Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Plasma Levels of Long-Acting Injectable Antipsychotics in Outpatient Care: A Retrospective Analysis
Authors Hýža M , Šilhán P , Češková E, Skřont T , Kacířová I , Uřinovská R, Grundmann M
Received 21 December 2020
Accepted for publication 25 February 2021
Published 14 April 2021 Volume 2021:17 Pages 1069—1075
DOI https://doi.org/10.2147/NDT.S298050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Martin Hýža,1,2 Petr Šilhán,1,2 Eva Češková,1,2 Tomáš Skřont,1 Ivana Kacířová,3,4 Romana Uřinovská,3 Milan Grundmann4
1Department of Psychiatry, University Hospital Ostrava, Ostrava, Czech Republic; 2Department of Clinical Neurosciences, Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic; 3Department of Clinical Pharmacology, Institute of Laboratory Diagnostics, University Hospital Ostrava, Ostrava, Czech Republic; 4Department of Clinical Pharmacology, Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic
Correspondence: Petr Šilhán
Department of Psychiatry, University Hospital Ostrava, 17. Listopadu 1790, Ostrava, 708 52, Czech Republic
Tel +420 59 737 3145
Fax +420 59 737 3314
Email [email protected]
Purpose: Antipsychotic efficacy in schizophrenia depends on its availability in the body. Although therapeutic outcomes remain still far from satisfactory, therapeutic drug monitoring is not a common part of clinical practice during treatment with long-acting injectable antipsychotics (LAI AP). The real effectiveness of LAI AP is thus uncertain.
Patients and Methods: We made a retrospective evaluation of plasma levels of LAI AP. Collection of blood samples was performed just before the drug application and one week later. Forty patients with a stabilized clinical condition and steady-state plasma levels were included.
Results: In the observed cohort of patients, flupentixol decanoate (n = 23) was the most often used drug, followed by fluphenazine decanoate (n = 7), haloperidol decanoate (n = 5), paliperidone palmitate (n = 3), and risperidone microspheres (n = 2). Just 5 of 40 patients were treated with a monotherapy. In the period before the application, 60% of the patients did not reach the therapeutic reference range (TRR) and 20% of the patients had an undetectable plasma level. At the time of collection of the second blood samples performed after 7 days, 24% of the patients were under the TRR.
Conclusion: We have found a surprisingly high incidence of plasma levels under the TRR in patients treated with LAI AP. Notwithstanding individual variability in pharmacokinetics, it seems that LAI AP may be underdosed in usual clinical practice.
Keywords: therapeutic drug monitoring, antipsychotics, psychotic disorders, schizophrenia
Introduction
Therapeutic drug monitoring (TDM) is a method of optimization and individualization of pharmacotherapy, the use of which in psychiatry is encouraged by the frequent need of treatment not only of the acute stage of the illness but also of long-lasting effective prophylaxis. Schizophrenia is one of the most serious psychiatric disorders and antipsychotics remain the first-choice drugs for its treatment.
