Back to Journals » Clinical Ophthalmology » Volume 14
Plasma Rich in Growth Factors for the Treatment of Cicatrizing Conjunctivitis
Authors de la Sen-Corcuera B , Montero-Iruzubieta J , Sánchez-Ávila RM , Orive G, Anitua E , Caro-Magdaleno M , Merayo-Lloves J
Received 4 March 2020
Accepted for publication 4 June 2020
Published 17 June 2020 Volume 2020:14 Pages 1619—1627
DOI https://doi.org/10.2147/OPTH.S252253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Borja de la Sen-Corcuera,1,2 Jesús Montero-Iruzubieta,3,4 Ronald M Sánchez-Ávila,1,5 Gorka Orive,1,2 Eduardo Anitua,1,2 Manuel Caro-Magdaleno,4 Jesús Merayo-Lloves5
1Biotechnology Institute (BTI), Vitoria, Spain; 2University Institute for Regenerative Medicine and Oral Implantology (UIRMI), Vitoria, Spain; 3Clínica Cartujavisión, Sevilla, Spain; 4Hospital Universitario Virgen de Macarena, Sevilla, Spain; 5Instituto Universitario Fernández-Vega, Fundación de Investigación Oftalmológica, Universidad de Oviedo, Oviedo, Spain
Correspondence: Ronald M Sánchez-Ávila
Instituto Universitario Fernández-Vega, Fundación de Investigación Oftalmológica, Avda Dres Fernández-Vega Num 34, Oviedo E-33012 Principado de Asturias, Spain
Tel +34 985240141
Fax +34 985233288
Email [email protected]
Purpose: The objective was to evaluate the clinical results obtained from the use of immunosafe plasma rich in growth factors (isPRGF) in the treatment of patients with cicatrizing conjunctivitis (CC) who had not responded to the usual therapy.
Patients and Methods: This is a retrospective study that included patients diagnosed with CC, in whom isPRGF was used in different phases (I: eye drops; II: eye drops and injectable; III: eye drops, injectable and surgical treatment) to achieve control of the inflammation. As a clinical follow-up of the patients, the better corrected visual acuity (BCVA), degree of inflammation (measured from 1 to 4), the severity of the CC, Schirmer I test, IOP and TBUT were analyzed. The adverse events were also evaluated.
Results: Ten eyes (6 patients) were evaluated, 50% corresponded to Stevens–Johnson Syndrome and 50% to ocular mucous membrane pemphigoid. The mean age was 59.7 ± 16.5 (39– 80) years, and 50% were women. Fifty per cent of the cases were initially considered severe CC, and 10% of the cases (one eye of one patient) were considered severe CC at the end of the treatment (p = 0.046). The initial degree of inflammation was 2 in 4 eyes, 3 in two eyes, and 4 in 4 eyes, and final inflammation degree was 1 in all cases (p = 0.004). Twenty per cent of the cases achieved stability in Phase I of the treatment with immunosafe PRGF, 70% with both Phases I and II, and only one case underwent Phase III to achieve stability. The IOP improved significantly (p = 0.027) though the BCVA, TBUT and Schirmer I test showed no significant changes. The follow-up time was 23.1 ± 6.7 (13.6– 30.3) months. No adverse effects were reported.
Conclusion: Treatment with PRGF technology in its injectable and topical immunosafe formulations may be a novel alternative for the treatment of patients with CC, given its complement activity modulating effect, as well as its anti-inflammatory, antifibrotic and regenerative properties.
Keywords: immunosafe plasma rich in growth factors, isPRGF, Stevens–Johnson syndrome, ocular mucous membrane pemphigoid, blood derivatives
Introduction
Cicatrizing conjunctivitis (CC) belongs to the group of rare diseases, which can lead to blindness due to its persistent conjunctival inflammation and progressive fibrosis.1 Corneal blindness can be the result of infectious keratitis, limbal stem cell deficiency (LSCD), persistent epithelial defects (PED), and even corneal perforations. The incidence of cicatrizing conjunctivitis is mostly unknown. Several studies in Europe have attempted to measure the incidence of the systemic disease that may cause CC with poor results. In some of these studies the incidence of CC has been estimated at 1.5/million inhabitants in Australia and New Zealand and 1.3/million inhabitants in the United Kingdom2 so it may be defined as a group of rare diseases. The main aetiology of CC is the Ocular mucous membrane pemphigoid (OcMMP) reaching 60% of cases.
