Back to Journals » Journal of Inflammation Research » Volume 14
Plasma Pentraxin-3 Combined with Plaque Characteristics Predict Cardiovascular Risk in ST-Segment Elevated Myocardial Infarction: An Optical Coherence Tomography Study
Authors Wang Y, Zhao X, Zhou P, Liu C, Sheng Z, Li J, Zhou J, Chen R , Chen Y, Song L, Zhao H, Yan H
Received 26 July 2021
Accepted for publication 24 August 2021
Published 2 September 2021 Volume 2021:14 Pages 4409—4419
DOI https://doi.org/10.2147/JIR.S330600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Ying Wang,1 Xiaoxiao Zhao,1 Peng Zhou,1 Chen Liu,1 Zhaoxue Sheng,1,2 Jiannan Li,1 Jinying Zhou,1 Runzhen Chen,1 Yi Chen,1 Li Song,1 Hanjun Zhao1 *, Hongbing Yan3 *
1Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College & Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Department of Cardiology, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 3Department of Cardiology, Fuwai Hospital, Chinese Academy of Medical Sciences, Shenzhen, People’s Republic of China
* These authors contributed equally to this work
Correspondence: Hongbing Yan
Department of Cardiology, Fuwai Hospital, Chinese Academy of Medical Sciences, No. 12, Langshan Road, Xili Street, Nanshan District, Shenzhen, 518000, People’s Republic of China
Tel +86-10-88322285
Email [email protected]
Hanjun Zhao
Department of Cardiology, Fuwai Hospital, Chinese Academy of Medical Sciences, No. 167 Beilishi Road, Xicheng District, Beijing, 100037, People’s Republic of China
Tel +86-15210020808
Email [email protected]
Background: Culprit‑plaque morphology [plaque rupture (PR) and plaque erosion (PE) identified by optical coherence tomography (OCT)] and biomarker of vascular inflammation, pentraxin-3 (PTX3), have been reported to influence clinical outcomes in coronary diseases. We aimed to investigate the prognostic implication of culprit-plaque morphology and plasma PTX3 for major adverse cardiovascular events (MACE) in patients with ST-segment elevation myocardial infarction (STEMI).
Methods: A total of 236 patients were enrolled and divided into four groups: PE/low-PTX3 (n = 57), PE/high-PTX3 (n = 47), PR/low-PTX3 (n = 78) and PR/high-PTX3 (n = 54). MACE was defined as the composite of all-cause death, recurrence of myocardial infarction, stroke and unplanned revascularization of any coronary artery.
Results: During the follow-up of 1.9 years, a total of 40 (16.9%) MACE were observed: 5.3% (3 patients) among patients with PE/low-PTX3, 21.3% (10 patients) among patients with PE/high-PTX3, 17.9% (14 patients) among patients with PR/low-PTX3 and 24.1% (13 patients) among patients with PR/high-PTX3 (Log rank P = 0.013). In fully adjusted analyses, patients with high-PTX3 were associated with higher MACE risk (HR: 2.40, 95% CI: 1.26– 4.57, P = 0.008). Patients with PR/high-PTX3 (HR: 5.63, 95% CI: 1.57– 20.16, P = 0.008) and PE/high-PTX3 (HR: 5.44, 95% CI: 1.46– 20.29, P = 0.012) presented higher MACE risk than those with PE/low-PTX3. Adding plasma PTX3 levels and PR to the risk prediction model increased the area under curves to 76.1% (95% CI: 67.6– 84.5%) and the NRI (28.1%, 95% CI: 0.3– 48.3%, P=0.040) and IDI (2.4%, 95% CI: 0.1– 12.9%, P = 0.040).
Conclusion: Patients with PR/high-PTX3 and PE/high-PTX3 presented a poorer prognosis than those with PE/low-PTX3. Combining the culprit-plaque morphology with PTX3 enhanced the predictive ability for MACE and contributed to better identification of high-risk patients.
Trial Registration Number: This study is registered at clinical trials.gov as NCT03593928.