However, there is strong interindividual variability in plasma levels of the psychopharmaceuticals administered in similar doses that can result in up to twentyfold differences.1 The fundamental recommendation for the use of TDM in psychiatry is based on the guidelines of “Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie” (AGNP) published in 2017.1 Generally, TDM is indicated in cases of the proven relation between plasma level and clinical effect; when there exists a distinctive variability in pharmacokinetics; in cases of a narrow therapeutic window; in patients with a suspicion of poor adherence; when it is not possible to optimize the dose on the basis of clinical observation only; when the toxic symptoms are difficult to recognize; and in cases of an altered clearance of the drug.1
A major problem in schizophrenic patients is their lack of insight and thus reduced adherence to treatment. Long-acting injectable antipsychotics (LAI AP) are used in the treatment of schizophrenia both for providing an effective antipsychotic treatment in non-adherent patients and for the reason of more advantageous pharmacokinetics – they avoid the “first-pass” effect and provide stable plasma levels during continuous release of the drug from the muscle depot. Apart from these advantages, this galenic form excludes the risk of overdosing in a suicide attempt and it generally reduces the risk of relapse and the number of rehospitalizations when compared to oral forms.2 In the mirror-analysis by Poloni et al,3 there was a substantial reduction for all considered variables (accesses to ER, number and length of hospitalizations and compulsory admissions) in the 12-month period following the introduction of LAI AP treatment compared to the period before it. However, the advantages of the LAI forms were recently questioned by the results of an extensive meta-analysis4 where neither better tolerance nor effectiveness were found for individual drugs when compared to oral forms. Aripiprazole (reduced number of drop-outs for any reason) and risperidone (reduced incidence of hyperprolactinemia) were the only exceptions. On the other hand, it is not possible to change the dosing of LAI AP flexibly if adverse effects occur.5
The LAI AP are currently prescribed to a broader spectrum of individuals, among patients with higher functional levels or shorter courses of disease and also to those with other diagnoses besides schizophrenia (eg bipolar disorder).6 Moreover, second-generation LAI AP are more frequent in younger patients, employed, with higher levels of an affective component, and a lower number of past LAI AP prescribed.6 Similarly, there are some differences in use among second-generation LAI AP. For instance, in the STAR Network Depot Study, treatment with paliperidone palmitate once-monthly was associated with higher levels of both positive and negative symptoms compared to the aripiprazole monohydrate.7
In outpatient care, LAI AP are perceived as a therapeutic tool that enhances the probability of effective prophylaxis because they overcome the problem of adherence control and provide more stable plasma concentrations. However, the plasma levels of all drugs tend to diverse inter-individually1 and the TDM of LAI AP is still not used as routinely as in oral AP. Therefore, we analysed the plasma levels of LAI AP obtained in outpatient settings in 2015, when we started TDM, to elucidate the dose-concentration relationship of LAI APs and to evaluate the accuracy of their dosage used in common clinical practice.
Patients and Methods
We conducted a retrospective evaluation of plasma levels of LAI AP in individuals treated in the outpatient ward of the psychiatric department of the University Hospital Ostrava (Czech Republic).
We selected the results of TDM in LAI AP obtained in 2015 within the usual regimen of TDM in LAI AP in our department; it means blood was taken twice within one cycle of drug administration – on the day of administration (before administration) and 7 days later. We included the results of TDM in patients of both sexes, over 18 years of age, and with a diagnosis of schizophrenia and related disorders (F2x.x). The diagnosis was assessed by a fully-qualified psychiatrist, according to the ICD-10.8 We evaluated the plasma levels of all LAI AP available in the Czech Republic at that time. Other inclusion criteria were at least three months' treatment with the agents, which is enough to reach the steady-state, and the absence of any treatment changes during this period. The patients had to adhere to the dates of application with a tolerance of ± 2 days.
Patients using the same drug in both LAI and oral form concurrently were excluded. A concurrent long-term use of different psychopharmaceuticals was permissible.
Inclusion Criteria
- Treatment with LAI AP for at least 3 months without dose/frequency changes
- A blood sample obtained within the usual regimen of TDM in LAI AP
- Age of at least 18 years
- Diagnosis of schizophrenia and related disorders (F2x.x)
- Adherence to the date of application with a tolerance of ± 2 days
Exclusion Criterion
- Using the same drug in both LAI and oral form concurrently
After obtaining the blood samples, the specimens were immediately sent for further processing to the Department of Clinical Pharmacology, where they were analysed using the ultra-performance liquid chromatography-tandem mass spectrometry method.9 This method has been fully validated and is used for routine analysis at the University Hospital Ostrava. In the case of risperidone, the sum of a parent compound and an active metabolite was taken into account.
Assessment of the plasma levels according to the schedule described above was a part of the usual clinical practice for TDM in both LAI and oral antipsychotics, and was routinely used for the evaluation of adequate pharmacotherapy. All patients whose data were included in the study signed an informed consent form at the time of their admission to outpatient care, in which they agreed to the processing of their data for research reasons. The use of anonymised data was approved by the Ethical Committee of the University Hospital Ostrava (1130/2019).