Mucous membrane pemphigoid (MMP) is a systemic autoimmune disease, which injures the mucous membranes of different tissues (conjunctiva, nasal cavity, oropharynx, genital, trachea and skin), leading to the formation of blisters, with chronic inflammation and progressive fibrosis, and secondary failure of the organs.3 Other causes of CC include Stevens–Johnson syndrome/Toxic Epidermal Necrolysis (SJS/NET), linear IgA disease, epidermolysis bullosa acquisita, CC induced by drugs, graft versus host disease (GVHD), mucocutaneous paraneoplastic disorders, atopic keratoconjunctivitis, ocular rosacea and squamous neoplasms of the ocular surface.1 The average time to OcMMP diagnosis is 2.5 years due to the difficulty in diagnosing CC diseases in a first consultation.3
Some guidelines are useful to make a better approximation to the diagnosis including microscopic evaluation of a conjunctival biopsy or the photographic screening (focusing on the shortening of the fornix, subconjunctival scarring, symblepharon, limbic insufficiency and the cicatricial state of the cornea). However, some others, such as the use of direct immunofluorescence (DIF), have a high false-negative rate.3,4
Different laboratory blood tests (detection of autoantibodies, inflammatory markers) are also among the aids for CC diagnoses. Immune-mediated inflammation, the activity of pro-fibrotic and pro-inflammatory cytokines, are some of the main factors that contribute to the progression of CC. These factors may lead to several conditions such as conjunctival scarring and their associated complications (entropion, trichiasis, lagophthalmos, dry eye), ocular surface diseases (PED, corneal infections, corneal neovascularization, corneal scarring) and LSCD. Likewise, all these factors may contribute to persistent ocular pain.5
The regenerative effect of plasma rich in growth factors (PRGF) in the ophthalmology field has been demonstrated in different pathologies of the ocular surface and cornea. Some examples include dry eye disease with different etiologies, neurotrophic keratopathies, corneal ulcers,6,7 patients with compromised inflammatory disorders (glaucoma),8 or even patients who have been previously treated with other blood derivatives or amniotic membranes having unsatisfactory results.11,12 Several in vitro and in vivo studies have been carried out to illustrate some of the most relevant biological effects of PRGF. Of particular importance, PRGF exerts anti-inflammatory, anti-fibrotic, anti-microbial and regenerative effects that are key for its therapeutic use.13 PRGF also increases the proliferation and migration activity of corneal keratocytes and conjunctival fibroblasts, reducing the TGFβ-1 activity, leading to a tissue regeneration approach that limits the fibrosis pathways.14–16
It has been reported that immunosafe PRGF (isPRGF) treatment, which implies a 1-hour extra heat treatment at 56º C, preserves the content of most of the proteins and morphogens involved in the wound healing process while reduces the content of IgE and complement activity drastically.20 In diseases related to an immune system unbalance, and an acute inflammatory response such as Sjögren Syndrome and GVHD, the effect of isPRGF has been evaluated with satisfactory results.9,10
In the present study, the biological role of isPRGF on CC was explored as a novel approach to this rare disease. To assess this disease, a case series study was carried out with a long-term follow-up to evaluate its efficacy and safety.
Patients and Methods
This is a retrospective, interventional, non-comparative study of the results and series of cases of patients diagnosed with CC of several origins and refractory to other therapies. It was performed at CartujaVisión Clinic (Seville, Spain) between January 2016 and June 2019, and the Institutional Review Board approved the study. The principles of the Declaration of Helsinki were followed, and all patients signed informed consent before being of PRGF therapy.