Keywords: pentraxin-3, plaque rupture, optical coherence tomography, cardiovascular risk, ST-segment elevation myocardial infarction
Introduction
Pentraxin-3 (PTX3), an acute phase protein implicated in innate immunity produced mainly by dendritic cells, macrophages, and endothelial cells in response to primary inflammatory stimuli,1 increases in 3–12h after acute myocardial infarction (AMI).2,3 Previous studies have reported that PTX3 levels are involved in the pathogenesis of atherosclerosis4 and influence clinical outcomes.5 Plaque rupture (PR) and plaque erosion (PE) are responsible for the majority of acute coronary events.6 PR identified by optical coherence tomography (OCT) has been demonstrated to be associated with clinical outcomes in patients with AMI.7 In addition, PTX3 has been proven to be a useful inflammatory marker that reflects coronary plaque vulnerability.8 Nevertheless, the combined effect of PTX3 and plaque morphology on prognostic implications remains unclear. The aim of this study was to investigate the prognostic implication of culprit-plaque morphology and plasma PTX3 for major adverse cardiovascular events (MACE) in patients with ST-segment elevation myocardial infarction (STEMI).
Methods
Study Population
As a post hoc analysis of the Optical Coherence Tomography Examination in Acute Myocardial Infarction (OCTAMI) registry (ClinicalTrials.gov: NCT03593928), this study consecutively enrolled 426 patients who were hospitalized for STEMI and underwent primary percutaneous coronary intervention (PCI) and pre-operative OCT examination of the culprit lesion from March 2017 to March 2019. The main exclusion criteria were: cardiogenic shock, severe liver dysfunction, end-stage renal disease, left main coronary artery disease, extremely tortuous or heavily calcified vessels and contraindication to aspirin or ticagrelor. STEMI was diagnosed according to previously established criteria.9 The flowchart is shown in Figure 1. For this analysis, 236 patients were consecutively enrolled and divided into four groups based on plaque morphology and PTX3: PE/low-PTX3 (n = 57), PE/high-PTX3 (n = 47), PR/low-PTX3 (n = 78) and PR/high-PTX3 (n = 54). Written informed consent was obtained from all included patients. The current registry complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Hospital (No. 2017-866).
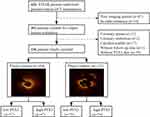 |
Figure 1 Study flow chart. Abbreviations: STEMI, ST-segment elevation myocardial infarction; OCT, optical coherence tomography; PTX3, pentraxin-3. |
Laboratory Tests
Venous blood samples were collected into tubes containing ethylene diamine tetraacetate before PCI. Then, these samples were centrifuged at 2000 ×g for 15 min at room temperature and the plasma was frozen for PTX3 and stored at −80°C until analysis. The levels of plasma PTX3 were detected using commercially available enzyme-linked immunosorbent assay (ELISA) kits in accordance with manufacturer’s instructions (DY1826, R&D Systems, Minneapolis, MN, USA).
Acquisition and Analysis of OCT Images
Based on angiographic results, electrocardiogram manifestations, and echocardiogram-derived regional wall motion abnormalities, the culprit artery was identified immediately after coronary angiography by at least two well-trained cardiologists. Thrombus aspiration and/or gentle pre-dilatation were performed to reduce the thrombus burden and restore antegrade coronary flow. The segments centered on the culprit lesion and extending bilaterally to ≥ 5 mm of the normal vessel segment were identified as the culprit plaques. OCT images of the culprit lesion were acquired using the frequency-domain ILUMIEN OPTIS OCT system and a dragonfly catheter (St. Jude Medical, Westford, MA, USA). All OCT images were analyzed and scrutinized using St Jude OCT Offline Review Workstation by three independent investigators. Definitions of OCT characteristics were based mainly on an established consensus.10 PR was identified as plaque with a disrupted fibrous cap and cavity formation, whereas a PE was defined based on OCT evidence of thrombus, an irregular luminal surface, and no evidence of fibrous cap disruption in multiple adjacent frames. Figure 1 demonstrates representative OCT images of PR and PE.
MACE and Follow-Up
MACEs were defined as composite all-cause death, recurrence of myocardial infarction, stroke and unplanned revascularization of any coronary artery. Follow-up was performed by well-trained physicians routinely at 1, 6, and 12 months after the discharge via outpatient visits or telephone interviews, and then annually, after 1-year follow-up.