Descriptive statistics were used for data processing. Patient representation was described by absolute and/or relative frequencies in each group, where 95% Clopper–Pearson confidence intervals (CI) were added.
Results
A total of 40 patients (23 men and 17 women), with an average age of 45±13 years, fulfilled the inclusion criteria. The second blood-taking was performed in 37 of them because 3 patients refused to have their blood sample collected or did not show up.
The included individuals were most often treated with flupentixol decanoate (n = 23), then fluphenazine decanoate (n = 7), haloperidol decanoate (n = 5), paliperidone palmitate (n = 3), and risperidone microspheres (n = 2). The average intervals between applications were 21 days in flupentixol, 24 days in fluphenazine, 18 days in paliperidone, and 14 days in risperidone.
Regarding the first blood samples obtained (just before the regular administration of LAI AP), the recommended therapeutic reference range (TRR) was not achieved in 24 patients (60.0%, CI 43.3–75.1%) and 8 of them (20.0%, CI 9.1–35.6%) actually had undetectable plasma levels. In the case of the second blood-taking (1 week later), 9 patients (24.3%, CI 11.8–41.2%) did not achieve the TRR, no patient had an undetectable level, and, in 1 patient, the plasma level exceeded the TRR. Only 5 patients (12.5%, CI 4.2–26.8%) were treated with a monotherapy of LAI AP: flupentixol decanoate was given to 4 of them and haloperidol decanoate was given to 1 patient. The rest of the cohort was also treated with an oral AP, with a different agent. The plasma levels of all 5 patients treated with the monotherapy were under the TRR at time of the first blood-taking; in the case of 1 patient treated with flupentixol decanoate and 1 patient with haloperidol decanoate, the levels were actually undetectable. Concise plasma levels are included in Table 1 and in a summary in Table 2. The socio-demographic characteristics of the cohort are provided in Table 3.
 |
Table 1 Concise Data of the 40 Patients Included in the Study |
 |
Table 2 Summarized Data Regarding Individual LAI AP |
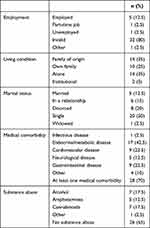 |
Table 3 Socio-Demographic Data, Medical Comorbidity, and Substance Abuse of the Cohort |
The vast majority of applied agents were indicated in accordance with the Summary of Product Characteristics. One diagnosis of acute and transient psychotic disorder and one of bipolar disorder were the only exceptions; however, we found an apparent development in relation to paranoid schizophrenia and schizoaffective disorder when we re-examined these cases in more detail.
Discussion
In the presented study, we performed a retrospective analysis of plasma levels of LAI AP. The results of the patients treated at the outpatient psychiatric ward of the University Hospital Ostrava were included. The choice of a specific LAI AP depends on the clinical features of the patient as well as on the experiences and preferences of individual physicians on outpatient or inpatient wards, because LAI AP are often administered for the first time during hospitalization.
The results obtained may be deemed surprising because the plasma levels before regular administration did not reach the lower borderline of the TRR in more than half of the patients, even though the recommended dosing was maintained. It is necessary to bear in mind the fact that the TRR has been established for oral preparations. In one-fifth of patients, the levels were actually undetectable, and thus the administration of LAI AP most certainly does not fulfil its basic aim in these cases, ie to ensure a permanent presence of the effective agent in the patient's body. Similar results have also been presented recently for oral antipsychotics,10 revealing a pseudo-resistance in patients with schizophrenia. However, the main reason for low concentrations of an oral medication, ie insufficient treatment adherence, can be excluded in the case of LAI AP.