The CC is a group of inflammatory diseases in which the OcMMP is the most frequent. Diagnosis for CC is based on clinical findings; the evolution of the disease may or may not be supported by tissue biopsies (DIF analysis), using a standardized evaluation protocol.17,18 All the cases included in this study suffered from trichiasis and chronic inflammation accompanying fibrotic changes that produced symblepharon affecting visual function. All patients had previously been on topical anti-inflammatory treatment, lubrication, oral tetracyclines, oral/topical antihistamines, and systemic immunomodulators, but with results were poor. The data were collected from medical records: gender, age, systemic diseases, systemic immunomodulatory treatments, and previous ophthalmological treatments (medical and surgical). The minimum follow-up time of the patients was 6 months, and the main variable was the control of inflammation in the CC. The conjunctival inflammation was evaluated on a scale of 0 to 4 depending on severity, 0 (absence of conjunctival inflammation), 1: mild inflammation (conjunctival hyperemia and mild stromal oedema), 2: moderate inflammation (marked conjunctival hyperemia with stromal oedema, and thickening tissue, not conjunctival ulcer or corneal limbitis), 3: Severe inflammation (inflammation in all four quadrants with severe corneal oedema, corneal limbitis and conjunctival ulceration may be present), 4: Very severe inflammation (severe inflammation of 4 quadrants, large tissue oedema, ulcers and corneal limbitis, high risk of eye perforation).17 The CC was classified as severe if it involved 5 mm of the central cornea (pupillary area) with neo-vessels, scarring, ulceration, conjunctivalization or keratinization.19 All patients underwent an initial exploration under a slit lamp of the ocular surface, cornea and fundus in pharmacological dilatation. The BCVA (Better Corrected Visual Acuity), IOP (intraocular pressure) was measured with Goldmann tonometer, the Tear Breakup time (TBUT) was measured in seconds and using fluorescein as staining, and the Schirmer I test was also performed and measured in millimetres for 5 minutes, these evaluations were carried out at the beginning and each follow-up visit.
Preparation and Use of Immunosafe PRGF
The protocol for the preparation of Endoret-PRGF eye drops (ePRGF) is described in the kit’s instructions for use, and it has been published by Anitua and Col.15,16,20 The procedure was carried out under sterile conditions in the surgical room. To prepare the ePRGF, 70 mL of peripheral blood was collected into 9 mL tubes with 3.8% sodium citrate as an anticoagulant. Subsequently, the blood was centrifuged at 580g at room temperature for 8 minutes, using the closed Endoret-PRGF system (BTI Biotechnology Institute, S.L., Miñano, Álava, Spain). The whole column of PRGF was collected after centrifugation using the Endoret-PRGF ophthalmology kit (BTI Biotechnology Institute) and avoiding the buffy coat that contains the leukocytes. PRGF eye drops were incubated with the Plasmaterm H Plus heater (BTI Biotechnology Institute) first at 37ºC for one hour. The supernatant was then heat-treated at 56ºC for one hour to reduce the immunologic components20 (immunosafe heat treatment). Finally, the plasma supernatant was filtered, aliquoted, and dispensed for use as eye drops. The immunosafe PRGF eye drop treatment (is-ePRGF) was then applied at a dose of 1 drop/per eye four times a day and deposited in the conjunctival sac. Interestingly each unit dose could remain up to 72 hours at room temperature without losing its regenerative properties while the whole rack containing the rest of the droppers was storage at the patient’s freezer (at −18/-20ºC). The injectable is-ePRGF was obtained from the is-ePRGF droppers and used in the surgical act (subconjunctival injection and areas of fibrosis) when needed.
Previous chronic treatments were maintained according to medical criteria to avoid systemic complications that could endanger the patient’s health and compromise the results of the is-ePRGF ocular treatment. Only once the disease’s clinical manifestations were effectively controlled, the patients were treated with is-ePRGF. The treatment was divided in three different phases:
Phase I = Treatment with topical is-ePRGF: application of the eye drops is-for one month (1 drop in conjunctival sac four times a day), associated with your previous treatments: topical anti-inflammatory treatment, lubrication, oral tetracyclines, oral/topical antihistamines, and systemic immunomodulators.