Statistical Analysis
Continuous variables are reported as mean ± standard deviation or median (interquartile range) and categorical variables are presented as numbers (percentages). One-way analysis of variance or Kruskal–Wallis tests were used for comparison of continuous variables. Categorical variables were compared using Pearson chi-square tests or or Fisher’s exact test when appropriate. Patients were dichotomized based on a cutoff PTX3 value determined by the Youden index, that is, patients with plasma PTX ≥6.6 ng/mL were identified as the high-PTX3 group, whereas those with plasma PTX3 level of <6.6 ng/mL were assigned to the low-PTX3 group. Survival curves were constructed using the Kaplan-Meier method, and compared using the Log rank test. Univariate and multivariable Cox proportional hazards regression model was used to assess the MACE risk of the four groups, and hazard ratio (HR) and 95% confidence interval (CI) were showed. Subgroup analysis was performed to assess the effect of PTX3 on cardiovascular risk in patients present with different plaque morphology. Time-dependent receiver operating characteristic (ROC) curves were used to show the predictive power of traditional risk factors and the new model combining traditional risk factors, PTX3, and PR. The area under the ROC curve (AUC) was presented. Using net reclassification improvement (NRI) and integrated discrimination improvement (IDI), we also calculated the ability of the new model to reclassify the risk of cardiovascular events in contrast to traditional risk factors. Furthermore, time-dependent ROC curves were used to compare the prognostic value of plasma PTX3 plus plaque morphology and SYNTAX score I and SYNTAX score II. SYNTAX score I and SYNTAX score II were calculated based on previously published studies.11,12
Analyses were conducted using IBM SPSS Statistics version 26.0 (IBM SPSS Statistics, IBM Corporation, Armonk, New York, USA) and R (http://www.r-project.org/) statistical packages. Statistical significance was set at P-value of <0.05.
Results
Baseline Characteristics
The mean age of the 236 enrolled participants was 57.5 ± 11.8 years, with 80.9% being male. Among the patients, 86.9% patients present with the history of dyslipidemia, which was the most prevalent risk factor for atherosclerotic disease, followed by hypertension (61.9%), current smoking (74.2%) and diabetes mellitus (31.4%). The median (interquartile range) plasma PTX3 levels were 6.3 (3.9–8.3) ng/mL. The baseline and angiographic characteristics of the four groups are summarized in Table 1. There were significant differences in body mass index and the distribution of culprit vessels among the four groups (P < 0.001 and P = 0.031, respectively). Other clinical features, laboratory parameters, and post-discharge medications were not significantly different among the four groups.
 |
Table 1 Baseline Characteristics |
OCT Findings
Table 2 summarizes the OCT characteristics, 55.9% of patients with STEMI in this study exhibited PR. Lipid-rich plaques were more prevalent in the PR group than in the PE group, in which fibrous plaques were more prevalent. Notably, patients with PR/high-PTX3 presented with a higher prevalence of thin-cap fibroatheroma (TCFA) and macrophage infiltration. Regarding quantitative OCT imaging, patients with PR tended to exhibit lower minimal fibrous cap thickness, higher maximal lipid arc and slightly larger minimal lumen area.
 |
Table 2 Optical Coherence Tomography Characteristics |
MACE During Follow-Up
As shown in Table 3, during the follow-up period of 1.9 years, a total of 40 (16.9%) MACE were observed: 5.3% (3 patients) among patients with PE/low-PTX3, 21.3% (10 patients) among patients with PE/high-PTX3, 17.9% (14 patients) among patients with PR/low-PTX3 and 24.1% (13 patients) among patients with PR/high-PTX3 (Log rank P=0.013). In fully adjusted analyses (Table 3), patients with high-PTX3 were associated with a higher risk of MACE (HR: 2.40, 95% CI: 1.26–4.57, P = 0.008). Strikingly, the risk of occurrence of MACE in patients with PR/high-PTX3 was 5.63 times greater than that in patients with PE/low-PTX3 (HR: 5.63, 95% CI: 1.57–20.16, P = 0.008), and the risk of MACE in patients with PE/high-PTX3 was 5.44 times greater than patients with PE/low-PTX3 (HR: 5.44, 95% CI: 1.46–20.29, P = 0.012). However, no significant difference in the risk of MACE was noted between PR and PE. When subgroup analysis was performed (Table 4), in the setting of PR, there was no significant difference on the risk of MACE and components when compared between high-PTX3 and low-PTX3. In the setting of PE, patients with high-PTX3 were related to 4.20 times higher risk of developing MACE than patients with low PTX3. KM curves showing the probability of event-free survival in the four groups are presented in Figure 2. There were differences in the risk of myocardial infarction and MACE but not in the risk of stroke and revascularization among the four groups.