Nevertheless, even subtherapeutic plasma levels might be effective in some patients, according to the definition of TRR.1 But the TRRs have been defined for oral preparations with their pharmacokinetic properties, and it is possible that the TRR of LAI AP could be different.11 This is in accordance with the results of Marder et al,12 who did not observe a connection between the plasma levels of fluphenazine decanoate and its clinical or adverse effects, and assumed that even the low (subtherapeutic) plasma levels of LAI fluphenazine may be effective. Also, the effect of low doses of flupentixol in the treatment of anxiety and depressive disorders is well known.13
Especially in cases of monotherapy associated with low levels of LAI AP, it is possible to ask whether the stabilized clinical condition is a consequence of an effective prophylaxis or whether it is achieved because of the natural course of the disease. The self-evident need of chronic antipsychotic prophylaxis was called into question, eg in the naturalistic observational study performed by Harrow et al.14 On the other hand, regular visits to the outpatient department are considered to be one of the favourable factors in patients treated with LAI AP. During these regular visits, the medical staff are also able to recognize the first signs of possible worsening of the clinical condition.
Combined therapy using LAI AP and oral AP is used in a third to a half of patients in clinical practice11,15 and actually accounted for 87.5% of our cohort. Despite general recommendations where monotherapy is preferred because of missing evidence regarding the combined treatment,16 a combination of LAI AP and oral AP does not necessarily have to be associated with more adverse events, and may even lead to a lower number of drop-outs.12 Considering the high rate of patients receiving combined treatment, we supposed a rather supportive role of LAI AP in a general treatment strategy, the aim of which was to reduce the risk of complete non-adherence. But the frequent use of a combination with the oral AP might also indicate a therapeutic hesitation regarding determination of a specific dose of LAI AP in a specific patient (if not using TDM) when relying on conversion recommendations only. This is contrary to the case of the short-acting agents, where the connection between the dose changes and the clinical effect is much more evident according to clinical practice. The more frequent use of the TDM in LAI AP treatment might help physicians to overcome this therapeutic uncertainty in this way.
The deviations in plasma levels can also be associated with drug or food interactions. In our study, we considered the comedication in patients with undetectable plasma levels only. We did not find the presence of any of the known inhibitors or inducers of the CYP 450 isoenzymes. Smoking, as a well-known inducer of CYP1A2, was not relevant to any of the followed antipsychotics.
Interesting results were found in the case of haloperidol. Its plasma levels declined surprisingly in the second blood sample (obtained one week after the initial administration) in 3 of 4 patients; in one patient, the plasma level was actually on the lower limit of the TRR (1.0 µg/L). An inconstant absorption from the muscle depot can be a possible explanation. In the case of fluphenazine, a subtherapeutic plasma level was observed in all patients even in the second blood samples, and so the overall dosing (including the single dose and interval of an application) should be considered insufficient with respect to all of the above-mentioned limitations.
The study has several obvious limitations. The first is the size of the cohort and its diagnostic heterogeneity. Some of the subgroups (risperidone, paliperidone) were too small to undergo the statistical analysis. The collection of blood samples and their evaluations were performed analogically in relation to the oral medication, and the established regimen of TDM at our department did not respect the pharmacokinetic differences of individual agents but fitted well for everyday clinical use. As mentioned, the TRRs are established for oral preparations only. Also, we did not follow the body weight of the patients, we did not analyse all possible drug and food interactions in the whole cohort, and we did not follow the possible polymorphism of CYP 450 isoenzymes.
Conclusion
LAI AP are primarily used to exclude non-adherence and they should guarantee a sustained therapeutic plasma level of the drug. However, our results show interindividual differences in pharmacokinetics of this antipsychotic medication and emphasize the importance of using TDM in patients treated with LAI AP, especially in monotherapy. Despite the small size and heterogeneity of the cohort, the obtained results raise a question regarding a possible underdosing of LAI AP, even when adhering to the dosing recommendations. This finding should form the basis for future research aimed at effective therapeutic plasma levels in LAI AP.
Data Sharing Statement
The datasets used and analysed during this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Approval was granted by the local ethics committee (of the University Hospital Ostrava) under reference number 1130/2019. This study was conducted in accordance with the Declaration of Helsinki.
