Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Plasma levels of IL-6 in patients with untreated major depressive disorder: comparison with catecholamine metabolites
Authors Yoshimura R , Kishi T , Iwata N
Received 20 November 2018
Accepted for publication 29 January 2019
Published 13 September 2019 Volume 2019:15 Pages 2655—2661
DOI https://doi.org/10.2147/NDT.S195379
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Reiji Yoshimura,1,* Taro Kishi,2,* Nakao Iwata2
1Department of Psychiatry, University of Occupational and Environmental Health, Kitakyushu, Fukuoka 8078555, Japan; 2Department of Psychiatry, Fujita Medical University, Toyoake Aichi 4701192, Japan
*These authors contributed equally to this work
Correspondence: Reiji Yoshimura
Department of Psychiatry, University of Occupational and Environmental Health, Kitakyushu Fukuoka 8078555, Japan
Tell +81936917253
Email [email protected]
Objective: IL-6 and catecholamines play roles in the pathophysiology of major depressive disorder (MDD).
Aim: The present study investigated associations between plasma IL-6 and plasma catecholamine metabolites in patients with MDD.
Participants and methods: A total of 148 patients (male/female 65/83, age 49.5±12.1 years) who met the criteria for MDD based on the Diagnostic and Statistical Manual of Mental Disorders IV and 40 participants as healthy controls (HC; male/female 23/17, age 44.0±10.5 years) were enrolled in the present study. Plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and homovanillic acid (HVA) were analyzed using high-performance liquid chromatography, and plasma IL-6 levels were measured using ELISA.
Results: No correlations were observed among plasma IL-6 levels, MHPG levels, and HVA levels in patients with MDD. Plasma IL-6 levels in patients with MDD were significantly higher than in the HC. A positive correlation was found between plasma IL-6 levels and Hamilton Rating Scale for Depression-17 scores.
Conclusion: No correlations existed between plasma IL-6 levels and plasma catecholamine metabolite levels in patients with MDD, and the severity of depressive state was related to plasma IL-6 levels in MDD.
Keywords: IL-6, 3-methoxy-4-hydroxyphenylglycol, homovanillic acid, major depressive disorder, Hamilton Rating Scale for Depression
Introduction
Major depressive disorder (MDD) is an important cause of reduced quality of life worldwide.1 It has been speculated that monoamines play a role in MDD,2 but the actions of monoamines during the pathophysiology of MDD remain incompletely understood. Drugs that inhibit the serotonin transporter (5-HTT) and/or the noradrenaline transporter exhibit antidepressive efficacy. Selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs) are first-line treatments for patients with MDD.3 MDD has been shown to be associated with increased levels of circulating cytokines and their soluble receptors.4–10 We recently reported that higher plasma IL-6 activity is associated with refractory depression, and plasma IL-6 levels might be a predictor of the response to SSRIs or SNRIs.11 We also reported that plasma IL-6 levels were significantly higher in patients with dysthymic disorder and MDD than in control subjects.12
We proposed plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG), a major metabolite of noradrenaline, as a candidate biomarker for predicting the response to antidepressants. Responders to milnacipran and duloxetine demonstrated lower plasma MHPG levels than nonresponders. In contrast, responders to paroxetine demonstrated higher serum MHPG levels than nonresponders.13,14 Since MDD is a heterogeneous disease, its pathophysiology might be divided into two groups by serum MHPG levels. We also reported that MDD patients who had higher scores for anxiety and agitation on the Hamilton Rating Scale for Depression-17 (HAM-D17) had better responses to paroxetine, whereas those who had higher scores for psychomotor retardation on the HAM-D17 had better responses to milnacipran.13 Taking these findings into account, it is possible that the dynamics of plasma IL-6 and serum MHPG are altered in patients with MDD, and these factors might be biomarkers for predicting the response to antidepressants. Brydon et al15 reported that stress altered plasma IL-6 levels, suggesting that psychological and immune stressors may act synergistically to promote inflammation and pathological behavior in humans. Parrado et al16 reported that dopamine agonists upregulate IL-6 production in human keratinocytes. Aguilar-Valles et al17 reported that IL-6 alters the function of mesolimbic dopamine neurons in rodents. Therefore, catecholamines influence IL-6 and vice versa.