 |
Table 3 Hazard Ratio to MACE According to Plaque Morphology and PTX3 |
 |
Table 4 Hazard Ratio to MACE According to PTX3 in Plaque Rupture and Plaque Erosion |
Predictive Role of PTX3 and Plaque Features
As shown in Figure 3, 1-year time-dependent ROC curves were plotted to assess the diagnostic value of traditional risk factors and the new model that combined traditional risk factors, PTX3 and PR. The AUC of risk factors (including age, sex, current smoking, body mass index, hypertension, diabetes mellitus and dyslipidemia) was 67.8% (95% CI: 57.3–78.4%). The AUC of the model combining PTX3 and PR with risk factors was 76.1% (95% CI: 67.6–84.5%). Discrimination and reclassification of 1-year MACE by different models are shown in Table 5. The addition of PTX3 and PR to the traditional risk factors model also resulted in a significant increase in NRI (28.1%, 95% CI: 0.3–48.3%, P=0.040) and IDI (2.4%, 95% CI: 0.1–12.9%, P=0.040). Furthermore, time-dependent ROC curves of plasma PTX3 plus PR, SYNTAX score I, and SYNTAX score II for 1-year MACE occurrences are shown in Figure 4. Levels of plasma PTX3 plus PR presented higher prognostic value (AUC: 67.7, 95% CI: 58.6–76.8%).
 |
Table 5 Discrimination and Reclassification of 1-Year MACE by Different Models |
 |
Figure 3 Time-dependent ROC curves of different models for predicting 1-year major adverse cardiac events. Abbreviations: PTX3, pentraxin-3; PR, plaque rupture; AUC, area under the ROC curve. |
 |
Figure 4 Time-dependent ROC curves by different models for 1-year major adverse cardiac events. Abbreviations: PTX3, pentraxin-3; PR, plaque rupture; AUC, area under the ROC. |
Discussion
To the best of our knowledge, this study is the first to explore the prognostic value of PTX3 and plaque morphology using OCT for predicting cardiovascular events in patients with STEMI. The main findings of this study can be summarized as follows: (1) patients with high-PTX3 were associated with higher risk of MACE, especially those exhibiting PE; (2) patients with PR/high-PTX3 and PE/high-PTX3 presented a poorer prognosis than those in the PE/low-PTX3 group; (3) combining the culprit-plaque morphology with PTX3 enhanced the predictive ability for MACE.
In the early 21st century, Peri et al2 confirmed that increased PTX3 levels were present in the blood of patients with AMI, suggesting that PTX3 is an early indicator of ischemic cardiac injury in humans. PTX3 is produced principally by macrophages and endothelial cells in atherosclerotic plaques.4 Circulating PTX3 levels were higher in AMI than in stable angina pectoris at 3 and 12h3 and the post-PCI plasma PTX3 levels were significantly higher at 24 h after drug eluting stent implantation in patients with stable coronary artery disease.13 In addition, PTX3 levels were found to be positively associated with parameters of coronary plaque vulnerably. Koga et al reported that higher levels of systemic PTX3 were associated with TCFA, indicating that PTX3 is a useful inflammatory marker that reflects coronary plaque vulnerability.8 Circulating inflammatory activity mediated endothelial dysfunction might is a possible major explanation for this phenomenon.14,15
The association of PTX3, as a significant marker of systemic inflammation, with increased risk of MACE has been widely established in previous studies which is in line with our results. A strong positive association between PTX3 and incident cardiovascular disease and all-cause death has been reported in a large cohort of 1583 healthy older adults from the Cardiovascular Health Study.16 In the present study, elevated PTX3 levels were independently associated with the risk of MACE, which was supported by previous researches that revealed patients with increased PTX3 levels had a higher risk of cardiac events in non-ST-segment elevation ACS17 and STEMI.18
Rupture and erosion of the atherosclerotic plaques are the leading causes of life-threatening acute coronary events.6,19,20 Compared with PE, patients with PR tend to present high-risk clinical characteristics, more complex angiographic features and high-risk plaque features, such as TCFA, large lipid core and inflammation activity.21,22 Niccoli et al demonstrated that patients with PR had a worse prognosis than those with an intact fibrous cap.7 Nevertheless, the current study failed to prove the independent prognostic role of PR, though the incidence of MACE was higher in patients with PR than PE (20.5% vs 12.5%). This discrepancy might mainly be due to the small sample size and insufficient follow-up time in this study. In addition, the findings of another study involving a small cohort with acute coronary syndrome cohort from China is consistent with our results.23 Due to the inconsistency of the results from these current studies on the cardiovascular effect of PR, further larger studies and/or pooled analyses are required.
