Back to Journals » Cancer Management and Research » Volume 12
Plasmacytoid Dendritic Cell Infiltration in Acute Myeloid Leukemia
Authors Zhu L, Wang P, Zhang W, Li Q, Xiong J, Li J, Deng X, Liu Y, Yang C, Kong P, Peng X, Zhong JF, Rao J, Zhang X
Received 5 May 2020
Accepted for publication 8 September 2020
Published 6 November 2020 Volume 2020:12 Pages 11411—11419
DOI https://doi.org/10.2147/CMAR.S260825
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Lidan Zhu,1,2,* Ping Wang,1,2,* Wei Zhang,1,2,* Qiong Li,1,2 Jingkang Xiong,1,2 Jiali Li,1,2 Xiaojuan Deng,1,2 Yao Liu,1,2 Chao Yang,1,2 Peiyan Kong,1,2 Xiangui Peng,1,2 Jiang F Zhong,3 Jun Rao,1,2 Xi Zhang1,2
1Medical Center of Hematology, Xinqiao Hospital, Army Medical University, Chongqing 400037, People’s Republic of China; 2State Key Laboratory of Trauma, Burns and Combined Injury, Army Medical University, Chongqing 400037, People’s Republic of China; 3Department of Otolaryngology, Keck School of Medicine, University of Southern California, Los Angeles, CA, 90033, USA
*These authors contributed equally to this work
Correspondence: Jun Rao; Xi Zhang Email [email protected]; [email protected]
Introduction: Increasing evidence has demonstrated that plasmacytoid dendritic cells (PDCs) in the tumor microenvironment (TME) play an important role in tumorigenesis and progression. PDC infiltration has been found in certain malignancies such as classic Hodgkin’s lymphoma and chronic myelomonocytic leukemia. Our previous work reported that PDC infiltration could occur in acute myeloid leukemia (AML), but the clinical significance of PDC in AML has not been thoroughly investigated.
Patients and Methods: Here, we evaluated the clinical significance of PDC to AML transition in a leukemia microenvironment. The frequency of PDCs in 80 acute myelomonocytic leukemia (AML-M4) and 83 acute monocytic leukemia (AML-M5) patients was determined by flow cytometry.
Results: We found 62 cases with PDC infiltration. These patients showed higher numbers of bone marrow blasts, higher mean Hb concentration, and required more cycles of chemotherapy before achieving complete remission (CR), but had lower white blood cell and platelet counts compared to patients without PDC infiltration. Drug sensitivity analysis showed that patients with PDC infiltration had lower sensitivity to standard chemotherapy regimens. Kaplan–Meier survival curves demonstrated that patients with PDC infiltration had a shorter overall survival (OS) time and progression-free survival time.
Discussion: These results suggested that PDC infiltration can be used for risk stratification of AML-M4/M5, and PDCs may transdifferentiate into leukemia in an AML microenvironment.
Keywords: acute myeloid leukemia; AML, tumor-forming plasmacytoid dendritic cells; TF-PDCs, prognosis, hematopoietic stem cell transplantation
Introduction
Acute myeloid leukemia (AML) is a heterogeneous clonal disorder characterized by abnormal differentiation and unregulated proliferation of hematopoietic progenitor cells (blasts), leading to defects in hematopoiesis.1,2 Immunophenotyping is a widely used method for identification of specific cell lineage as well as maturation stage in the leukemia process, which can complement morphology, cytochemistry, and karyotype to make a more precise diagnosis and classification of AML.3–5 Accurate immunophenotyping is essential for fully understanding the heterogeneity and interactions among leukemia cells and accessory cells in the tumor microenvironment (TME).