Phase II = Treatment with injected is-ePRGF: Applied in different eye areas as needed (sack funds, symblepharon bridges, subconjunctival, perilimbal, lacrimal gland, and Intrapalpebral), this procedure is performed monthly and repeated according to evolution up to a maximum of 6 times, and the treatment provided in Phase I was maintained.
Phase III = When the disease has stabilized, and extensive scar sequelae remain, surgical intervention is considered for visual rehabilitation (phototherapeutic keratectomy – PTK, Keratoprosthesis, phacoemulsification with Intraocular Lens (IOL) implantation) and sequential surgery of the ocular surface, where a therapeutic keratectomy is performed, resection of fibrotic zones, the subconjunctival and intralesional is-ePRGF injection was then made, and Phase I treatment maintained on an outpatient care for at least three months.
The safety profile of the use of topical and injected is-ePRGF was evaluated. Inflammatory recurrences, rescue treatments, additional surgeries during follow-up and infectious complications were agreed to be reported.
Statistical Analysis
Descriptive statistics were performed using absolute and relative frequencies for qualitative variables, and for quantitative variables, the mean and standard deviation were used. The non-parametric Wilcoxon statistical test was used for quantitative variables when required, the McNemar test and Chi-square for categorical variables. The level of statistical significance was at p <0.05. The statistical software used was SPSS v22.0 for Windows (SPSS Inc., Chicago, IL, USA), Excel software version 14.0 (Microsoft Office 2011, Microsoft Corp) was also used.
Results
Ten eyes from six Caucasian patients with CC were evaluated in this study (Table 1). Three patients (50%) were male, and three were female (50%). Two patients (one male and one female) had only one eye affected, while the remaining ones (four) had both eyes affected. The mean age of the patients was 59.7 years (SD = 16.5 range 39–80). The pre-treatment diagnosis included SJS (3 patients and five eyes) and OcMMP (3 patients and five eyes). All patients were diagnosed at least 60 months before the beginning of the study. No results of conjunctival biopsies were reported neither at the beginning nor during this study.
 |
Table 1 Characteristics of Patients with Cicatrizing Conjunctivitis |
In addition to the ophthalmological involvement of autoimmune disease, other tissues were compromised: mouth and skin (three patients), face and scalp (one patient). All patients had previously received topical treatment cycles (dexamethasone, artificial tear, moxifloxacin, fusidic acid, azelastine) and immunomodulatory treatment (azathioprine 50 mg/day), without achieving clinical stability on their ocular manifestations. All treatments were maintained throughout the study (Table 1). The patients presented in addition to the CC other ocular diagnoses: glaucoma (2 eyes of 2 patients), aphakia (1 eye of 1 patient), pseudophakia (6 eyes of 4 patients), LSCD (2 eyes of 1 patient) and cataract (2 eyes of 1 patient). In the fundus, no abnormalities were detected in the initial examination or during the follow-up.
A total of 50% per cent of the cases were considered severe CC, that is, with the four affected quadrants, presenting oedema, and also being able to suffer from limbitis and ulcerations. The initial inflammation ranged between grades 2 to 4; with four eyes (40%) with the degree of inflammation 2 being classified, two eyes (20%) with the degree of inflammation 3 and four eyes (40%) with the degree of inflammation 4 (Table 2). The mean follow-up time was 23.1 months (± 6.7, range 13.6–30.3). A total of 20% of cases (2 eyes) required only Phase I treatment to achieve clinical stability, 70% of cases (7 eyes) required Phases I and II to achieve stability, while only 10% of cases (1 eye) required the 3 phases of is-ePRGF treatment to achieve clinical stability.
 |
Table 2 Results of Treatment with PRGF in Phases |
The initial degree of inflammation was: 2 in four eyes (40%), 3 in two eyes (20%), and grade 4 in four eyes (40%); and the degree of inflammation at the end of the follow-up was: 1 in all eyes (100%), with a statistically significant decrease in inflammation (p = 0.004). Overall, the values of the degree of inflammation went from 2.9 ± 1.1 to 1.0 ± 0.0, which implies going from an inflammation considered severe to a mild inflammation (Table 3). From a total of 3 patients (5 eyes) who were initially considered as severe CC, only one eye of one patient became catalogued as severe CC at the end of the treatment (p = 0.046).