Furthermore, we revealed that the risk of inflammation for cardiac events varied among patients with different plaque pathological types, briefly, in patients with plaque erosion, the higher plasma PTX3 levels were associated with poor prognosis, but this association was not significant in the plaque rupture cohort. This might reflect the different mechanisms and inflammation risk involved in different plaque types, implying that risk stratification in STEMI based solely on plaque morphology is inadequate. Instead, assessing to cardiovascular risk from both plaque vulnerability and inflammation activity is a more comprehensive risk stratification strategy.
PTX3 plays a role as a specific biomarker of vascular inflammation reflecting the risk of clinical events, and identification of plaque pathological characteristics is of great significance for the precise treatment of AMI. The predictive value of SYNTAX score I and SYNTAX score II for clinical outcomes was investigated.11,12,24 Our study demonstrated that plasma PTX3 plus PR presented higher prognostic value than SYNTAX score I and SYNTAX score II. Furthermore, the current research was conducted to verify the combination of PTX3 levels with plaque morphology to identify high-risk patients and revealed that plasma PTX3 and PR added important prognostic information compared with traditional tradition cardiovascular risk factors in patients with STEMI.
There are several limitations in the present study. First, the reported results should be considered preliminary, as our study was limited by a relatively small sample size. And selection bias is probably inevitable due to the single-center, retrospective design with strict inclusion and exclusion criteria. Moreover, the assessment of the plaque morphology was, to a certain extent, subjective and affected by pre-OCT operation including thrombus aspiration and pre-dilatation. Finally, due to the small sample size and short follow-up time, the event rate was relatively low, especially when subgroup analysis was performed. Therefore, further investigations with a larger population and prospective designs are warranted.
Conclusion
In patients with STEMI, those with PR/high-PTX3 and PE/high-PTX3 presented a poorer prognosis than those with PE/low-PTX3. Combining the culprit-plaque morphology with PTX3 enhanced the predictive ability for MACE and contributed to better identification of high-risk patients.
Abbreviations
PTX3, Pentraxin-3; AMI, acute myocardial infarction; OCT, optical coherence tomography; MACE, major adverse cardiovascular events; STEMI, ST-segment elevation myocardial infarction; NRI, net reclassification improvement; IDI, integrated discrimination improvement; TCFA, thin-cap fibroatheroma.
Data Sharing Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The current registry complied with principles of the Declaration of Helsinki and was approved by the Ethics Committee of Fuwai Hospital (No. 2017-866). All patients provided written informed consent.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgments
We gratefully acknowledge all individuals who participated in this study. We are grateful to the Department of Cardiology, Cardiovascular Institute of Fuwai Hospital for its help in recruiting patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is supported by the National Natural Science Foundation of China (81970308), Shenzhen Key Medical Discipline Construction Fund (SZXK001), the Fund of “Sanming” Project of Medicine in Shenzhen (SZSM201911017) and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-1-009).
Disclosure
The authors report non-financial competing interests include family associations, political, religious, academic or any other.
The Authors declare that there is no conflict of interest.