Dendritic cells (DCs) are “assistants” in the immune system which can initiate adaptive immunity and shape immune responses.6,7 DCs can be divided into conventional myeloid DC (MDC) and plasmacytoid DC (PDC) lineages. PDCs lack the cell surface markers for T and B cells, and strongly express CD123, HLA-DR and BDCA-2.8–11 Stimulated PDCs have strong antigen-presenting ability, which plays a critical role in the recruitment of natural killer cells, activation of T cells, and polarization of Th1 and Th2 cells. Some studies have demonstrated that PDCs can be found in a wide range of human disorders, including Kikuchi Fujimoto lymphadenopathy, hyaline-vascular Castleman disease, and certain malignancies such as classical Hodgkin’s lymphoma and chronic myelomonocytic leukemia.12–14 In particular, PDC infiltration has been found in the bone marrow and lymph nodes of patients with myeloid neoplasm; this rare condition is described as involving “tumor-forming PDCs” (TF-PDCs)13,15–17 Our previous reports described 3 cases of TF-PDCsassociated with AML. These PDCs had similar chromosomal abnormalities to those observed in leukemia cells, suggesting that TF-PDCs may be derived from undifferentiated myeloid leukemia. However, the detection ratio of TF-PDCs was low among all AML patients, so the associated clinicopathological features and significance for prognosis could not be investigated thoroughly.16
PDCs can be generated from non-leukemic cells, such as CD34+ myeloid progenitors and CD14+ monocytes, and our previous work demonstrated that TF-PDCs are likely to originate from the monocytic lineage. Acute myelomonocytic/monocytic leukemia (AML-M4/M5, respectively) may therefore provide an excellent opportunity to study the prognostic significance of TF-PDCs. In this study, we examined TF-PDCs in the bone marrow of 163 AML-M4/M5 patients. The results showed that the presence of TF-PDCs was significantly correlated with patients’ WBC count, Hb concentration, platelet count, bone marrow blasts and number of chemotherapy cycles needed to achieve complete remission (CR). The survival time of patients with TF-PDCs was shorter than that of TF-PDC negative patients, and patients with TF-PDCs also showed lower median sensitivity to standard chemotherapy regimens. Multivariate analysis showed that the presence of TF-PDCs represents an independent prognostic indicator of overall survival (OS). We also found that allogeneic hematopoietic stem cell transplantation (allo-HSCT) can significantly reduce leukemia residual disease and improve TF-PDC positive patients’ survival, suggesting that allo-HSCT might be an optimal choice for patients with TF-PDC infiltration due to its ability to change the microenvironment to be less conducive to PDC-to-leukemia transition.
Patients and Methods
Patient Selection
Between February 2015 and July 2018, 163 previously untreated AML-M4/M5 patients were retrospectively analyzed. All consecutive patients who were appropriate for this study during the period were included without selection. Histological diagnoses were made independently by at least two experienced senior pathologists based on the French-American-British (FAB) classification. Laboratory examinations of morphology, cytochemistry, cytogenetic and molecular biology testing were performed following standard protocols. The sample collection and following study were completed with the approval of the ethics committee in our institution, and all patients gave written informed consent. The response criteria for CR were assessed by conventional diagnostic methods using the International Working Group criteria. OS was defined as time from diagnosis to the date of death or last follow-up, and relapse-free survival (RFS) was defined as the time interval from when CR was obtained to the date of disease relapse after primary treatment.
Flow Cytometric Analysis
The identification schema of TF-PDC was the same as in our previous work.16 A two-step FCM analysis strategy was followed for all AML patients. First, using CD117, HLA-DR, CD34, and CD123, we identified myeloid primordial cells and made a diagnosis of AML. Second, gating on the CD123 positive cells, by using CD304, CD56, CD11c, CD13, CD33, CD15, CD14, CD64, CD34, CD117, CD38, we identified TF-PDCs as Lin−HLA-DRbri+CD123bri+CD304+CD56+CD11c−CD13+CD33+CD15−CD14−CD64−CD34−CD117−CD38+. TF-PDC status was classified as detectable (TF-PDC positive) or undetectable (TF-PDC negative) based on clear population assayed by flow cytometry.
