME, Dekker GA, Heard AR. Risk factors for intrauterine growth restriction in a socio-economically disadvantaged region. J Mater Fetal Neonatal Med. 2005;18(1):23–30. doi:10.1080/14767050500127674
9. Kanat‐Pektas M, Yesildager U, Tuncer N, Arioz DT, Nadirgil‐Koken G, Yilmazer M. Could mean platelet volume in late first trimester of pregnancy predict intrauterine growth restriction and pre‐eclampsia? J Obstetrics Gynaecol Res. 2014;40(7):1840–1845. doi:10.1111/jog.12433
10. Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstetrics Gynecol. 2010;116(2):402–414. doi:10.1097/AOG.0b013e3181e9322a
11. Scharf RE. Platelet signaling in primary haemostasis and arterial thrombus formation: part 1. Hamostaseologie. 2018;38(4):203–210. doi:10.1055/s-0038-1675144
12. Missfelder‐Lobos H, Teran E, Lees C, Albaiges G, Nicolaides K. Platelet changes and subsequent development of pre‐eclampsia and fetal growth restriction in women with abnormal uterine artery Doppler screening. Ultrasound Obstetrics Gynecol. 2002;19(5):443–448. doi:10.1046/j.1469-0705.2002.00672.x
13. Gioia S, Piazze J, Anceschi M, et al. Mean platelet volume: association with adverse neonatal outcome. Platelets. 2007;18(4):284–288. doi:10.1080/09537100601078448
14. Karin N. The multiple faces of CXCL12 (SDF‐1α) in the regulation of immunity during health and disease. J Leukoc Biol. 2010;88(3):463–473. doi:10.1189/jlb.0909602
15. Jalili A, Shirvaikar N, Marquez-Curtis L, et al. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38(4):321–332. doi:10.1016/j.exphem.2010.02.002
16. Quinn KE, Reynolds LP, Grazul-Bilska AT, Borowicz PP, Ashley RL. Placental development during early pregnancy: effects of embryo origin on expression of chemokine ligand twelve (CXCL12). Placenta. 2016;43:77–80. doi:10.1016/j.placenta.2016.05.008
17. Wang L, Li X, Zhao Y, et al. Insights into the mechanism of CXCL12-mediated signaling in trophoblast functions and placental angiogenesis. Acta Biochim Biophys Sin (Shanghai). 2015;47(9):663–672. doi:10.1093/abbs/gmv064
18. Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065. doi:10.1038/nm1452
19. Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635. doi:10.1038/382635a0
20. Tseng -J-J, Chen Y-F, Hsieh Y-T, Chou -M-M. Elevated amniotic fluid stromal cell-derived factor-1α (SDF-1α) concentration in mid-gestation as a predictor of adverse birth outcomes. J Chin Med Assoc. 2009;72(12):638–642. doi:10.1016/S1726-4901(09)70446-0
21. Figueras F, Gratacos E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36(2):86–98. doi:10.1159/000357592
22. Albu AR, Anca AF, Horhoianu VV, Horhoianu IA. Predictive factors for intrauterine growth restriction. J Med Life. 2014;7(2):165–171.
23. ACOG Practice bulletin no. 134. fetal growth restriction. Obstet Gynecol. 2013;121(5):1122–1133.
24. Hendrix N, Berghella V, editors. Non-Placental Causes of Intrauterine Growth Restriction. Seminars in Perinatology. Elsevier; 2008.
25. Tomaiuolo M, Brass LF, Stalker TJ. Regulation of platelet activation and coagulation and its role in vascular injury and arterial thrombosis. Intervent Cardiol Clin. 2017;6(1):1–12. doi:10.1016/j.iccl.2016.08.001
26. Norris LA, Sheppard BL, Burke G, Bonnar J. Platelet activation in normotensive and hypertensive pregnancies complicated by intrauterine growth retardation. Br J Obstet Gynaecol. 1994;101(3):209–214. doi:10.1111/j.1471-0528.1994.tb13111.x
27. Piazze J, Gioia S, Maranghi L, Anceschi M. Mean platelet and red blood cell volume measurements to estimate the severity of hypertension in pregnancy. J Perinat Med. 2006;34(3):246–247. doi:10.1515/JPM.2006.044
28. Thalor N, Singh K, Pujani M, Chauhan V, Agarwal C, Ahuja R. A correlation between platelet indices and preeclampsia. Hematol Transfusion Cell Ther. 2019;41(2):129–133. doi:10.1016/j.htct.2018.08.008
29. Müllers SM, Burke N, Flood K, et al. Altered platelet function in intrauterine growth restriction: a cause or a consequence of uteroplacental disease? Am J Perinatol. 2016;33(08):791–799. doi:10.1055/s-0036-1572428
30. Ahmed Y, Sullivan MH, Pearce JM, Elder MG. Changes in platelet function in pregnancies complicated by fetal growth retardation. Eur J Obstetrics Gynecol Reprod Biol. 1991;42(3):171–175. doi:10.1016/0028-2243(91)90215-7
31. Groom KM, David AL. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am J Obstet Gynecol. 2018;218(2s):S829–S840. doi:10.1016/j.ajog.2017.11.565
32. Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30(1–2):260–279. doi:10.3109/07420528.2012.717455
33. McCowan LM, Figueras F, Anderson NH. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am J Obstet Gynecol. 2018;218(2s):S855–S868. doi:10.1016/j.ajog.2017.12.004
34. Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi:10.1056/NEJMoa055352
35. Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PlGF) and soluble Flt-1 by oxygen—a review. Placenta. 2000;21:S16–S24. doi:10.1053/plac.1999.0524
36. Mayhew T, Charnock-Jones D, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25(2–3):127–139.
37. Jaleel MA, Tsai AC, Sarkar S, Freedman PV, Rubin LP. Stromal cell-derived factor-1 (SDF-1) signalling regulates human placental trophoblast cell survival. Mol Hum Reprod. 2004;10(12):901–909. doi:10.1093/molehr/gah118
38. Hwang HS, Kwon HS, Sohn IS, Park YW, Kim YH. Increased CXCL12 expression in the placentae of women with pre-eclampsia. Eur J Obstetrics Gynecol Reprod Biol. 2012;160(2):137–141. doi:10.1016/j.ejogrb.2011.10.007
39. Red-Horse K, Drake PM, Gunn MD, Fisher SJ. Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am J Pathol. 2001;159(6):2199–2213. doi:10.1016/S0002-9440(10)63071-4
40. Schanz A, Winn VD, Fisher SJ, et al. Pre-eclampsia is associated with elevated CXCL12 levels in placental syncytiotrophoblasts and maternal blood. Eur J Obstetrics Gynecol Reprod Biol. 2011;157(1):32–37. doi:10.1016/j.ejogrb.2011.02.023
41. Rajakumar A, Michael HM, Rajakumar PA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26(7):563–573. doi:10.1016/j.placenta.2004.09.001
42. Massberg S, Konrad I, Schürzinger K, et al. Platelets secrete stromal cell–derived factor 1α and recruit bone marrow–derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203(5):1221–1233. doi:10.1084/jem.20051772
43. Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. The Lancet. 2003;362(9385):697–703. doi:10.1016/S0140-6736(03)14232-8
44. Dimova I, Karthik S, Makanya A, et al. SDF-1/CXCR4 signalling is involved in blood vessel growth and remodelling by intussusception. J Cell Mol Med. 2019;23(6):3916–3926. doi:10.1111/jcmm.14269
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.