References
1. Goodman AR, Cardozo T, Abagyan R, Altmeyer A, Wisniewski HG, Vilcek J. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996;7(2):191–202. doi:10.1016/1359-6101(96)00019-6
2. Peri G, Introna M, Corradi D, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102(6):636–641. doi:10.1161/01.CIR.102.6.636
3. Helseth R, Solheim S, Opstad T, Hoffmann P, Arnesen H, Seljeflot I. The time profile of pentraxin 3 in patients with acute ST-elevation myocardial infarction and stable angina pectoris undergoing percutaneous coronary intervention. Mediators Inflamm. 2014;2014:608414. doi:10.1155/2014/608414
4. Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22(5):e10–4. doi:10.1161/01.ATV.0000015595.95497.2F
5. Kimura S, Sugiyama T, Hishikari K, et al. Relationship of systemic pentraxin-3 values with coronary plaque components on optical coherence tomography and post-percutaneous coronary intervention outcomes in patients with stable angina pectoris. Atherosclerosis. 2020;292:127–135. doi:10.1016/j.atherosclerosis.2019.11.022
6. Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984;310(18):1137–1140. doi:10.1056/NEJM198405033101801
7. Niccoli G, Montone RA, Di Vito L, et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J. 2015;36(22):1377–1384. doi:10.1093/eurheartj/ehv029
8. Koga S, Ikeda S, Yoshida T, et al. Elevated levels of systemic pentraxin 3 are associated with thin-cap fibroatheroma in coronary culprit lesions: assessment by optical coherence tomography and intravascular ultrasound. JACC Cardiovasc Interv. 2013;6(9):945–954. doi:10.1016/j.jcin.2013.04.024
9. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177.
10. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. 2012;59(12):1058–1072. doi:10.1016/j.jacc.2011.09.079
11. Kappetein AP, Feldman TE, Mack MJ, et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur Heart J. 2011;32(17):2125–2134. doi:10.1093/eurheartj/ehr213
12. Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381(9867):639–650. doi:10.1016/S0140-6736(13)60108-7
13. Haibo L, Xiaofang G, Chunming W, et al. Prognostic value of plasma pentraxin-3 levels in patients with stable coronary artery disease after drug-eluting stent implantation. Mediators Inflamm. 2014;2014:963096. doi:10.1155/2014/963096
14. Gustin C, Delaive E, Dieu M, Calay D, Raes M. Upregulation of pentraxin-3 in human endothelial cells after lysophosphatidic acid exposure. Arterioscler Thromb Vasc Biol. 2008;28(3):491–497. doi:10.1161/ATVBAHA.107.158642
15. Carrizzo A, Lenzi P, Procaccini C, et al. Pentraxin 3 induces vascular endothelial dysfunction through a p-selectin/matrix metalloproteinase-1 pathway. Circulation. 2015;131(17):1495–1505;discussion 1505. doi:10.1161/CIRCULATIONAHA.114.014822
16. Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2009;29(4):594–599. doi:10.1161/ATVBAHA.108.178947
17. Matsui S, Ishii J, Kitagawa F, et al. Pentraxin 3 in unstable angina and non-ST-segment elevation myocardial infarction. Atherosclerosis. 2010;210(1):220–225. doi:10.1016/j.atherosclerosis.2009.10.033
18. Kimura S, Inagaki H, Haraguchi G, et al. Relationships of elevated systemic pentraxin-3 levels with high-risk coronary plaque components and impaired myocardial perfusion after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. Circ J. 2014;78(1):159–169. doi:10.1253/circj.CJ-13-0329
19. Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992;326(4):242–250. doi:10.1056/NEJM199201233260406
20. Narula J, Nakano M, Virmani R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61(10):1041–1051. doi:10.1016/j.jacc.2012.10.054
21. Kim HO, Kim CJ, Kurihara O, et al. Angiographic features of patients with coronary plaque erosion. Int J Cardiol. 2019;288:12–16. doi:10.1016/j.ijcard.2019.03.039
22. Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62(19):1748–1758. doi:10.1016/j.jacc.2013.05.071
23. Hu S, Zhu Y, Zhang Y, et al. Management and outcome of patients with acute coronary syndrome caused by plaque rupture versus plaque erosion: an Intravascular Optical Coherence Tomography Study. J Am Heart Assoc. 2017;6(3):e004730. doi:10.1161/JAHA.116.004730
24. Hayıroğlu M, Keskin M, Uzun AO, et al. Predictive value of SYNTAX score II for clinical outcomes in cardiogenic shock underwent primary percutaneous coronary intervention; a pilot study. Int J Cardiovasc Imaging. 2018;34(3):329–336. doi:10.1007/s10554-017-1241-9
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