Treatment Strategy
After calculating the cytogenetic risk-group categories for all patients at the time of diagnosis, all patients were administered an induction therapy regimen consisting of cytarabine at a dosage of 100–200 mg/m2 daily as a continuous infusion for 7 days, in combination with daunorubicin at a daily dosage of 60–90mg/m2 on days 1–3. The post-induction phase was carried out with one consolidation cycle (same drugs and doses as the induction phase) and two intensification cycles consisting of three days of idarubicin (10mg/m2/day) and high-dose cytarabine (3g/m2/day). For those patients who underwent allo-HSCT, donor selection, stem cell mobilization and collection, conditioning regimens and GVHD prophylaxis were performed according to reported criteria.18–20
Drug Sensitivity Analysis
Drug sensitivity was analyzed using the High-throughput Drug sensitivity and functional Genes analysis Strategy (HDGS) (PRECDO Bio-pharmaceutical, Hefei, China). Briefly, 49 chemotherapy drugs, 9 targeted drugs, and 35 combined therapy strategies were included. Sensitivity was identified according to the inhibition ratio: Low sensitivity was defined as 30% ≤ inhibition ratio < 50%; median sensitivity was defined as 50% ≤ inhibition ratio <70%; and high sensitivity was defined as inhibition ratio ≥ 70%.
Statistical Analysis
Statistical analysis was conducted using SPSS software (Version 18.0, LEAD cop). The normality distribution was estimated with the Kolmogorov-Smirnov test. The correlation of TF-PDC positivity with clinicopathologic features of patients was assessed by the Pearson χ2 test. Survival estimates were obtained using the Kaplan–Meier method, and comparisons were made using Log rank tests when analyzing the survival differences between TF-PDC positive and negative AML patients; patients who underwent bone marrow transplant were excluded. Unpaired Student’s t-test for comparisons of two groups and one-way ANOVA for multiple group data were applied in this study. A COX proportional regression model was used to calculate the survival hazard ratio (HR). Statistical differences were considered significant if the P-value was less than 0.05.
Results
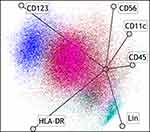
One hundred sixty-three newly diagnosed AML patients were analyzed using flow cytometry. A detection scheme was performed according to our previous work. In order to identify abnormal cell clusters, a radar plot was generated according to different expression of cell surface markers. In Figure 1, the blue cluster represents TF-PDCs. In order to distinguish blastic plasmacytoid dendritic cell neoplasm (BPDCN), TCL1 expression was also evaluated. In TF-PDC positive patients, TCL1 expression was negative in the PDC population. TF-PDC patients’ demographics and clinical characteristics are summarized in Table 1. The age of patients ranged from 8–60 years, with a mean age of 46.9 ± 13.2 years. Sixty-two cases were TF-PDC positive, accounting for 38.03% of patients. The PDCs constituted an average of 2.8% of the mononuclear cells in the bone marrow in these patients. In addition, the presence of TF-PDCs was significantly correlated with the number of chemotherapy cycles required to achieve CR, ELN risk classification, FLT3-ITD mutation, patients’ WBC count, Hb concentration, platelet count and bone marrow blasts. The presence of TF-PDCs did not significantly correlate with the gender, age, cytogenetic abnormality, or FAB classification.
 |
Table 1 Relationship Between TF-PDC and Clinicopathological Features of AML Patients |
In order to explore the origins of TF-PDCs, karyotype analysis of leukemia blasts and sorted TF-PDCs were performed. In most of the patients, the same chromosomal abnormalities were detected in PDCs and leukemia blasts, such as AML1 amplification, BCR amplification, CBFB-MYH11 fusion, MLL amplification, ETO amplification, etc. (Figure 2), suggesting these types of PDCs might originate from leukemia blasts.