 |
Table 3 Overall Results of Treatment with PRGF |
The IOP changed from initial values of 21.8 ± 4.2 mmHg to final values of 13.3 ± 2.4 mmHg (p = 0.027). TBUT changed from pre-treatment values of 9.4 (SD ± 1.3) to post-treatment values of 9.7 (SD ± 0.8), showing no statistically significant improvement. The initial Schirmer I test was 15.0 ± 5.3, and at the end of the follow-up, it was 21.4 ± 6.3, with an improvement of 42.7% (p = 0.057) (Table 3).
The BCVA improvement was observed in two cases (20%), it remained stable in 7 cases (70%), and worsened only in one case (10%). In the patient 1, the BCVA changed from 0.2 decimal (0.699 LogMAR) to 0.1 decimal (1000 LogMAR), worsening 43.1%, however, the right eye of patient 3 improved the BCVA by 66.7% and in the left eye of patient 6 improved 94.8%. Overall, visual acuity did not suffer statistically significant improvements, from values of 1400 (SD ± 1413) LogMAR before treatment to values of 0.946 (SD ± 1.163) LogMAR after treatment.
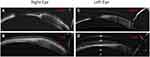
In the analysis by groups according to the aetiology of the CC, it is obtained that 50% corresponds to SJS and the other 50% corresponds to OcMMP, which show identical results in the initial degree of inflammation 3.0 ± 1.0 and the degree of final inflammation 1.0 ± 0.0, obtaining a statistically significant reduction in both groups (p = 0.041) (Figure 1). The corneal tissue regeneration can be assessed using the optical coherence tomography, as was observed in a case with SJS who received treatment with is-ePRGF for one year, requiring only Phase I and II of treatment to achieve clinical stability (Figure 2).
 |
Figure 1 Degree of inflammation according to etiology of cicatrizing conjunctivitis. Note: *p < 0.05. Abbreviations: SJS, Stevens–Johnson syndrome; OcMMP, ocular mucous membrane pemphigoid. |
Regarding safety, no adverse effects were reported at any of the phases during this study; this includes both topical and injected is-ePRGF.
Discussion
The CC is diseases that need a systemic immunomodulatory and ophthalmological approach (repair, antifibrotic and regenerative). In many cases, therapeutic failure is due to non-controlled inflammatory or fibrotic process.1,3,4 PRGF is an autologous blood derivative with great versatility in ophthalmology. Indeed, the different PRGF-based formulations including injectable liquid, eye drops, clot and membrane (for outpatient treatment and surgical use) exert regenerative, anti-inflammatory, antifibrotic, anti-bacterial, proliferative and cell migration properties.14–16 Therefore, CC therapy should be focused on regeneration and on avoiding inflammation and fibrosis before reaching the risk of corneal blindness or other ocular surface complications. In this particular study, and intending to avoid the potential risk of certain side effects related to the PRGF’s IgE levels and complement activity in autoimmune diseases or other refractory cases to the treatment, we decided to apply Immunosafe PRGF formulations.