Funding
Supported by Ministry of Health, Czech Republic - conceptual development of research organization (FNOs/2017).
Disclosure
Dr Petr Šilhán reports receiving grants from the Ministry of Health of the Czech Republic during the conduct of the study, grants from Janssen-Cilag s.r.o., and personal fees from Lundbeck Czech Republic s.r.o., outside the submitted work. Professor Eva Češková reports receiving personal fees from Angelini Pharma Česká republika s.r.o., outside the submitted work. All other authors report no conflicts of interest in this work.
References
1. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–2):9–62. doi:10.1055/s-0043-116492
2. Mac Ewan JP, Kamat SA, Duffy RA, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67(11):1183–1188. doi:10.1176/appi.ps.201500455
3. Poloni N, Ielmini M, Caselli I, et al. Oral antipsychotic versus long-acting injections antipsychotic in schizophrenia spectrum disorder: a mirror analysis in a real-world clinical setting. Psychopharmacol Bull. 2019;49(2):17–27.
4. Ostuzzi G, Bighelli I, So R, Furukawa TA, Barbui C. Does formulation matter? A systematic review and meta-analysis of oral versus long-acting antipsychotic studies. Schizophr Res. 2017;183:10–21. doi:10.1016/j.schres.2016.11.010
5. Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64(Suppl 16):18–23.
6. Ostuzzi G, Mazzi MA, Terlizzi S, et al. Factors associated with first- versus second- generation long-acting antipsychotics prescribed under ordinary clinical practice in Italy. PLoS One. 2018;13(8):e0201371. doi:10.1371/journal.pone.0201371
7. Bartoli F, Ostuzzi G, Crocamo C, et al. Clinical correlates of paliperidone palmitate and aripiprazole monohydrate prescription for subjects with schizophrenia-spectrum disorders: findings from the STAR Network Depot Study. Int Clin Psychopharmacol. 2020;35(4):214–220. doi:10.1097/YIC.0000000000000317
8. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Description and Diagnostic Guidelines. Geneva: World Health Organization; 1992.
9. Urinovska R, Brozmanova H, Sistik P, et al. Liquid chromatography–tandem mass spectrometry method for determination of five antidepressants and four atypical antipsychotics and their main metabolites in human serum. J Chromatogr B. 2012;907:101–107. doi:10.1016/j.jchromb.2012.09.009
10. McCutcheon R, Beck K, D´Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39–46. doi:10.1111/acps.12825
11. Barnes TR, Shingleton-Smith A, Paton C. Antipsychotic long-acting injections: prescribing practice in the UK. Br J Psychiatry Suppl. 2009;52(S52):S37–42. doi:10.1192/bjp.195.52.s37
12. Marder SR, Aravagiri M, Wirshing WC, Wirshing DA, Lebell M, Mintz J. Fluphenazine plasma level monitoring for patients receiving fluphenazine decanoate. Schizophr Res. 2002;53(1–2):25–30. doi:10.1016/S0920-9964(00)00184-5
13. Altamura AC, Sassella F, Santini A, Montresor C, Fumagalli S, Mundo E. Intramuscular preparations of antipsychotics: uses and relevance in clinical practice. Drugs. 2003;63(5):493–612. doi:10.2165/00003495-200363050-00004
14. Harrow M, Jobe TH, Faull RN. Does treatment of schizophrenia with antipsychotic medications eliminate or reduce psychosis? A 20-year multi-follow-up study. Psychol Med. 2014;44(14):3007–3016. doi:10.1017/S0033291714000610
15. Cordiner M, Shajahan P, McAvoy S, Bashir M, Taylor M. Effectiveness of long-acting antipsychotics in clinical practice: 2. Effects of antipsychotic polypharmacy on risperidone long-acting injection and zuclopenthixol decanoate. Ther Adv Psychopharmacol. 2016;6(2):66–76. doi:10.1177/2045125315623584
16. Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35(3):661–681. doi:10.1016/j.psc.2012.06.007
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