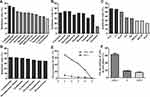
We next examined whether PDCs could influence the drug sensitivity of the patients. Drug sensitivity was evaluated through High-throughput Drug sensitivity and functional Genes analysis Strategy (HDGS) and we found that leukemia cells from TF-PDC positive patients showed low sensitivity to cytarabine, idarubicin, cladribine, homoharringtonie, fludarabine, decitabine, venetoclax, bortezomib, tretinoin, vincristine, cyclophosphamide, methotrexate and methylprednisolone; medium sensitivity to vinflunine and carboplatin; and resistance to pirarubicin and sorafenib (Figure 3A–C). Of note, the leukemia cells of TF-PDC positive patients only showed a moderate level of sensitivity to a standard chemotherapy regimen (IA, DAC), and resistance to the HD-DA, HOAP, HA regimen (Figure 3D). Results of serial monitoring of patients’ bone marrow blast cells and PDC cells showed that the percentages of both types of cells dropped over time, but the blast cells decreased more than the PDCs (Figure 3E). Furthermore, cell cycle analysis by flow cytometry indicated that PDCs were in G0/G1 phase (Figure 3F), consistent with the fact that PDCs possess a low proliferation index. These results suggested that TF-PDC positive AML patients might have primary chemotherapeutic resistance, and TF-PDC in the AML microenvironment might be the cause of drug resistance and disease relapse.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) can significantly reduce leukemia residual disease and has been able to cure many AML patients. In our cohort, in order to avoid the bias due to HSCT, survival analysis was performed on patients who did not undergo HSCT. The OS for TF-PDC negative patients was significantly longer than that of TF-PDC positive patients (Figure 4A), suggesting that TF-PDC might be a poor prognostic factor for AML patients. In this study, we also demonstrated that survival of patients who underwent allo-HSCT was superior to that of those who underwent chemotherapy alone (Figure 4B). Interestingly, for TF-PDC positive patients, we found that allo-HSCT lengthened survival relative to chemotherapy alone (Figure 4C, P<0.001) and patients who underwent allo-HSCT were able to remain negative for minor residual disease (MRD) during the follow-up, suggesting that intensive chemotherapy is suitable for TF-PDC positive patients and allo-HSCT might be an optimal therapy strategy for them. In addition, survival analysis also showed that allo-HSCT provided a prognosis advantage in TF-PDC negative patients (Figure 4D). DFS analysis showed that survival of TF-PDC negative patients was superior (Figure 4E), and the incidence of relapse in TF-PDC positive patients was much higher than in TF-PDC negative patients (Figure 4F). The results of univariate and multivariate analysis for risk factors contributing to OS in patients are summarized in Table 2. Multivariate analysis of pretreatment factors revealed that cytogenetic abnormality, platelet count, FLT3-ITD mutation and TF-PDC expression were independent prognostic indicators of OS, and multivariate analysis of treatment factors showed that disease relapse was a sole prognostic factor (Table 3). In summary, all our results demonstrated that TF-PDC in the BM microenvironment is a poor prognostic factor for AML patients.
 |
Table 2 Univariate and Multivariate Analyses of Pretreatment Factors on OS of the patients |
 |
Table 3 Univariate and Multivariate Analyses of Treatment Associated Factors on OS of the Patients |
Discussion
The BM microenvironment plays an important role in regulation of leukemia cell growth, progression and drug resistance. Multiple lines of evidence have demonstrated that cellular components (such as bone marrow stromal cells, macrophages and osteoclasts) can regulate tumor growth. In this study, we evaluated the prognostic significance of another BM cellular component, namely TF-PDCs, to leukemia transition in AML patients.
This study is the first to explore the diagnostic value of TF-PDCs in a large cohort. We found that TF-PDC positive patients had shorter survival times than the TF-PDC negative cohort and moderate sensitivity to standard chemotherapy regimens. Moreover, we observed that allo-HSCT could maintain MRD negative status and improve the prognosis of TF-PDC positive patients. All these results suggested that TF-PDCs might play a protective role and transdifferentiate into leukemia cells in the AML microenvironment. Allo-HSCT might be an optimal therapeutic strategy for patients with TF-PDC infiltration.