The synergistic effect between immune activation by lipopolysaccharide and methamphetamine, a dopamine stimulant could have important implications in the treatment of neuroimmune-compromised and dopamine dysfunctional populations such as MDD or schizophrenia.18 Raison et al19 reported peripherally administered interferon-alpha increased cerebrospinal fluid (CSF) IL-6 levels, and which were associated with decreases in the serotonin metabolite, 5-hydroxy-indoleacetic acid (5-HIAA), but not MHPG or homovanillic acid (HVA). This in turn was correlated with depressive symptoms. Thus, we hypothesized that interactions exist between catecholamines and IL-6 in patients with MDD. If so, drugs targeting IL-6 might be effective for MDD. Zhang et al20 demonstrated blockade of IL-6 receptor promotes rapid and sustained antidepressant actions in mice via influencing gut–brain axis. We investigated the associations between serum catecholamine metabolite and plasma IL-6 levels in patients with MDD to confirm this hypothesis.
Subjects and methods
Participants
A total of 148 patients (male/female 65/83, age 49.5±12.1 years) were continuously recruited from 2004 to 2012 at the Neuropsychiatry Branch of the University Hospital of University of Occupational and Environmental Health, Kitakyushu, Japan. The patients were screened for axis I psychiatric disorders using the Japanese version of the Mini-International Neuropsychiatric Interview and blood sampling was performed by Reiji Yoshimura (a board certified psychiatrist with over 30 years of experience) on the first day the patients visited our branch of the hospital. In short, major depressive episodes were diagnosed using the Diagnostic and Statistical Manual of Mental Disorders IV, Text Revision.21 The severity of depression was evaluated using the HAM-D17.22 All patients had not taken antidepressants for at least 1 month before the evaluation. MDD patients whose HAM-D17 scores were 17 or greater were enrolled in the present study. The exclusion criteria included any history of neurological disease or other physical diseases and comorbidity with other psychiatric disorders. Forty participants as healthy controls (HC) were enrolled in the present study. All of them were recruited from nearby communities via an interview conducted by the same psychiatrist using Structured Clinical Interview for DSM-IV Axis I Disorders/Non patients (SCID-I/NP). The HC consisted of not only staff from our institution but also their relatives (marriage) and close friends. Biologically related relatives were excluded from the HC. No HC had a history of severe medical or neuropsychiatric illness or a family history of major psychiatric or neurological illnesses. They also had no history of serious physical diseases and use of drugs (steroid, aspirin, or nonsteroidal anti-inflammatory drugs).
The demographic data for the people with MDD and the HC are described in Table 1. The study protocol was approved by the Ethics Committee of the University of Occupational and Environmental Health, Japan. Written informed consent was obtained from all subjects who participated in this study and the study was conducted in accordance with the Declaration of Helsinki.
 |
Table 1 Demographic data of the MDD patients and healthy controls |
Assays of plasma levels of MHPG, HVA, and IL-6
Circadian variation was demonstrated in catecholamine and their metabolites. Plasma levels of catecholamines and their metabolites are higher during the day than those during the night.23–27 Thus, blood sampling was performed from 9 am to 11 am in the morning after at least 30 minutes after lying in bed. The method used was high-performance liquid chromatography (HPLC). Plasma levels of MHPG and HVA were measured according to the method of Minegishi and Ishizaki28 and Yeung et al,29 respectively. Plasma levels of IL-6 were measured with the methods we previously described.30
Statistical analysis
The Shapiro–Wilk test indicated that IL-6 levels were normally distributed. A generalized linear model was used to explore the association of IL-6 levels with HVA and MHPG levels using potentially confounding variables (age, gender, first episode or not, melancholia or not, duration of untreated illness, and HAM-D17 scores at baseline) as covariates, and the adjusted results were regarded as the study outcomes. All statistical tests were performed using JMP (JMP 12, SAS Japan Inc., Tokyo, Japan), and P<0.05 was considered to indicate statistical significance. Non-paired t-test was performed comparing plasma levels of IL-6 between the MDD patients and HC.