The treatment of CC should be oriented towards the following key points: 1) Control of ocular surface disease (ex: trichiasis, entropion, PED, dry eye); 2) Control of immune-mediated inflammation with systemic drugs (prednisolone, immunoglobulins IV, cyclophosphamide, mycophenolate mofetil, azathioprine, methotrexate, dapsone, sulfapyridine, rituximab [Anti-CD20], infliximab [anti-TNFα]); 3) Control of fibrosis (reconstructive surgical treatment, topical anti-inflammatory, regenerative therapies); 4) Prophylaxis in corneal ulcers and exposure; 5) improved vision in patients with corneal blindness.5,21 Antimetabolites therapy (Mitomycin C: MMC, 5 fluorouracil: 5-FU) is among the conjunctival antifibrotic strategies most used to treat CC, although this treatment has some drawbacks such as the risks of three scleral thinning and the anterior chamber toxicity. Over-expression of TGFβ in patients with CC increases fibroblast proliferation, migration, collagen contraction, myofibroblastic transformation and increased secretion of extracellular matrix (collagen I, III and fibronectin). Hence, the development of a therapeutic tool to modulate TGFβ response could be an alternative for the treatment of patients with CC.22 Clinical investigations include the use of antibodies against TGF and the use of Rho kinase inhibitors.23,24
A preclinical study showed that PRGF treatment reduced drastically the number of α-smooth muscle actin (α-SMA) positive cells in human keratocytes and human conjunctival fibroblasts cell cultures treated for three days with TGFβ-1. According to this study, PRGF reduces fibrosis involved in the wound healing process by significantly inhibiting TGF β1–induced myofibroblast differentiation.25 Besides, for ocular autoimmune-related diseases, it has been demonstrated that an extra 1-h heat treatment at 56ºC of the autologous PRGF eye drop formulation does not alter some of its protein and growth factor content, both involved in the regeneration process, nor its therapeutic potential,20 and they can be correlated with the results obtained by Freire et al.26 At the same time, PRGF inactivation preserves the content of most of the proteins and morphogens involved in the wound healing effects while reducing the content of IgE and complement activity drastically.20 The latter could be useful for the treatment of ocular disorders in those patients where immunoglobulin IgE has the leading role in the pathogenesis of their diseases.9,10,20
The objective of this study was evaluating the efficacy and safety of isPRGF using its injectable and eye drop formulations as a novel approach in patients with CC, with at least 60 weeks of evolution with poor results in their before treatments and high risk of ophthalmologic complications. To the best of our knowledge, this is the first report using isPRGF in an up to three phases treatment for the control of the pathology’s ocular symptoms. Moreover, this is the first report using these two combined PRGF applications (topical and injected is-ePRGF) for any ocular surface treatment. The use of topical is-ePRGF in the pre-surgical phase improves inflammation control and reduces fibrosis, on the other hand, the use of injectable is-ePRGF in subconjunctival and intralesional injections at the surgical phase, also contribute to the control inflammation and decrease the risk of fibrotic tract formation. The use of topical is-ePRGF after the surgical treatment is also recommended to control better the inflammation and fibrosis listed above.
In this study, the inflammation and severity outcomes showed a significant improvement (p < 0.05) for the 6 patients treated during the follow up (mean 23.1 weeks). Ten out of 10 eyes treated presented an inflammation grade improvement (p < 0.004) and from 5 eyes initially considered as severe CC, only 1 remained with this status (p < 0.046). The IOP also showed a significant improvement (p < 0.027), results similar to those obtained in other PRGF related studies,8,10,25 being some of these directly or indirectly related to glaucoma.27 Further studies may be beneficial to clarify the role of PRGF for the management of the IOP and its clinical applications.
The study has certain limitations: first, it is a retrospective and non-comparative study. Second, being CC considered as a rare disease, the recruitment of patients for the study has been challenging, and thus only a limited number of patients were included. However, according to our understanding, this study with is-ePRGF can be considered an initial clinical proof of content which may help to promote new clinical trials in this particular topic in the future.
Conclusion
This preliminary study suggests that is-ePRGF (through its role in the modulation of TGFβ in the ocular surface and corneal tissues, and control of inflammatory processes) can decrease/reverse fibrosis in patients with CC. It also suggests that for those patients with autoimmune diseases and difficult management due to a high risk of fibrosis and inflammation recurrence, lower IgE levels and decreased complement activity may also help obtain long term fibrosis and inflammation reduction outcomes. Hence, though studies with a larger cohort of patients are needed, medical treatment with is-ePRGF, as an adjuvant to systemic immunomodulation and surgery in patients with CC, may be considered as a new therapeutic option, promoting cell migration and proliferation, and therefore enhancing tissue regeneration.