Based on our previous case reports and the existing literature, PDCs can be generated from non-leukemic cells (such as CD34+ myeloid progenitors and CD14+ monocytes), but crucially can also differentiate from leukemic blasts of AML and MDS under rare conditions.21–23 Our previous work demonstrated that TF-PDCs are prone to monocytic lineage pathology and share the same chromosomal abnormalities as leukemia blasts. From this we inferred that AML-M4 and -M5 may be optimal diseases through which to explore the clinical significance of TF-PDCs. In a previous study that examined the interval from from November 2013 to September 2016, 1.13% of patients with AML were detected to have TF-PDC infiltration. In this study, we used the same scheme to examine the TF-PDCs in the patients with AML-M4 and AML-M5. The detection ratio increased to 38.03%, suggesting that TF-PDCs in AML (M4 and M5) might be differentiated from leukemia blasts or leukemia stem cells. Evidence has established that DC progenitors require factor FMS-like tyrosine kinase 3 ligand (FL) to expand and differentiate. FL is proposed to be a critical regulator that maintains the hemostasis and frequency of DCs.24,25 An internal tandem duplication (ITD) mutation in FLT3 is closely correlated with a particularly poor prognosis and increased incidence of relapse in AML patients.26–28 Rickmann et al29 analyzed the frequency of PDCs in diagnostic samples from 47 FLT3-ITD− and 40 FLT3-ITD+ AML patients, and demonstrated that the frequency of PDCs in FLT3-ITD+ AML patients is higher than in ITD− AML patients and normal healthy controls; interestingly, PDCs sorted from ITD+ AML patients had the same ITD insert mutation seen in their leukemia cells. FLT3 is homologous to c-Kit and highly expressed in hematopoietic progenitor cells. Some studies have shown that FLT3 ligand injection can lead to expansion of PDCs in lymphoid and non-lymphoid organs, and furthermore, in mice, genetic deletion of FLT3 ligand or treatment with FLT3 inhibitors can decrease the frequency of PDCs.24,30,31 These results demonstrated that the FL/FLT3 signaling pathway might be involved in expansion of tumor-associated PDCs in AML patients, suggesting these cells have close crosstalk with leukemia stem cells or leukemia cells.
DCs present antigens and stimulate specific T-cell response; they are responsible for recruitment and activation of NK cells and differentiation of naïve T cells. In AML patients, tumor-associated PDCs have been found to express some leukemia-related proteins in studies by our group and others. However, this does not mean PDCs can be efficiently presented to T cells. Some studies showed that AML PDCs cannot acquire co-stimulatory molecules CD80 and CD86, and expression of HLA-DR in PDC-positive AML was found to be significantly lower than in healthy controls.32 On the other hand, PDCs are known to secrete large amounts of type I IFN after microbial infection. Type I IFN plays a crucial role in enhancing the cytotoxic activity of NK cells and macrophages, maintaining the survival and development of T cells.33,34 Type I IFN has been observed to treat AML in some xenograft studies and clinical trials. Long-acting IFN can have significant antileukemic activity in vivo. In the AML patients, the secretion of IFN by AML PDCs is significantly decreased,32 suggesting that TF-PDCs in the TME might protect leukemia cells or leukemia stem cells from elimination by antileukemic immunity. In summary, since AML PDCs have the same cytogenetic abnormalities as leukemia cells and interfere in innate and adaptive immune responses against tumors, we suggest that they can be defined as malignant clones.
Current standard chemotherapy for AML is IA (cytarabine and idarubicin), but in our cohort, we found that patients with TF-PDC were likely resistant to chemotherapeutics, and in particular had low sensitivity to cytarabine, idarubicin, cladribine, homoharringtonie, fludarabine, decitabine, venetoclax, bortezomib, tretinoin, vincristine, cyclophosphamide, methotrexate and methylprednisolone; medium sensitivity to vinflunine and carboplatin; and resistance to pirarubicin and sorafenib. Of note, patients showed a moderate level of sensitivity to the standard chemotherapy regimen for AML and ALL. Therefore, in our clinical practice, in order to determine the optimal treatment for these patients, we chose chemotherapeutic drugs to which they were moderately sensitive and then performed allogeneic stem cell transplantation after achieving the first CR. In this way, the survival time of patients with TF-PDCs associated with AML could be prolonged significantly, suggesting that allo-HSCT might be an optimal therapeutic strategy.