Results
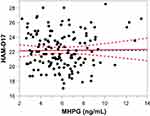
The plasma IL-6 level of the patients with MDD (3.11±1.03 ng/mL) was significantly higher than that of the HCs (2.69±0.83 ng/mL) (P=0.00096) (Figure 1 and Table 3). A significant positive correlation was found between plasma IL-6 levels and HAM-D17 scores in MDD patients (R2=0.32904, P<0.0001) (Figure 2) but not between plasma levels of MHPG (P=0.8630) (Figure 3) or HVA (P=0.9860) (Figure 4) and HAM-D17 scores in MDD patients. No correlations were found among plasma IL-6 levels, plasma MHPG levels, and plasma HVA levels using both raw (Table 4) and adjusted (Table 5) measures in patients with MDD. We also calculated the power of the three measures (Table 6). Moreover, we investigated the correlation between sub-items scores of HAM-D17 according to Seretti et al31 and IL6, MHPG, or HVA. A significant correlation was found all sub-items scores and IL-6. On the other hand, no correlations were found between each-item score and MHPG or HVA (Table 2).
 |
Table 2 Relations between HAM-DI7 sub-items scores and IL-6, MHPG, or HVA |
 |
Table 3 Comparison of IL-6 between the MDD patients and HC |
 |
Table 4 The raw correlations among the biological measures |
 |
Table 5 The adjusted correlations among the biological measures |
 |
Table 6 Calculated power for three biomarkers |
 |
Figure 1 Dot plot of IL-6 between MDD and HC (P=0.00096). Abbreviations: MDD, major depressive disorder; HC, healthy controls. |
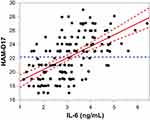 |
Figure 2 Partial regression residual leverage plot of plasma IL-6 levels and HAM-D17 scores (P<0.0001, R2=0.33). Abbreviation: HAM-D17, 17-item Hamilton Depression Rating Scale. |
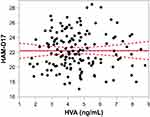 |
Figure 4 Partial regression residual leverage plot of plasma HVAlevels and HAM-D17 scores (P=0.9860). Abbreviations: HAM-D17, 17-item Hamilton Depression Rating Scale; HVA, homovanillic acid. |
Discussion
No correlations were found between plasma IL-6 levels and plasma MHPG levels or plasma HVA levels in patients with MDD. The results do not support the hypothesis that interactions exist between catecholamines and IL-6 in patients with MDD. It has been reported that noradrenaline induces the expression of IL-6 in cell lines.32,33 Handley et al34 reported that IL-6 levels were also lower after treatment with haloperidol than after treatment with placebo, but this effect was not observed for aripiprazole. This result suggests that full antagonism of the dopamine two receptor can lead to a reduction of IL-6 levels.