Abbreviations
BCVA, better corrected visual acuity; CC, cicatrizing conjunctivitis; DIF, direct immunofluorescence; PRGF, plasma rich in growth factors; ePRGF, plasma rich in growth factors eye drops; isPRGF, immunosafe Plasma rich in growth factors; is-ePRGF, immunosafe PRGF eye drop formulation; GVHD, graft versus host disease; IIF, indirect immunofluorescence; IOL, intraocular lens; IOP, intraocular pressure; LSCD, limbal stem cell deficiency; MMP, mucous membrane pemphigoid; NET, toxic epidermal necrolysis; OcMMP, ocular mucous membrane pemphigoid; PED, persistent epithelial defects; PTK, phototherapeutic keratectomy; SJS, Stevens–Johnson syndrome; TBUT, tear breakup time.
Ethics Approval and Informed Consent
The Institutional Review Board approved this study. The principles of the Declaration of Helsinki were followed, and all patients signed informed consent before being of PRGF therapy.
Disclosure
The authors declare the following competing financial interest(s): EA is the Scientific Director of Biotechnology Institute; RMSA, GO, and BDLSC are scientists at BTI Biotechnology Institute. RMSA reports personal fees from BTI Biotechnology Institute. The other authors JMI, MCM and JML declare no conflicts of interest in developing this study.
References
1. Bobba S, Devlin C, Di N, et al. Incidence, clinical features and diagnosis of cicatrising conjunctivitis in Australia and New Zealand. Eye. 2018;32(10):1636–1643. doi:10.1038/s41433-018-0155-7
2. Radford CF, Rauz S, Williams GP, Saw VPJ. Incidence, presenting features, and diagnosis of cicatrising conjunctivitis in the United Kingdom. Eye. 2012;26(9):1199–1208. doi:10.1038/eye.2012.119
3. Georgoudis P, Sabatino F, Szentmary N. Ocular mucous membrane pemphigoid: current state of pathophysiology, diagnostics and treatment. Ophthalmol Ther. 2019;8(1):5–17. doi:10.1007/s40123-019-0164-z
4. Wang K, Seitzman G, Gonzales JA. Ocular cicatricial pemphigoid. Curr Opin Ophthalmol. 2018;29(6):543–551. doi:10.1097/ICU.0000000000000517
5. Dart JK. The 2016 Bowman lecture conjunctival curses: scarring conjunctivitis 30 years on. Eye. 2017;31(2):301–332. doi:10.1038/eye.2016.284
6. Merayo-Lloves J, Sanchez-Avila RM, Riestra AC, et al. Safety and efficacy of autologous plasma rich in growth factors eye drops for the treatment of evaporative dry eye. Ophthalmic Res. 2016;56(2):68–73. doi:10.1159/000444496
7. Sanchez-Avila RM, Merayo-Lloves J, Riestra AC, et al. Treatment of patients with neurotrophic keratitis stages 2 and 3 with plasma rich in growth factors (PRGF-Endoret) eye-drops. Int Ophthalmol. 2017. doi:10.1007/s10792-017-0582-7
8. Sánchez-Avila RM, Merayo-Lloves J, Fernández ML, et al. Plasma rich in growth factors eye drops to treat secondary ocular surface disorders in patients with glaucoma. Int Med Case Rep J. 2018;11. doi:10.2147/IMCRJ.S153918
9. Sanchez-Avila RM, Merayo-Lloves J, Riestra AC, et al. The effect of immunologically safe plasma rich in growth factor eye drops in patients with Sjogren syndrome. J Ocul Pharmacol Ther. 2017;33(5):391–399. doi:10.1089/jop.2016.0166
10. Sanchez-Avila RM, Merayo-Lloves J, Muruzabal F, Orive G, Anitua E. Plasma rich in growth factors for the treatment of dry eye from patients with graft versus host diseases. Eur J Ophthalmol. 2018;1120672118818943. doi:10.1177/1120672118818943
11. Merayo-Lloves J, Sanchez RM, Riestra AC, et al. Autologous plasma rich in growth factors eyedrops in refractory cases of ocular surface disorders. Ophthalmic Res. 2015;55(2):53–61. doi:10.1159/000439280