In summary, patients positive for TF-PDCs showed shorter survival times compared to those without evidence of PDCs. TF-PDC positive patients might have primary resistance to standard AML chemotherapy regimens, which may underlie why the presence of TF-PDCs correlates with poorer prognosis for AML patients. Our preliminary work explored the therapeutic strategies for these patients and found that allo-HSCT may be an optimal therapy, but multicenter prospective trials are required to confirm.
Ethics Statement
The study was conducted according to the principles of the Declaration of Helsinki and was approved by the ethics committee of Xinqiao Hospital, Chongqing 400037, China.
Acknowledgments
This Study is supported by grants from the National Natural Science Fund (Nos. 81600166, 81873424), Science and Technology Innovation Promotion Project of AMU (2019XLC3020), Project of Key Laboratory of Tumor Immunopathology and Ministry of Education of China (2018jsz102).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McCulloch EA, Kelleher CA, Miyauchi J, et al. Heterogeneity in acute myeloblastic leukemia. Leukemia. 1988;2(12Suppl):38S–49S.
2. Takahashi K, Wang F, Morita K, et al. Integrative genomic analysis of adult mixed phenotype acute leukemia delineates lineage associated molecular subtypes. Nat Commun. 2018;9(1):2670. doi:10.1038/s41467-018-04924-z
3. Terstappen LW, Safford M, Konemann S, et al. Flow cytometric characterization of acute myeloid leukemia. Part II. Phenotypic heterogeneity at diagnosis. Leukemia. 1992;6(1):70–80.
4. Gorczyca W, Sun ZY, Cronin W, Li X, Mau S, Tugulea S. Immunophenotypic pattern of myeloid populations by flow cytometry analysis. Methods Cell Biol. 2011;103:221–266.
5. Buccheri V, Matutes E, Dyer MJ, Catovsky D. Lineage commitment in biphenotypic acute leukemia. Leukemia. 1993;7(6):919–927.
6. Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi:10.1146/annurev.immunol.18.1.767
7. Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122(2):128–130. doi:10.1016/j.imlet.2008.11.017
8. Rovati B, Mariucci S, Manzoni M, Bencardino K, Danova M. Flow cytometric detection of circulating dendritic cells in healthy subjects. Eur J Histochem. 2008;52(1):45–52. doi:10.4081/1185
9. Obregon C, Kumar R, Pascual MA, Vassalli G, Golshayan D. Update on dendritic cell-induced immunological and clinical tolerance. Front Immunol. 2017;8:1514. doi:10.3389/fimmu.2017.01514
10. Rasaiyaah J, Yong K, Katz DR, Kellam P, Chain BM. Dendritic cells and myeloid leukaemias: plasticity and commitment in cell differentiation. Br J Haematol. 2007;138(3):281–290. doi:10.1111/j.1365-2141.2007.06622.x
11. Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16(6):392–404. doi:10.1097/PAP.0b013e3181bb6bc2
12. Baddoura FK, Hanson C, Chan WC. Plasmacytoid monocyte proliferation associated with myeloproliferative disorders. Cancer. 1992;69(6):1457–1467. doi:10.1002/1097-0142(19920315)69:6<1457::AID-CNCR2820690625>3.0.CO;2-F
13. Song HL, Huang WY, Chen YP, Chang KC. Tumorous proliferations of plasmacytoid dendritic cells and Langerhans cells associated with acute myeloid leukaemia. Histopathology. 2012;61(5):974–983. doi:10.1111/j.1365-2559.2012.04282.x
14. Vermi W, Facchetti F, Rosati S, et al. Nodal and extranodal tumor-forming accumulation of plasmacytoid monocytes/interferon-producing cells associated with myeloid disorders. Am J Surg Pathol. 2004;28(5):585–595.