Miller et al35 reported that cytokines and their signaling pathways, including p38 mitogen-activated protein kinase, have significant effects on the metabolism of multiple neurotransmitters, such as serotonin, dopamine, and glutamate, by affecting their synthesis, release, and reuptake. Felger et al36 reported that connectivity between the striatum and the ventromedial prefrontal cortex was associated with increased plasma levels of IL-6, IL-1 beta, and IL-1 receptor antagonists, which suggests that decreased corticostriatal connectivity may serve as a target for anti-inflammatory or prodopaminergic treatment strategies to improve motivational and motor deficits in patients with increased inflammation, including depression. These findings were inconsistent, and we considered that noradrenaline and dopamine may enhance IL-6 levels. We reported that plasma IL-6 levels were significantly higher in patients with MDD and dysthymic disorder than in HC.37
We also reported that among patients with MDD, patients who responded to paroxetine and sertraline had higher plasma IL-6 levels than nonresponders to those antidepressants.11 Fluvoxamine decreased plasma IL-6 levels, which was associated with improved depressive symptoms, as evaluated by the HAM-D17.38 Manoharan et al39 also reported a significant correlation between the percentage change in plasma IL-6 levels and the percentage change in HAMD scores among MDD patients who responded to treatment with fluoxetine. In contrast, Fornaro et al40 reported that plasma IL-6 levels increased significantly only in responders to duloxetine using a very small sample of only nine patients. Based on our previous unpublished experiments, no significant differences were observed in plasma MHPG and plasma HVA levels between MDD patients and HC because of their broad distribution (data not shown). A recent report demonstrated that HVA levels, but not MHPG, and 5-HIAA in the CSF were significantly decreased in MDD patients.41 Another report also demonstrated a significant reduction in HVA levels, but not MHPG in the CSF between MDD patients and HC.42 Sasayama et al43 reported that both the patients with schizophrenia and MDD had significantly higher CSF IL-6 levels compared to the HC. IL-6 levels were significantly higher in the CSF than in the serum. The authors also demonstrated that no significant correlation was observed between CSF and serum IL-6 levels. The present findings suggest that IL-6 of central origin is associated with the pathophysiology of schizophrenia and MDD. We interpret the results as follows. Since MDD is heterogeneous, it is reasonable that the levels of plasma IL-6, MHPG, and HVA vary widely. Moreover, individual levels of IL-6, MHPG, and HVA are regulated by both genetic factors and epigenetic factors. Therefore, complicated mechanisms might link IL-6 and catecholamines. We can categorize MDD patients into several subtypes according to plasma levels of IL-6, MHPG, and HVA. SSRIs and SNRIs both work on the serotonin transporter. We reported no differences in baseline IL-6 levels or the change in plasma IL-6 levels between patients who were serotonin transporter (5-HTT) gene L carriers and those who had the S/S genotype, which indicates that the plasma IL-6 level is independent of the 5-HTT genotype.11
We confirmed our preliminary results that plasma IL-6 levels are significantly higher in MDD patients than in HC and that there is a positive correlation between plasma IL-6 levels and HAM-D17 scores. Furthermore, we additionally reported significant correlations between sub-items scores of HAM-D17 according to Seretti et al31 and plasma IL-6 levels. Several recent reports demonstrated a positive correlation between plasma IL-6 levels and HAM-D17 scores.38,44–46 The results of the present study were in accordance with those of recent studies, including our previous study. Thus, it is possible that plasma IL-6 is a biomarker of MDD severity. This preliminary study had several serious limitations, such as a small sample, heterogeneous participants, and a cross-sectional design. In addition, we should correct the plasma IL-6 levels with body mass index, because body mass index was reported to influence plasma IL-6 levels.47 Thus, we are performing a longitudinal study with a large sample, with age-, sex-, and BMI-matched control groups, to reconfirm these preliminary results.
Conclusion
No correlations existed between plasma IL-6 levels and plasma MHPG or HVA levels in patients with MDD. Plasma IL-6 levels were significantly higher in MDD patients than in HC, and a positive correlation was observed between plasma IL-6 levels and HAM-D17 scores in patients with MDD.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800.
2. Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63.
3. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366.
4. Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(1):11–38.
5. Maes M. The immune pathophysiology of major depression. In: Honing A, van Praag H, editors. Depression Neurobiological Psychophysiological and Therapeutic Advances. Chester: Wiley; 1997:197–215.
6. Sluzewska A, Rybakowski J, Bosmans E, et al. Indicators of immune activation in major depression. Psychiatry Res. 1996;64(3):161–167.
7. Berk M, Wadee AA, Kuschke RH, O’Neill-Kerr A. Acute phase proteins in major depression. J Psychosom Res. 1997;43(5):529–534.
8. Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247(4):228–233.
9. Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157(5):683–694.
10. Shiepers O, Wichers M, Maes M. Cytokines and major depression, and antidepressants medications: meta-analysis and implication. Biol Psychiatry. 2008;64:527–532.
11. Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Ueda N, Nakamura J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(4):722–726.