12. Sanchez-Avila RM, Merayo-Lloves J, Riestra AC, et al. Plasma rich in growth factors membrane as coadjuvant treatment in the surgery of ocular surface disorders. Medicine (United States). 2018;97:17. doi:10.1097/MD.0000000000010242
13. Anitua E, Muruzabal F, de la Fuente M, Riestra A, Merayo-Lloves J, Orive G. PRGF exerts more potent proliferative and anti-inflammatory effects than autologous serum on a cell culture inflammatory model. Exp Eye Res. 2016;151:115–121. doi:10.1016/j.exer.2016.08.012
14. Anitua E, Muruzabal F, Tayebba A, et al. Autologous serum and plasma rich in growth factors in ophthalmology: preclinical and clinical studies. Acta Ophthalmol. 2015;93(8):e605–e614. doi:10.1111/aos.12710
15. Anitua E, Muruzabal F, De Fuente M, et al. Plasma rich in growth factors for the treatment of ocular surface diseases plasma rich in growth factors for the treatment of ocular surface diseases. Curr Eye Res. 2016;(February):1–8. doi:10.3109/02713683.2015.1104362
16. Anitua E, de la Fuente M, Muruzabal F, et al. Differential profile of protein expression on human keratocytes treated with autologous serum and plasma rich in growth factors (PRGF). PLoS One. 2018;13(10):e0205073. doi:10.1371/journal.pone.0205073
17. Elder MJ, Bernauer W. Monitoring of activity and progression in cicatrising conjunctivitis. Dev Ophthalmol. 1997;28:111–122.
18. Studie K. Control of ocular disease in mucous membrane pemphigoid. Klinische Monatsblätter für Augenheilkunde. 2014;231:331–334.
19. Ong HS, Setterfield JF, Minassian DC, Dart JK. Mucous membrane pemphigoid with ocular involvement: the clinical phenotype and its relationship to direct immunofluorescence findings. Ophthalmology. 2018;125(4):496–504. doi:10.1016/j.ophtha.2017.10.004
20. Anitua E, Muruzabal F, De la Fuente M, Merayo-Lloves J, Orive G. Effects of heat-treatment on plasma rich in growth factors-derived autologous eye drop. Exp Eye Res. 2014;119:27–34. doi:10.1016/j.exer.2013.12.005
21. Queisi MM, Zein M, Lamba N, Meese H, Foster CS. Update on ocular cicatricial pemphigoid and emerging treatments. Surv Ophthalmol. 2016;61(3):314–317. doi:10.1016/j.survophthal.2015.12.007
22. Zada M, Pattamatta U, White A. Modulation of fibroblasts in conjunctival wound healing. Ophthalmology. 2017;1–14. doi:10.1016/j.ophtha.2017.08.028
23. Khaw P, Grehn F, Hollo G, et al. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 2007;114(10):1822–1830. doi:10.1016/j.ophtha.2007.03.050
24. Futakuchi A, Inoue T, Fujimoto T, Inoue-Mochita M, Kawai M, Tanihara H. The effects of ripasudil (K-115), a Rho kinase inhibitor, on activation of human conjunctival fibroblasts. Exp Eye Res. 2016;149:107–115. doi:10.1016/j.exer.2016.07.001
25. Anitua E, Sanchez M, Merayo-Lloves J, De la Fuente M, Muruzabal F, Orive G. Plasma Rich in Growth Factors (PRGF-Endoret) stimulates proliferation and migration of primary keratocytes and conjunctival fibroblasts and inhibits and reverts TGF-β1–induced myodifferentiation. Invest Ophthalmol Vis Sci. 2011;52(9):6066–6073. doi:10.1167/iovs.11-7302
26. Freire V, Andollo N, Etxebarria J, Durán JA, Morales M-C. In vitro effects of three blood derivatives on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2012;53(9):5571–5578. doi:10.1167/iovs.11-7340
27. Rodriguez-Agirretxe I, Freire V, Muruzabal F, et al. Subconjunctival PRGF fibrin membrane as an adjuvant to nonpenetrating deep sclerectomy: a 2-year pilot study. Ophthalmic Res. 2018;59(1):45–52. doi:10.1159/000481535
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.