15. Dargent JL, Delannoy A, Pieron P, Husson B, Debecker C, Petrella T. Cutaneous accumulation of plasmacytoid dendritic cells associated with acute myeloid leukemia: a rare condition distinct from blastic plasmacytoid dendritic cell neoplasm. J Cutan Pathol. 2011;38(11):893–898. doi:10.1111/j.1600-0560.2011.01777.x
16. Ping Wang YF, Deng X, Liu S, et al. Tumor-forming plasmacytoid dendritic cells in acute myelocytic leukemia: a report of three cases and literature review. Int J Clin Exp Pathol. 2017;10(7):7285–7291.
17. Wang M, Chen YJ, Wang LR, Wang YZ, Lu J. Plasmacytoid dendritic cells proliferation coexisted with acute myeloid leukemia. Chin Med J (Engl). 2018;131(15):1866–1867. doi:10.4103/0366-6999.237404
18. Gao L, Gong Y, Zhang C, Chen XH, Zhang X. Reduced-intensity conditioning therapy with fludarabine, idarubicin, busulfan and cytarabine for allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia and myelodysplastic syndrome. Leuk Res. 2013;37(11):1482–1487. doi:10.1016/j.leukres.2013.08.016
19. Zhang X, Li Y, Zhang Y, et al. Etoposide in combination with low-dose CAG (cytarabine, aclarubicin, G-CSF) for the treatment of relapsed or refractory acute myeloid leukemia: a multicenter, randomized control trial in southwest China. Leuk Res. 2013;37(6):657–664. doi:10.1016/j.leukres.2013.03.005
20. Gao L, Zhang Y, Hu B, et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol. 2016;34(24):2843–2850. doi:10.1200/JCO.2015.65.3642
21. Gluckman JC, Canque B, Chapuis F, Rosenzwajg M. In vitro generation of human dendritic cells and cell therapy. Cytokines Cell Mol Ther. 1997;3(3):187–196.
22. Kremser A, Dressig J, Grabrucker C, et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: an evaluation of different methods. J Immunother. 2010;33(2):185–199. doi:10.1097/CJI.0b013e3181b8f4ce
23. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi:10.1146/annurev-immunol-020711-074950
24. Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198(2):305–313. doi:10.1084/jem.20030323
25. Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99(1):310–318.
26. Li L, Piloto O, Kim K-T, et al. FLT3/ITD expression increases expansion, survival and entry into cell cycle of human haematopoietic stem/progenitor cells. Br J Haematol. 2007;137(1):64–75. doi:10.1111/j.1365-2141.2007.06525.x
27. Bruserud O, Hovland R, Wergeland L, Huang TS, Gjertsen BT. Flt3-mediated signaling in human acute myelogenous leukemia (AML) blasts: a functional characterization of Flt3-ligand effects in AML cell populations with and without genetic Flt3 abnormalities. Haematologica. 2003;88(4):416–428.
28. Munoz L, Aventin A, Villamor N, et al. Immunophenotypic findings in acute myeloid leukemia with FLT3 internal tandem duplication. Haematologica. 2003;88(6):637–645.
29. Rickmann M, Krauter J, Stamer K, et al. Elevated frequencies of leukemic myeloid and plasmacytoid dendritic cells in acute myeloid leukemia with the FLT3 internal tandem duplication. Ann Hematol. 2011;90(9):1047–1058. doi:10.1007/s00277-011-1231-2
30. Anandasabapathy N, Breton G, Hurley A, et al. Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant. 2015;50(7):924–930. doi:10.1038/bmt.2015.74
31. Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184(5):1953–1962. doi:10.1084/jem.184.5.1953
32. Mohty M, Jarrossay D, Lafage-Pochitaloff M, et al. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood. 2001;98(13):3750–3756. doi:10.1182/blood.V98.13.3750
33. Joslyn RC, Forero A, Green R, Parker SE, Savan R. Long noncoding RNA signatures induced by toll-like receptor 7 and type I interferon signaling in activated human plasmacytoid dendritic cells. J Interferon Cytokine Res. 2018;38(9):388–405. doi:10.1089/jir.2018.0086
34. Breton G, Lee J, Zhou YJ, et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med. 2015;212(3):401–413. doi:10.1084/jem.20141441
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.