12. Yoshimura R, Umene-Nakano W, Suzuki A, et al. Rapid response to paroxetine is associated with plasma paroxetine levels at 4 but not 8 weeks of treatment, and is independent of serotonin transporter promoter polymorphism in Japanese depressed patients. Hum Psychopharmacol. 2009;24(6):489–494.
13. Shinkai K, Yoshimura R, Ueda N, Okamoto K, Nakamura J. Associations between baseline plasma MHPG (3-methoxy-4-hydroxyphenylglycol) levels and clinical responses with respect to milnacipran versus paroxetine treatment. J Clin Psychopharmacol. 2004;24(1):11–17.
14. Atake K, Yoshimura R, Hori H, Katsuki A, Nakamura J. Catechol-O-methyltransferase Val158Met genotype and the clinical responses to duloxetine treatment or plasma levels of 3-methoxy-4-hydroxyphenylglycol and homovanillic acid in Japanese patients with major depressive disorder. Neuropsychiatr Dis Treat. 2015;11:967–974.
15. Brydon L, Walker C, Wawrzyniak A, et al. Synergistic effects of psychological and immune stressors on inflammatory cytokine and Sickness responses in humans. Brain Behav Immun. 2009;23(2):217–224.
16. Parrado AC, Canellada A, Gentile T, Rey-Roldán EB. Dopamine agonists upregulate IL-6 and IL-8 production in human keratinocytes. Neuroimmunomodulation. 2012;19(6):359–366.
17. Aguilar-Valles A, Jung S, Poole S, Flores C, Luheshi GN. Leptin and interleukin-6 alter the function of mesolimbic dopamine neurons in a rodent model of prenatal inflammation. Psychoneuroendocrinology. 2012;37(7):956–969.
18. Petrulli JR, Kalish B, Nabulsi NB, Huang Y, Hannestad J, Morris ED. Systemic inflammation enhances stimulant-induced striatal dopamine elevation. Transl Psychiatry. 2017;7(3):e1076.
19. Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303.
20. Zhang J-C, Yao W, Dong C, et al. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry. 2017;7(5):e1138.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). Washington, D.C.: American Psychiatric Association; 2000.
22. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
23. Turton MB, Deegan T. Circadian variations of plasma catecholamine, cortisol and immunoreactive insulin concentrations in supine subjects. Clin Chim Acta. 1974;55(3):389–397.
24. Kendler KS, Mohs RC, Davis KL. The effects of diet and physical activity on plasma homovanillic acid in normal human subjects. Psychiatry Res. 1983;8(3):215–223.
25. Drici MD, Roux M, Candito M, Rimailho A, Morand P, Lapalus P. Influence of beta-blockade on circulating plasma levels of 3-methoxy- 4-hydroxy phenylethylene glycol (MHPG) during exercise in moderate hypertension. Clin Exp Pharmacol Physiol. 1991;18(12): 807–811.
26. Atuk NO, Hanks JB, Weltman J, Bogdonoff DL, Boyd DG, Vance ML. Circulating dihydroxyphenylglycol and norepinephrine concentrations during sympathetic nervous system activation in patients with pheochromocytoma. J Clin Endocrinol Metab. 1994;79(6):1609–1614.
27. Ishikawa A, Miyatake T. Effects of smoking in patients with early-onset Parkinson’s disease. J Neurol Sci. 1993;117(1–2):28–32.
28. Minegishi A, Ishizaki T. Determination of free 3-methoxy-4-hydroxyphenylglycol with several other monoamine metabolites in plasma by high-performance liquid chromatography with amperometric detection. J Chromatogr. 1984;311(1):51–57.
29. Yeung PK, Buckley SJ, Pedder SC, Dingemanse J. Determination of 3,4-dihydroxyphenylacetic acid and 5-hydroxyindoleacetic acid in human plasma by a simple and rapid high-performance liquid chromatography assay. J Pharm Sci. 1996;85(4):451–453.
30. Hori H, Yoshimura R, Katsuki A, et al. Relationships between serum brain-derived neurotrophic factor, plasma catecholamine metabolites, cytokines, cognitive function and clinical symptoms in Japanese patients with chronic schizophrenia treated with atypical antipsychotic monotherapy. World J Biol Psychiatry. 2017;18(5):401–408.
31. Serretti A, Mandelli L, Lorenzi C, et al. Serotonin transporter gene influences the time course of improvement of “core” depressive and somatic anxiety symptoms during treatment with SSRIs for recurrent mood disorders. Psychiatry Res. 2007;149(1–3):185–193.
32. Yang R, Lin Q, Gao HB, Zhang P. Stress-related hormone norepinephrine induces interleukin-6 expression in ges-1 cells. Braz J Med Biol Res. 2014;47(2):101–109.
33. Li M, Yao W, Li S, Xi J. Norepinephrine induces the expression of interleukin-6 via β-adrenoreceptor-NAD(P)H oxidase system-NF-κB dependent signal pathway in U937 macrophages. Biochem Biophys Res Commun. 2015;460(4):1029–1034.
34. Handley R, Mondelli V, Zelaya F, et al. Effects of antipsychotics on cortisol, interleukin-6 and hippocampal perfusion in healthy volunteers. Schizophr Res. 2016;174(1–3):99–105.
35. Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30(4):297–306.
36. Felger JC, Li Z, Haroon E, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21(10):1358–1365.
37. Yoshimura R, Umene-Nakano W, Hoshuyama T, et al. Plasma levels of brain-derived neurotrophic factor and interleukin-6 in patients with dysthymic disorder: comparison with age- and sex-matched major depressed patients and healthy controls. Hum Psychopharmacol. 2010;25(7–8):566–569.
38. Yoshimura R, Katsuki A, Atake K, Hori H, Igata R, Konishi Y. Influence of fluvoxamine on plasma interleukin-6 or clinical improvement in patients with major depressive disorder. Neuropsychiatr Dis Treat. 2017;13:437–441.
39. Manoharan A, Rajkumar RP, Shewade DG, Sundaram R, Muthuramalingam A, Paul A. Evaluation of interleukin-6 and serotonin as biomarkers to predict response to fluoxetine. Hum Psychopharmacol. 2016; 31(3):178–184.
40. Fornaro M, Martino M, Battaglia F, Colicchio S, Perugi G. Increase in IL-6 levels among major depressive disorder patients after a 6-week treatment with duloxetine 60 mg/day: a preliminary observation. Neuropsychiatr Dis Treat. 2011;7:51–56.
41. Ogawa S, Tsuchimine S, Kunugi H. Cerebrospinal fluid monoamine metabolite concentrations in depressive disorder: a meta-analysis of historic evidence. J Psychiatr Res. 2018;105:137–146.
42. Pech J, Forman J, Kessing LV, Knorr U. Poor evidence for putative abnormalities in cerebrospinal fluid neurotransmitters in patients with depression versus healthy non-psychiatric individuals: a systematic review and meta-analyses of 23 studies. J Affect Disord. 2018;240:6–16.
43. Sasayama D, Hattori K, Wakabayashi C, et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. 2013;47(3):401–406.
44. Xia Q-R, Liang J, Cao Y, Shan F, Liu Y, Xu Y-Y. Increased plasma nesfatin-1 levels may be associated with corticosterone, IL-6, and CRP levels in patients with major depressive disorder. Clin Chim Acta. 2018; 480:107–111.
45. Fan N, Luo Y, Ou Y, He H. Altered serum levels of TNF-α, IL-6, and IL-18 in depressive disorder patients. Hum Psychopharmacol. 2017;32(4): e2588.
46. Yoshimura R, Hori H, Ikenouchi-Sugita A, et al. Plasma levels of interleukin-6 and selective serotonin reuptake inhibitor response in patients with major depressive disorder. Hum Psychopharmacol. 2013; 28(5):466–470.
47. Tucker P, Pfefferbaum B, Nitiéma P, Khan Q, Aggarwal R, Walling EE. Possible link of interleukin-6 and interleukin-2 with psychiatric diagnosis, ethnicity, disaster or BMI. Cytokine. 2017;96:247–252.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.